Abstract
To assess the impact of human bocavirus (HBoV) virus load on epidemiologic and clinical characteristics in children with lower respiratory tract infection (LRTI). Clinical records of a total of 654 patients with HBoV infection during January 2013 and December 2014 were retrospectively reviewed. Patients with high HBoV virus load infection had a similar age distribution with the total HBoV infection, which had a peak age group of 6–24 months. Patients with high virus load are significantly younger (P < 0.01) than those with low load. The patients who had wheeze and tachypnea/dyspnea at presentation were more strongly affiliated with the patients with high virus load (both P < 0.01). Co-infection was found significantly more frequently among patients with low virus load than those with high virus load (57.0% vs 38.9%; P < 0.01). High virus load was a significant predictor of severe LRTI (P < 0.05). HBoV infections are found in an important proportion of the hospitalized children with respiratory illnesses (8.85% in our series). A high HBoV virus load could be an etiologic agent for LRTI, which may lead to more severe lower respiratory tract symptom and severe disease.
The discovery of human bocavirus (HBoV) was the result of a viral study of respiratory secretions from Swedish children with symptoms of acute respiratory infection (ARI) reported in 20051. Increasing evidences are emerging to support its role as an etiologic pathogen in lower respiratory tract infection (LRTI). Its epidemiologic and clinical characteristics have also been assessed2,3,4,5,6,7. These studies reported a prevalence of HBoV infection of 1.5%–21.5% and a coinfection rate with other viruses from 33% to 67% of specimens. The most frequent clinical diagnoses associated with respiratory HBoV infection are upper respiratory tract infections, bronchiolitis, pneumonia, bronchitis and asthma exacerbation8. Nevertheless, few data are available related to HBoV virus load and clinical features for children with HBoV-positive LRTIs.
In this study we aimed to assess the relationship between the HBoV virus load in respiratory tract and clinical characteristics. Samples were referred from January 2013 and December 2014, in Suzhou, China.
Results
Quantitative analysis of HBoV DNA in nasopharyngeal aspirates
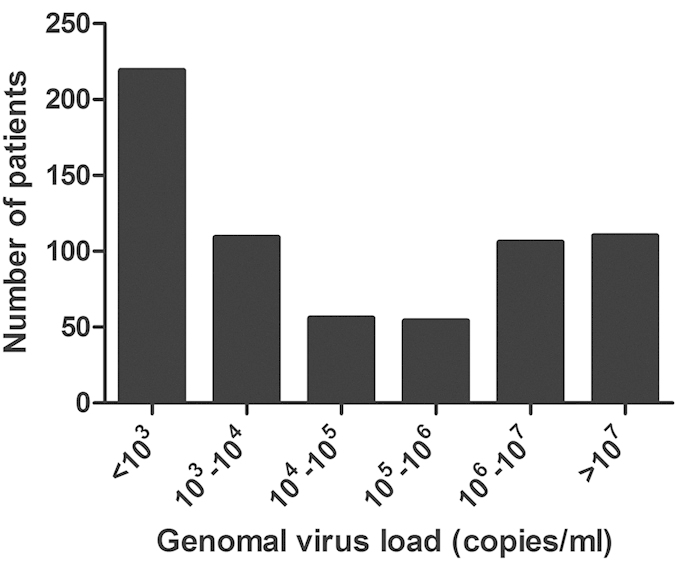
Of the 7393 patients with LRTI, HBoV were positive in 654 patients with a positive rate of 8.85%. The virus load ranged from <103 to 3.97 × 109 copies/ml with a median of 1.2 × 103copies/ml. According to the distribution of genomal virus load (Fig. 1), we classified all patients into high virus load (>104 copies/ml) group (n = 326) and low virus load (≤104 copies/ml) group (n = 328).
Figure 1. Distribution of different virus load in patients with human bocavirus infection.
Demographic characteristics
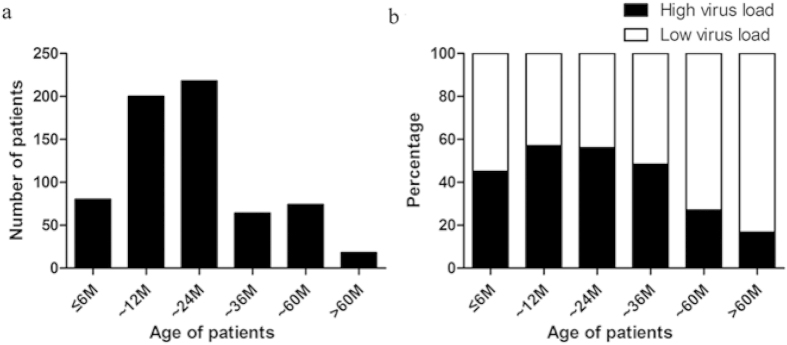
Of the 654 patients with HBoV infection, 431 (65.90%) were males and 223 (34.10%) were females. The male to female ratio was 1.93:1. The median age was 16 months (range from 1 month to 14 years). The age distribution of the patients is shown in Fig. 2A, 418 (63.91%) of the patients were aged 6-24 months.
Figure 2.
(a) The age distribution of the patients with human bocavirus (HBoV) infection. (b) Different virus load individuals categorized according to age group.
There were no significant differences between high and low virus load in different age groups less than 36 months (p > 0.05, Fig. 2B). However, the virus load decreased with their age, with a statistical significance for age distribution (p < 0.001) in patients older than 36 months (Fig. 2B).
Seasonal distribution of HBoV infections
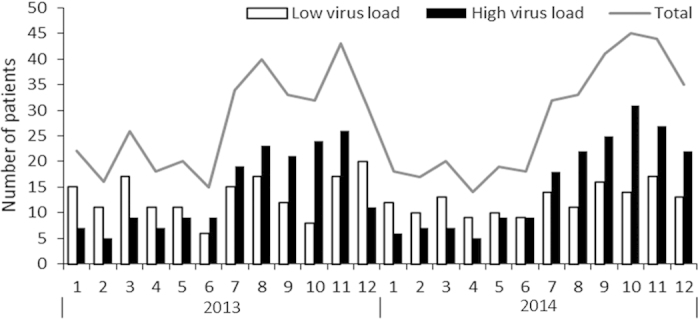
The monthly distribution of HBoV infection is shown in Fig. 3. It occurred throughout the year with a higher proportion from July to December and it peaked in October or November. The frequency of patients with high virus load also increased from July to December and peaked in October and November. Interestingly, the frequency of patients with high virus load was higher from July to December, but lower from January to June.
Figure 3. Monthly occurrence of total episodes of human bocavirus infection and cases with different virus load from 2013 to 2014.
Comparison of clinical characteristics and laboratory values by PCR status
Clinical characteristics and laboratory values of children infected by HBoV with high virus load and low virus load are shown in Table 1. To exclude the interaction between parameters, mean age, fever, wheezing and tachypnea/dyspnea at presentation were tested by logistic regression analysis. On clinical manifestations, patients with high virus load were significantly younger (P < 0.01) than those with low load. The frequency of fever was significantly lower in patients with high virus load compared with low load (P < 0.01). The patients who had wheeze and tachypnea/dyspnea at presentation were more strongly affiliated with the patients with high virus load (both P < 0.01).
Table 1. Clinical characteristics and laboratory values of children infected by human bocavirus (HBoV) with high virus load and low virus load.
| Parameters | high virus load (n = 326) | low virus load (n = 328) | P value |
|---|---|---|---|
| Age (m), median | 16.8 ± 12.2 | 22.2 ± 18.4 | <0.001 |
| Clinical features | |||
| Cough | 319 (97.9) | 320 (97.6) | 0.80 |
| Wheezing | 118 (36.2) | 41(12.5) | <0.001 |
| Fever | 169 (51.8) | 199 (60.7) | 0.001 |
| Rhinorrhea | 136 (41.7) | 135 (41.2) | 0.89 |
| Vomiting/diarrhea | 75 (23.0) | 85 (25.9) | 0.39 |
| Tachypnea/dyspnea | 43 (13.2) | 17 (5.2) | <0.001 |
| Median stay of hospitalization (d) | 7 | 7 | 0.193 |
| Laboratory values | |||
| White blood cells, median (×109/L) | 9.6 ± 4.9 | 9.3 ± 4.7 | 0.354 |
| Neutrophils, median (%) | 47.3 ± 19.2 | 39.9 ± 18.2 | 0.024 |
| Platelet (×109/L) | 370.2 ± 111.2 | 323 ± 114.3 | 0.083 |
| C-reaction protein, median (mg/L) | 1.3 ± 0.9 | 2.5 ± 1.1 | 0.113 |
Mean age, fever, wheezing and tachypnea/dyspnea at presentation were tested by logistic regression analysis.
Comparison of co-infection by PCR status
Of the 654 children with HBoV infection, 321 (49.1%) patients were co-infected with other respiratory pathogens, including 236 (36.1%) patients with Mycoplasma pneumoniae (MP), 46 (7.0%) with respiratory syncytial virus (RSV), 20 (3.1%) with parainfluenza virus (PIV) 1-3, 11 (1.7%) with influenza virus (IV)-A, 8 (1.2%) with adenovirus (ADV). Among the 321 cases, 36 patients were with two other pathogens.
Co-infection was found significantly more frequently among patients with low virus load than those with high virus load (57.0% vs 38.9%; P < 0.01). Co-infection with RSV was more strongly affiliated with the patients with low virus load (both P < 0.01, Fig. 4).
Figure 4. Co-infection with other pathogens in human bocavirus infection patients with different virus load.
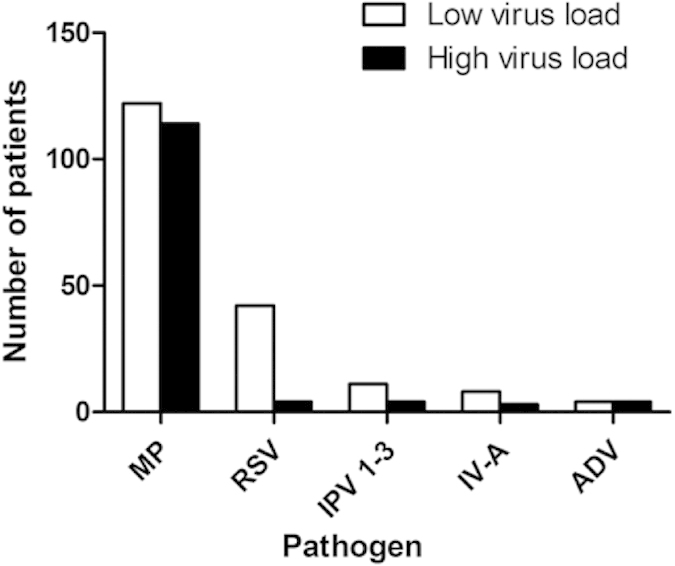
MP stands for Mycoplasma pneumoniae, RSV stands for respiratory syncytial virus, PIV stands for parainfluenza virus, IV stands for influenza virus, ADV stands for adenovirus.
Risk Factors for disease severity
Fifty-one (7.8%) patients received O2 (38 with high virus load and 13 with low virus load). None of the patients transferred to ICU. Requirement for O2 was used to evaluate disease severity. In unadjusted analysis, requirement for O2 was associated with young age, HBoV single infection and high virus load (all P < 0.05). Other variables (sex, white blood cells, percentage of neutrophils, platelets and C-reaction protein [CRP]) showed no difference.
The multivariable logistic regression model for requirement for O2 is shown in Table 2. Controlling for 4 demographic and clinical characteristics, significant predictors of requirement for O2 were age <2 months and high virus load (both P < 0.05).
Table 2. Multivariable predictors of requirement for O2 among children with human bocavirus (HBoV) infection.
| Characteristics | OR (95% CI) | P value |
|---|---|---|
| Age in months | ||
| <6 | 2.54 (1.12–5.73) | 0.02 |
| 6–24 | 0.93 (0.74–1.11) | 0.64 |
| >24 | 1.00 (reference) | – |
| Sex | ||
| Male | 0.98 (0.82–1.49) | 0.52 |
| Female | 1.00 (reference) | – |
| HBoV single or co-infection | ||
| Single infection | 1.39 (0.87–2.22) | 0.17 |
| Co-infection Virus load | 1.00 (reference) | − |
| High | 3.39 (1.9-6.1) | <0.01 |
| Low | 1.00 (reference) | − |
Adjusted for all the variables listed in the table.
Discussion
As far as we know, this is by far the largest study focus on the clinical significance of different virus load of HBoV in patients with LRTI. We included a series of patients in the Suzhou region over two consecutive years. Also, this is the first study comprehensively describe the impact of HBoV virus load on epidemiology, clinical features, laboratory values and microbiological evaluation in children with HBoV LRTI.
During the last decade since its discovery, HBoV is found to be one of the most common pathogens that leads children to acute respiratory tract infection, especially LRTI4,9. Previous studies have shown that rates of detection of HBoV in ARI varied from 1.5% to 19.0%. In the present study, the overall detection rate of HBoV in LRTI patients was 8.85% which was similar to other regions of the world.
Age profile noted for individuals with HBoV was similar to RSV infections, with infections almost completely confine to infants and young children (<24 months). Our study are in line with most of the previous studies1,2,10. However, our study disagree with the Canadian study, in which there was much less difference in the prevalence of HBoV according to age11. Interestingly, our study also demonstrated that patients with high virus load had a similar age distribution with the total HBoV infection, which had a peak age group of 6-24 months. This interesting phenomenon highlighted the demographic importance of high virus load in HBoV LRTI.
Seasonal peaks of HBoV infection vary among different counties because of climate and geographic factors. Previous studies suggested that HBoV infection had a higher detection rate in winter12,13 or in summer2,5. In our study, a higher frequency of HBoV was observed between July and December with a peak in November. Interestingly, the frequency of patients with high virus load was also higher from July to December, and its seasonal distribution correlated with the total distribution, which highlighted the importance of high HBoV virus load in the seasonal distribution of HBoV virus.
In our study, wheezing and tachypnea occurred more frequently in children with high virus load. Actually, wheezing and tachypnea have been recognized as important clinical manifestations in HBoV infection2,3,4,14,15,16, while the clinical significance of different virus load in manifestations has been less studied. Allander etal found that high virus load of HBoV were noted mainly in the absence of other viral agents, suggesting a causative role for acute wheezing4. Deng etal found that wheezing was one of the most common symptoms presented by patients with positive HBoV, and the days of wheezing correlated with virus load. Different from the previous study, we use logistic regression analysis to exclude the interaction between clinical parameters and supported the fact that wheezing and tachypnea were most common and important symptoms presented in high HBoV load LRTI.
Co-infection was found significantly more frequently among patients with low virus load than those with high virus load (57.0% vs 38.9%; P < 0.01), indicating that patients with low HBoV virus load were more likely to be co-infected with other pathogens, which is consistent with the studies of Brieu17 and Kaida18. Although HBoV has been regarded as an infectious agent present, its pathogenic role in respiratory disease is still debatable. This virus is frequently detected in co-infection with other respiratory viruses of well-established pathogenic role8,10. In our study, high virus load were noted mainly in the absence of other respiratory viruses and suggesting a causative role for HBoV. However, low virus load were more detected in virus co-infection, and co-infection with RSV was more strongly affiliated with the patients with low virus load, suggesting that a low virus load in the nasopharynx seemed to be associated with long term shedding of HBoV DNA, unrelated to current illness. Actually, a recent study has suggested that asymptomatic viral infection in infants is associated with low viral load19.
Indeed, HBoV has been detected in infants and children with and without respiratory symptoms19,20,21,22. Byington reported that approximately half of the episodes of HBoV were asymptomatic. Furthermore, they found 14% of 151 episodes remained positive for 3 or more weeks21. Chonmaitree reported prolonged detection of bocavirus of up to 28 days19. HBoV DNA was also found in nasopharyngeal aspirates from 43% of asymptomatic children undergoing elective surgery23, and in lymphocytes from palatine tonsils of 32% of children undergoing tonsillectomy24, suggesting that HBoV could establish latent or persistent infection in respiratory tract. Actually, in our study, 18 patients readmitted within 30 days (median duration: 17 days) after discharge, while 14 (77.8%) of the readmitted patients had HBoV positive duration the second hospitalization (data not shown). This interesting phenomenon may also suggest that HBoV could establish persistent infection in respiratory tract.
Previous studies6,25 showed that the high HBoV virus load played an important role in the severity of LRTIs. Deng etal also found that high HBoV virus load led to more severe lower respiratory tract symptoms and longer hospitalization16. In our study, we found that age <2 months and high virus load were associated with severe LRTI (requirement for O2), while virus co-infection would not increase disease severity. Therefore the more severe lower respiratory tract symptom presented in high HBoV virus load patients may solely depend on HBoV virus load. Different from the previous study, our study use multivariable logistic regression which highlight the role of high virus load in severe LRTI.
The present study has potential limitations. First, quantitative PCR combined with serology would give a better idea of whether the viral infection was active or incipient26, we used only endpoint PCR. We found a high proportion of HBoV infections that had coinfection with other viruses. However, we may have missed additional viral infections not detected by PCR or tissue culture. As diagnostic testing for bacterial pathogens was not performed, we do not know whether HBoV-bacterial coinfections occurred among children. Because all our study subjects were hospitalized patients with LRTIs, the results are not necessarily generalizable to outpatient clinics.
In conclusion, HBoV infections are found in an important proportion of the hospitalized children with respiratory illnesses (8.85% in our series). A high HBoV virus load could be an etiologic agent for LRTI, which may lead to more severe lower respiratory tract symptom and severe disease.
Methods
Study Patients
All experiments were performed following the relevant guidelines and regulations of Soochow University. The methods were carried out in accordance with the approved guidelines. The study was approved by the Medical Ethics Committee of Soochow University. The parents of all study participants gave both verbal and written informed consent before study enrollment. A total of 7393 patients with a clinical and radiological diagnosis of LRTI in Children’s Hospital of Soochow University during January 2013 and December 2014 were enrolled in the study.
Respiratory tract aspirates preparation and nucleic acid extraction
Nasopharyngeal aspirates were obtained from all patients within 24 hours of admission. This involved passing a suction catheter through the nose with the intent of passing it into the lower part of the pharynx. The depth of penetration for the nasopharyngeal aspirate catheter was set at 7–9 cm. A total of 2 ml nasopharyngeal aspirates was obtained and centrifuged at 500 × g for 10 minutes and resuspended in 2 ml saline and divided into 2 aliquots for pathogen detection using direct immunofluorescence assay (DFA) and PCR. One of the equally divided samples of nasopharyngeal aspirate was centrifuged at 12000 × g for 5 minutes, followed by extraction of DNA from a 400-ul KL sample using DNA-EZ Reagents (Sangon Biotech, Shanghai, China) or TRIzol Reagent (Life Technologies, Carlsbad, USA) in accordance with the manufacturer’s instructions. A final 200 KL of DNA was eluted and DNA sample was divided into 2 aliquots for HBoV and Mycoplasma pneumoniae (MP) gene amplification via PCR.
Viral isolation
Direct immunofluorescence assay was done directly on nasal aspirate specimens by use of murine monoclonal antibodies (Chemicon). The specimens were tested for respiratory syncytial virus (RSV), adenovirus (ADV), parainfluenza virus (PIV) types 1–3, and influenza virus (IV) types A and B. All staining procedures were performed according to the manufacturer’s instructions. Immunostained preparations were viewed with a fluorescence microscope (Leica 020-518.500, Germany). Sputum DNA was extracted as described above, and hBoV-DNA was detected by real-time fluorescent PCR.
Mycoplasma pneumoniae (MP) detection
Specimens were tested for the presence of MP by real-time PCR, acute IgM and IgG serology were also evaluated using enzyme-linked immunosorbent assay (ELISA). MP infection was confirmed if a positive PCR and an elevated IgM at admission or a fourfold increase in IgG at follow-up was detected.
Data collection
Demographic, clinical features and laboratory tests including white blood cells (WBC), neutrophils, platelet, C-reaction protein (CRP) and nasopharyngeal aspirates tests were routinely performed of each patient.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS; version 17.0). Data were expressed as number with percentage, mean and standard deviation (SD) or median as appropriate. Normally distributed continuous variables were compared using the Student t test and non-normally distributed variables were analyzed using Mann-Whitney U test. Categorical data were analyzed using the chi-squared (χ2) test or Fisher’s exact test. P value < 0.05 was considered statistically significant. Multivariable logistic regression analyses were conducted to identify different clinical characteristics associated with different virus load, and to evaluate independent predictors of requirement for O2. Factors were considered for inclusion in the model if they were found to be associated with the outcome in unadjusted analyses (P < 0.20) or were potentially clinically important.
Additional Information
How to cite this article: Jiang, W. et al. Clinical significance of different virus load of human bocavirus in patients with lower respiratory tract infection. Sci. Rep. 6, 20246; doi: 10.1038/srep20246 (2016).
Footnotes
Author Contributions W.J.J. and W.J. wrote the main manuscript text and F.Y. and Y.D.Y. collected and analyzed data. W.F.Z. detected human bocavirus. All authors reviewed the manuscript.
References
- Allander T. et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 102, 12891–12896 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E. H. et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis 43, 585–592 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J. Y., Han T. H., Kim C. K. & Kim S. W. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis 12, 1254–1256 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T. et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 44, 904–910 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. K. et al. Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. BMC Infect Dis 11, 345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. et al. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol 44, 1132–1134 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund-Venermo M. et al. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg Infect Dis 15, 1423–1430 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen O. et al. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev 21, 291–304 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen O. Human bocavirus: increasing evidence for virulence. Pediatr Pulmonol 45, 118–119 (2010). [DOI] [PubMed] [Google Scholar]
- Chow B. D. & Esper F. P. The human bocaviruses: a review and discussion of their role in infection. Clin Lab Med 29, 695–713 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N., Brandt K., Dust K., Ward D. & Li Y. Human Bocavirus infection, Canada. Emerg Infect Dis 12, 848–850 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B. D., Huang Y. T. & Esper F. P. Evidence of human bocavirus circulating in children and adults, Cleveland, Ohio. J Clin Virol 43, 302–306 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N. et al. Detection of human bocavirus in Canadian children in a 1-year study. J Clin Microbiol 45, 610–613 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbrich B. et al. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis 6, 109 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghipour M., Cuevas L. E., Bakhshinejad T., Dove W. & Hart C. A. Human bocavirus in Iranian children with acute respiratory infections. J Med Virol 79, 539–543 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y. et al. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One 7, e34353 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieu N., Guyon G., Rodiere M., Segondy M. & Foulongne V. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J 27, 969–973 (2008). [DOI] [PubMed] [Google Scholar]
- Kaida A., Kubo H., Takakura K. & Iritani N. Detection and quantitative analysis of human bocavirus associated with respiratory tract infection in Osaka City, Japan. Microbiol Immunol 54, 276–281 (2010). [DOI] [PubMed] [Google Scholar]
- Chonmaitree T. et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clin Infect Dis 60, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. T., Fairchok M. P., Stednick Z. J., Kuypers J. & Englund J. A. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis 207, 982–989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byington C. L. et al. Community Surveillance of Respiratory Viruses Among Families in the Utah Better Identification of Germs-Longitudinal Viral Epidemiology (BIG-LoVE) Study. Clin Infect Dis 61, 1217–1224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch G. A. Editorial Commentary: Plethora of Respiratory Viruses and Respiratory Virus Data. Clin Infect Dis 61, 1225–1227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtin J. et al. Human bocavirus infections in hospitalized children and adults. Emerg Infect Dis 14, 217–221 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Gooding L. R. & Erdman D. D. Human bocavirus in tonsillar lymphocytes. Emerg Infect Dis 14, 1332–1334 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A., Nordbo S. A., Krokstad S., Rognlien A. G. & Dollner H. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol 49, 158–162 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jartti T., Soderlund-Venermo M., Hedman K., Ruuskanen O. & Makela M. J. New molecular virus detection methods and their clinical value in lower respiratory tract infections in children. Paediatr Respir Rev 14, 38–45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]