Abstract
Coating on the sperm surface, glycocalyx, plays a key role in sperm motility, maturation and fertilization. A comprehensive profile of sperm surface glycans will greatly facilitate both basic researches and clinical studies. Because of the capability of recognizing different glycan moieties, lectins are widely used in glycobiology. However, lacking high-throughput technology, limited lectins have been reported for analyzing the glycan of human sperm. In this study, we employed a lectin microarray for profiling the surface glycans of human sperm, on which 54 out of 91 lectins showed positive binding. Based on this technique, we compared lectin binding profiling of sperm with homozygous DEFB126 mutation (del/del) with that of wild type (wt/wt). DEFB126 was reported to contribute to the sialylation on sperm surface and its homozygous mutation was related to male subfertility. Six lectins (Jacalin/AIA, GHA, ACL, MPL, VVL and ABA) were found to develop lower binding affinity to sperm with del/del. Further validation showed that these lectins, especially ABA and MPL, can be potential biomarkers for clinical diagnosis of subfertility due to the mutation of DEFB126. Our research provides insight into the detection of some unexplained male subfertility, and the lectin microarray is generally applicable for infertility/subfertility sperm biomarker discovery.
The membrane surface of mature sperm has been found to be coated with a thick layer of glycans, i.e., the sperm glycocalyx, including O- and N-glycans, which protects sperm during transit in the female reproductive tract and assists with other key functions, including attachment of sperm to oviduct epithelium, regulation of capacitation, and sperm-egg interaction1,2,3,4,5,6. The glycans play crucial role in the binding of sperm with egg7. The elaborate glycocalyx of sperm is a conserved feature of epididymal maturation in mammals and the surface of mammalian sperm experiences dramatic changes which are vital for sperm to keep viability and functions in the female reproductive tract1,8,9,10. However, it is still not well understood how the sperm glycocalyx contributes to male fertility. In the macaque, β-defensin 126 (DEFB126), originally named epididymis-specific protein ESP13.2, is a multifunctional glycoprotein consisting of a conserved β-defensin core and a C-terminal glycosylated peptide tail with 20 O-glycosylation sites linking oligosaccharides in contributing to glycocalyx formation11,12. DEFB126 is expressed and secreted by the principal cells of epididymal distal corpus and proximal cauda epithelium and the highly sialylated DEFB126 protein is recruited to the surface of sperm during transit through the epididymal duct11,13,14, where the protein constitutes a major part of the sperm glycocalyx12. DEFB126 was reported to be essential for sperm efficiently advancing into the upper female reproductive tract, protecting sperm from immune recognition by the female immune system15, facilitating sperm penetration of cervical mucus16, and mediating attachment of sperm to oviduct epithelia17.
It was reported that about 20% of the Chinese males in reproductive ages had two-nucleotide deletion in DEFB126 (rs11467417) gene on both chromosomes (del/del)8. Homozygous DEFB126 mutant (del/del) shows significantly poorer penetration of Hyaluronic acid (HA, a substitute of cervical mucus), thus making the married men with DEFB126 mutation (del/del) take longer time to get their wives pregnant than those with either DEFB126 wild type (wt/wt) or heterozygous mutation (wt/del)8. And interestingly, sperm with del/del showed lower Agaricus bisporus (ABA) lectin binding8. It is highly possible that the DEFB126 mutation (del/del) associated sperm surface glycan changes may relate to the capability of fertilization. Thus, it will be extremely valuable to get the whole profile of the sperm surface glycans, and based on this, one could easily identify surface glycan difference that is clinically meaningful, e.g., difference/s between del/del, and wt/del, wt/wt of DEFB126 in a fast and efficient method.
As a group of natural glycan binders, lectins labeled with different conjugates including enzyme, fluorescence or biotin to detect individual glycans by immunocytochemistry, immunohistochemistry or flow cytometry are the major tools to explore the composition of the sperm glycocalyx18. In 2005, a novel high-throughput technique-lectin microarray came into being19,20,21,22. Owing to its high speed, accuracy and sensitivity, the technique has been extensively utilized in the analysis of bacteria23,24, fungi22,25, virus26 and mammalian cell surface glycome27,28; therefore, it can be a promising and powerful tool to characterize the glycome on the surface of diverse cells.
Taking advantage of the lectin microarray technology, we reported the establishment of a standard and general procedure for profiling the human sperm surface glycome in a high-throughput fashion and with less than 3 h. A detailed lectin binding profile of human sperm with 91 lectins was generated. We employed the standard procedure for fast identification of lectin binding difference/s by comparing sperm samples of three different genotypes of DEFB126 (i.e., wt/wt, wt/del and del/del). Several candidate lectins, i.e., Jacalin/AIA, GHA, ACL, MPL, VVL and ABA, with significant binding difference were successfully identified. ABA and MPL were further validated by a variety of assays, and the results were all found to be consistent with those from the lectin microarray. Thus, the candidate lectins may serve as novel biomarkers for the diagnosis of male subfertility.
Results
An optimized approach for direct profiling of the sperm surface glycome
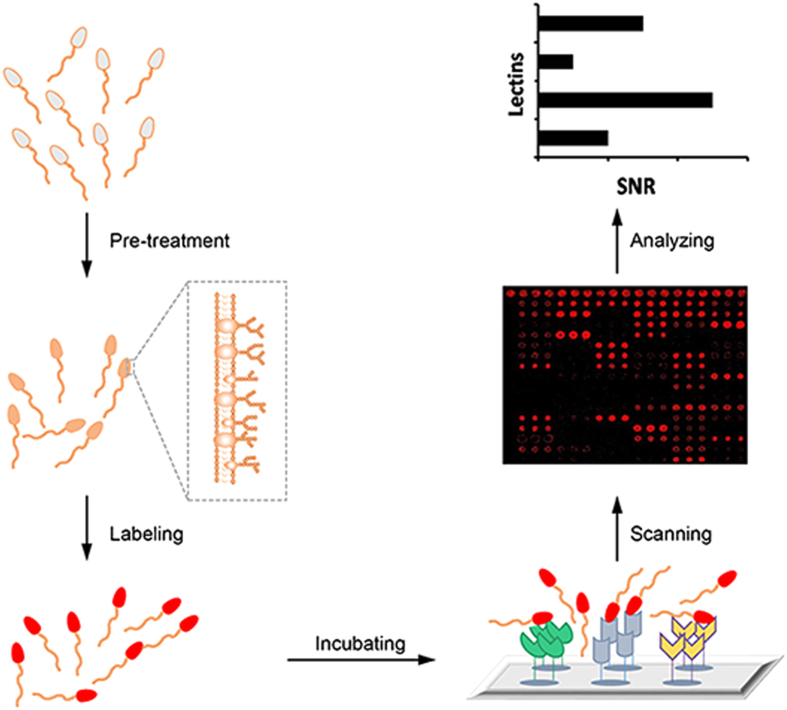
In order to investigate the glycocalyx of human sperm and the changes of glycans in sperm from men with del/del, we firstly optimized the approach of lectin microarray for dectecting human sperm. As shown in Fig. 1, the schematic was composed of 5 typical steps: sperm pre-treatment, labeling, incubating, scanning and data analysis.
Figure 1. Schematic diagram for detecting human sperm surface glycans by lectin microarray.
The ejaculated sperm were pre-treated and labeled with appropriate fluorescent dye, and incubated with lectin microarray. After the unbound sperm were washed off, the binding signals of sperm-lectin were then visualized, recorded and processed by a fluorescence microarray scanner coupled with appropriate software.
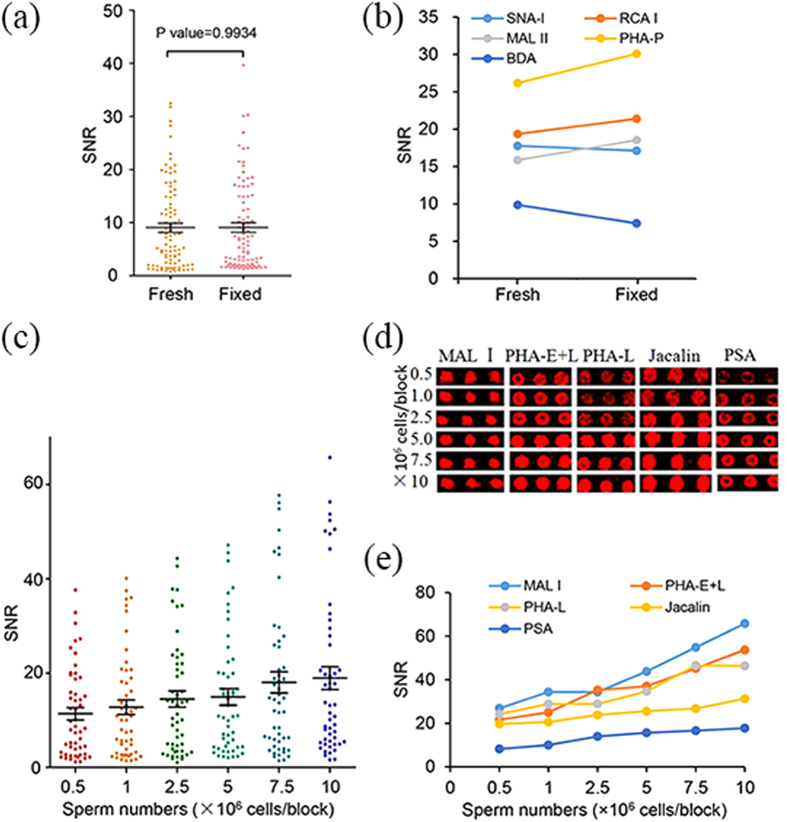
Since sperm have different characteristics when compared with common mammalian cells, the basic steps of the lectin microarray analysis were optimized in the sample preparation. To reduce the effect of the fast motility of live sperm on sperm-lectin binding, as the binding intensity of sperm after cryopreservation was low, we compared the lectin binding patterns of the fixed sperm with the fresh samples, finding that the lectin binding patterns and fluorescence intensity were similar between them (Fig. 2a,b, P > 0.05).
Figure 2. Optimization of the lectin microarray based strategy for sperm surface glycan analysis.
(a,b) Effect of fixation on sperm-lectin binding; the average SNRs of the total lectins (a) and the five representative lectins (b) showing non-significant differences between the fixed sperm and the fresh ejaculated sperm; (c–e) the optimization of sperm concentration for lectin microarray analysis from 0.5–10 × 106 sperm/block; the average SNRs of the total lectins (c) and the five representative lectins (d,e) depending on the sperm number.
In order to prevent the sperm-lectin binding signal saturation and save the clinic sperm samples, sperm at a range of 0.5–10 × 106 sperm/block were applied to lectin microarray for optimizing sperm counts. The signal intensity to the local background noise ratio (SNR) of the total lectins (Fig. 2c; Supplementary Fig. S1) and 5 representative lectins (Fig. 2d,e) were plotted against the sperm number. The data indicated that SNRs enhanced with the increasing sperm counts (Fig. 2c,e) and the signal of some lectins like PHA-L reached saturation at 7.5 × 106 cells/block, whereas most lectins located in the linear range of 2.5–7.5 × 106 sperm/block (Fig. 2e). In the current study, the sperm at a concentration of 5 × 106 sperm/block, which lay in the middle of the linear range, were analyzed.
Additionally, to examine whether the storage time of fixed sperm affected the lectin binding profiling, the fixed sperm stored at 4 °C from 0 day to 6 months were employed on lectin microarray. No significant difference was observed, the SNRs keeping almost the same among the samples (Supplementary Fig. S2).
The lectin binding profiling of human sperm
The human sperm surface is decorated with a thick layer of glycans, i.e., the glycocalyx. Though it is known that some lectins have positive bindings to human sperm10,29,30,31, the overall picture of the glycan composition of human sperm glycocalyx is still unclear. We took advantage of the established lectin microarray based strategy to profile the human sperm surface glycan composition.
To test the reproducibility, sperm samples from 10 donors with normal semen parameters and wt/wt genotype of DEFB126 were repeatedly probed in four blocks, the sperm-lectin binding pattern of each block showing constant repeatability and almost unanimous binding patterns (Supplementary Fig. S3, R = 0.9496). As shown in Fig. 3, 91 lectins binding signal intensity of sperm were analyzed, the results showing that 54 lectins (SNR ≥ 2 being cut-off) were positive in binding sperm, which covered a wide range of glycan specificity containing galactose (Gal), N-acetylgalactosamine (GalNAc), N-acetylglucosamine (GlcNAc), mannose/glucose (Man/Glc), sialic acids (Sia), fucose (Fuc) and complex-type glycan. Of 91 lectins, many were found for the first time to be strong in the binding, such as MPL and MNA-G (Gal binders), VVL and WFA (GalNAc binders), BPL (Galβ1-3GalNAc binder), DSL and STL (GlcNAc binders), MNA-M (Man/Glc binder) and PHA-P (unknown in specificity). Additionally, α2-3-Sia (MALII and MAA) and α2-6-Sia specific lectins (SNA and SNA-I) presented strong binding intensity to the sperm. The lectin binding pattern of sperm from donors with wt/wt genotype was almost consistent with the previous report from donors with normal semen parameters32. The findings indicated the human sperm glycocalyx was composed of a variety of glycans.
Figure 3. The lectin binding profiling of human sperm.
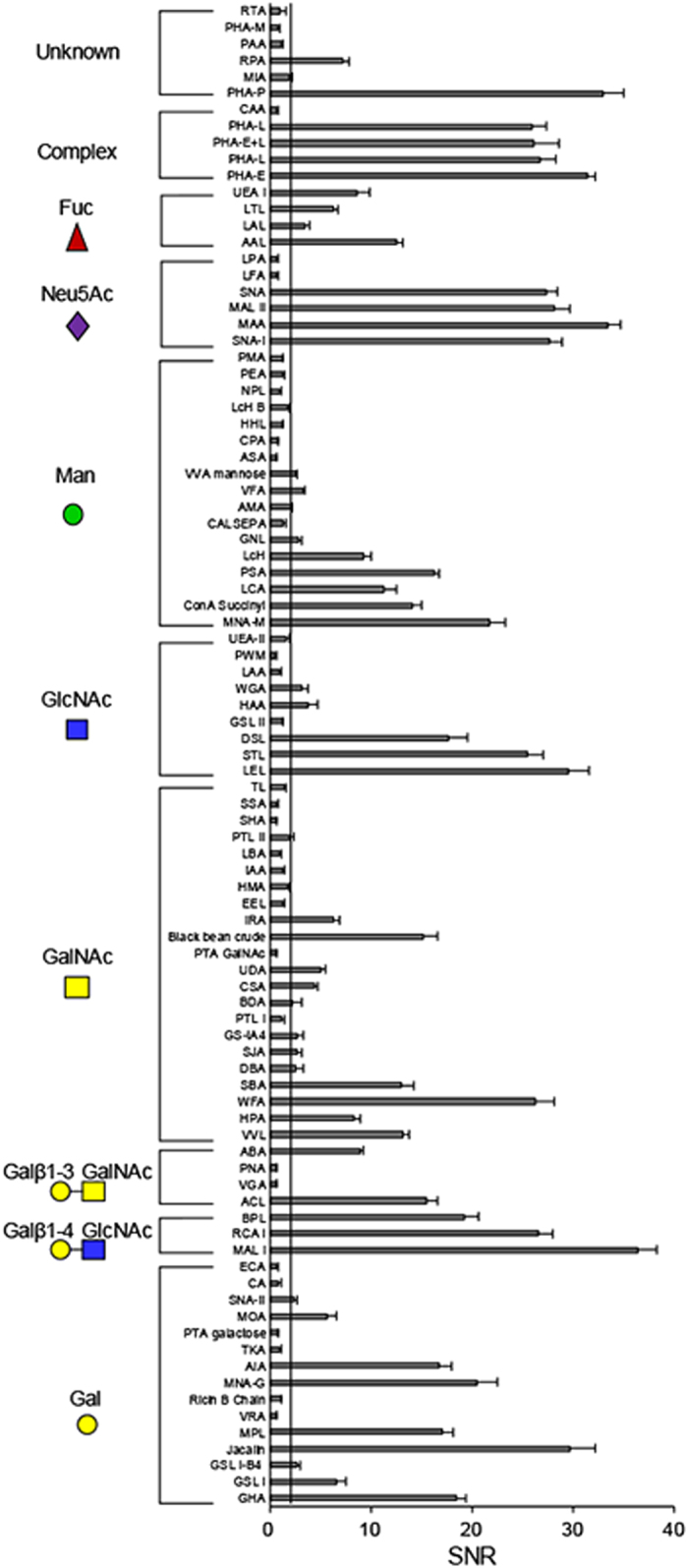
The sperm-lectin binding profiling presented by the average SNR; the data being the average ± SEM of 10 samples.
Expression and location of DEFB126
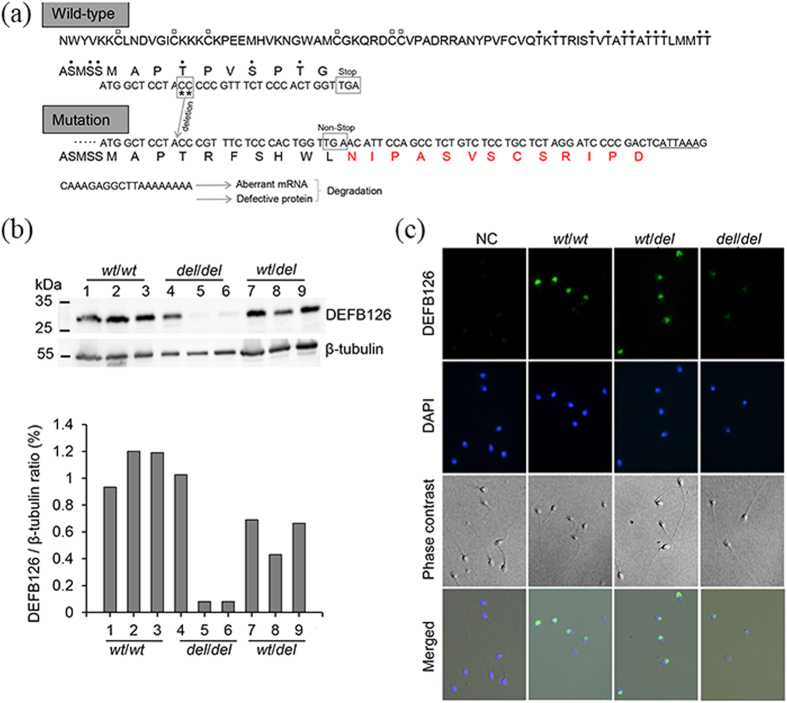
Beta-defensin126 (DEFB126) has numerous potential sites (serine and threonine) for O-glycosylation in at the carboxyl terminus, and is a major component of the sperm glycocalyx in mouse and macaque12,13. In human, DEFB126 had 17 potential O-glycosylation residues predicted by NetOGlyc 3.1 (Fig. 4a). The reported two-nucleotide deletion of DEFB126 would generate a non-stop mRNA and cause the aberrant mRNAs and peptides degradation by a non-stop decay (NSD) pathway and protein quality control system (Fig. 4a)33,34,35.
Figure 4. The expression and location of DEFB126 on human sperm.
(a) The wild-type DEFB126 and its two-nucleotide deletion mutant deduced from the mRNA nucleotide sequence; the six highly conserved cysteines (C) marked with empty squares45; seventeen potential residues (serine and threonine, dotted) at carboxyl terminus of DEFB126 predicted to be O-linked glycosylated (NetOGlyc 3.1)43; the two missed nucleotides (CC) labeled with asterisk and its frame-shifted version shown in the lower panel; the additional amino acids in the mutant protein displayed with the red front; the regulatory element of polyA signal sequence labeled with underline. (b) Representative Western blot from the ejaculated sperm of different genotypes (wt/wt, n = 3; wt/del, n = 3; del/del, n = 3), showing unstable expression of DEFB126 in sperm with del/del compared to the sperm with the other two genotypes (wt/wt or wt/del); β-tubulin used as loading control. (c) Localization of DEFB126 (green) on human sperm of different genotypes (wt/wt, wt/del and del/del); the sperm smears stained with polyclonal antibody against DEFB126 (rabbit anti-human β-defensin 126, sc-85535; 1:200 dilution) followed by an Alexa Fluor 488 conjugated donkey anti-rabbit IgG (1:200 dilution); sperm nuclei stained with DAPI (blue); Negative control (NC) sperm staining with rabbit IgG showing no immunoreactive staining.
To examine whether DEFB126 could bind with human sperm, the total sperm proteins were analyzed by western blot, thus producing the results that sperm with wt/wt or wt/del genotype exhibited clear and specific bands at about 30 kDa region, larger than the theoretical mature molecular weight (10 kDa; http://www.ncbi.nlm.nih.gov/protein/NP_112193.1), while the expression of DEFB126 in sperm with del/del genotype was unstable (Fig. 4b). Some of the sperm with del/del developed a considerable amount of DEFB126, as in the case of the sperm with wt/wt or wt/del, whereas some presented very weak bands (Fig. 4b). The data of sperm immunofluorescence also showed the same results. The sperm which showed weak bands demonstrated reduced fluorescence when compared with that with wt/wt or wt/del (Fig. 4c; Supplementary Fig. S4). As not in the case of mouse and macaque11,13, DEFB126 mainly located on human sperm acrosome (Fig. 4c; Supplementary Fig. S4).
Different surface glycan profiling of the sperm with the common mutation of DEFB126
To test whether DEFB126 mutation is related to sperm surface glycan aberrance, the sperm samples from 30 donors with wt/wt, wt/del or del/del were collected and probed on the lectin microarray following the established protocols.
Upon an analysis of significant differences of lectin binding patterns by lectin microarray with software SPSS16.0, six lectins containing Artocarpus integrifolia agglutinin (Jacalin/AIA), Gossypium hirsutum agglutinin (GHA), Amaranthus caudatus lectin (ACL), Maclura pomifera lectin (MPL), Vicia villosa lectin (VVL) and Agaricus bisporus agglutinin (ABA) showed significantly reduced capacity of binding to the sperm with del/del genotype in comparison with the sperm from the wild type (Fig. 5a).
Figure 5. Comparison of the lectin bindings among human sperm of three genotypes, i.e., wt/wt, wt/del and del/del
 ; 10 samples of each genotype).
; 10 samples of each genotype).
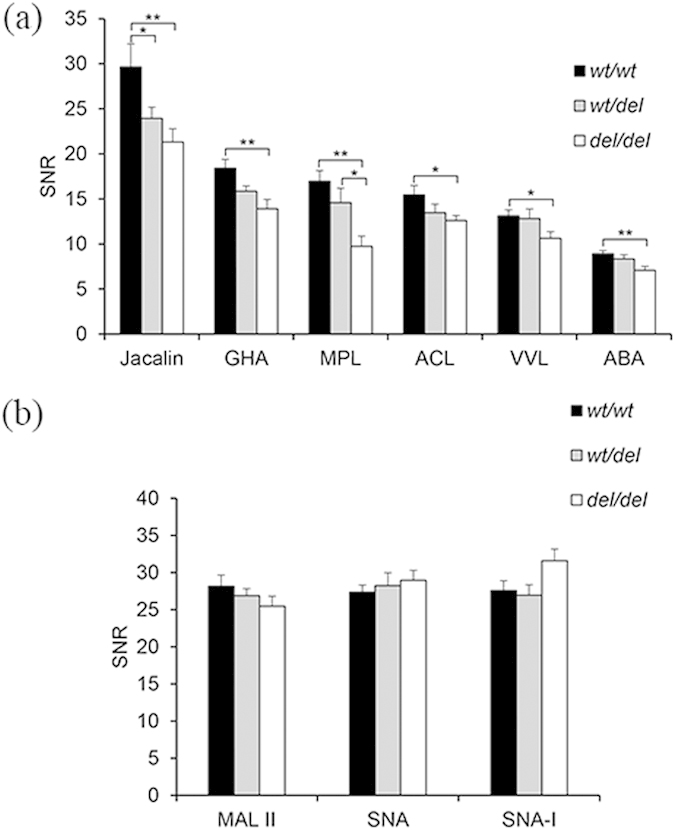
(a) Lectins showing statistically significant sperm binding differences among human sperm with wt/wt, wt/del or del/del genotype; (b) Sialic acid specific lectins demonstrating similar sperm-lectin bindings among the three genotypes, *P < 0.05, **P < 0.01. Error bars showing mean values with SEM.
Intriguingly, the sialic acid specific lectins of MAL II (α2-3-Sia), SNA and SNA-I (α2-6-Sia) presented no significant difference in the sperm of wt/wt, wt/del and del/del in terms of binding intensity (Fig. 5b).
To rule out the differences due to the sperm heterogeneity, the semen parameters of sperm with different genotypes of DEFB126 (wt/wt, wt/del and del/del), containing the sperm motility and viability, were analyzed and they all demonstrated no significant difference among the three groups (Supplementary Table. S1). Moreover, the binding signals of pissum sativum lectin (PSA), a well known lectin used to access acrosome reaction36,37, also showed no significant difference among the three groups (Supplementary Fig. S5).
Validation of the different lectins binding of sperm with DEFB126 mutation
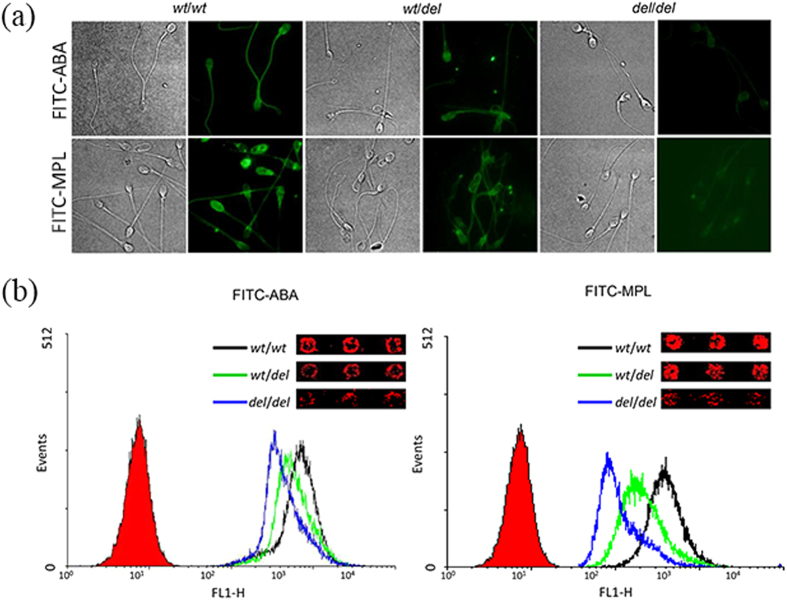
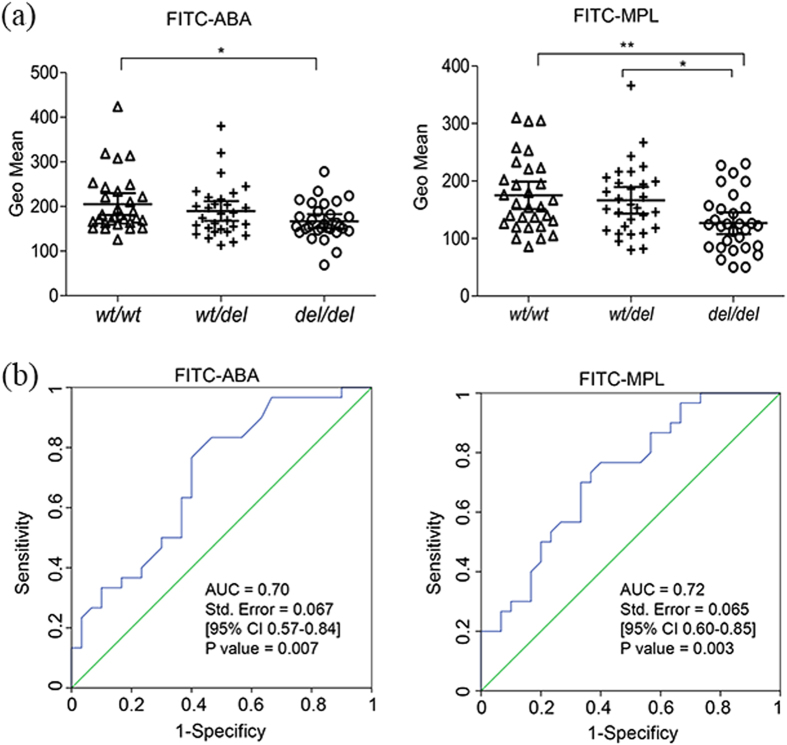
To validate the different lectins binding of the sperm with wt/wt, wt/del and del/del genotypes, we used fluorescein isothiocyanate (FITC)-labeled ABA and MPL to analyze the binding signal of sperm by the fluorescence microscope and flow cytometry (FACS). The fluorescence signals of FITC-ABA and FITC-MPL binding sperm with del/del genotype were weaker than those of sperm with the other two genotypes (Fig. 6a). As indicated by the data from the flow cytometry, moreover, the intensity of the fluorescence was directly associated with the degree of binding capacity on the microarray (Fig. 6b).
Figure 6. Validation of sperm-lectin binding by fluorescence microscope and FACS.
(a) The different fluorescence signal of sperm with wt/wt, wt/del or del/del genotype labeled with FITC-ABA or FITC-MPL; the fluorescence micrographs of sperm with each genotype shown in right panel and its corresponding phase contrast shown in left panel. (b) FACS analysis of sperm with the three genotypes labeled with FITC-ABA or FITC-MPL; the mean channel fluorescence (MCF) of sperm with wt/wt genotype (black line) shifts being larger than those of sperm with wt/del (green line) or del/del (blue line); insets being the lectin microarray images for the sperm with respective genotypes.
To further test the capability of the identified lectins as potential biomarkers for diagnosing subfertility due to the mutation of DEFB126, the fluorescence geometric mean (Geo mean) of FITC-ABA and FITC-MPL was measured among 90 donors (wt/wt, n = 30; wt/del, n = 30; del/del, n = 30) and the statistic difference between them showed high consistency with the results obtained by lectin microarray (Fig. 7a). In addition, no significant association was observed between the different DEFB126 genotypes of the sperm and the semen parameters by the computer-assisted sperm analysis (CASA) (Table 1). Statistical analysis with receiver operating characteristic (ROC) curve demonstrated that the area under the curves (AUC) of ABA and MPL was 0.70 ± 0.067 (95% CI: 0.57–0.84, P < 0.01) and 0.72 ± 0.065 (95% CI: 0.60–0.85, P < 0.01), respectively (Fig. 7b). Such results indicated that ABA and MPL could serve as potential biomarkers for clinical diagnosis of the impaired glycocalyx due to the homozygous mutation of DEFB126. The cut-off values of ABA and MPL based on these data were 157.5 with 83.3% specificity (95% CI: 0.65–0.94) and 53.3% sensitivity (95% CI: 0.34–0.72), and 125.5 with 76.7% specificity (95% CI: 0.58–0.90) and 60.0% sensitivity (95% CI: 0.41–0.77), respectively.
Figure 7. Evaluation of ABA and MPL being biomarker candidates by FACS.
(a) The statistic difference of Lectins (ABA and MPL) verified by FACS showing complete consistency with lectin microarray; Geo Mean of lectins binding sperm with different DEFB126 genotypes (wt/wt, n = 30; wt/del, n = 30; del/del, n = 30) analyzed by SPSS16.0. *P < 0.05, **P < 0.01. (b) The performance of ABA and MPL as biomarkers for assessing the sperm fertility; sperm with wt/wt genotype as the control group (n = 30) and sperm with del/del genotype as mutation group (n = 30). The ROC curves and the corresponding AUCs were calculated by SPSS16.0.
Table 1. Association between the three DEFB126 genotypes and the general semen parameters.
| wt/wt(n = 30) | wt/del(n = 30) | del/del(n = 30) | P | |
|---|---|---|---|---|
| Sperm concentration (×106/ml) | 54.60 ± 3.15 | 59.05 ± 4.18 | 59.27 ± 3.80 | 0.60 |
| Total motility (%) | 39.97 ± 2.29 | 43.42 ± 2.46 | 42.71 ± 2.51 | 0.57 |
| Sperm viability (%) | 58.41 ± 2.64 | 62.25 ± 2.63 | 63.33 ± 2.37 | 0.35 |
| Round cell concentration (×106/ml) | 0.58 ± 0.05 | 0.52 ± 0.04 | 0.59 ± 0.05 | 0.48 |
All values are means ± SEM.
Discussion
Sperm glycocalyx plays an important role in sperm motility, maturation and fertilization2,9. However, the glycan profiling of sperm glycocalyx is largely unknown because of the lacking of powerful technology. In the current study, therefore, we pioneered in optimizing the procedures of lectin microarray for the global profiling of sperm surface glycans. DEFB126, a major carrier of glycocalyx carbohydrates in macaque and mouse, has been reported to be a common mutation related to sperm subfertility8,12,13. With the sperm of different DEFB126 genotypes (wt/wt, wt/del, and del/del) compared, six lectins (Jacalin/AIA, GHA, ACL, MPL, VVL and ABA) displayed statistically significant differences, and the sperm with del/del showed the lower lectin binding ability. Further validation with flow cytometry showed that these lectins, especially ABA and MPL, could be biomarkers for clinical diagnosis/identification of unexplained subfertile sperm due to the mutation of DEFB126.
It is well recognized that DEFB126 is synthesized and secreted in the epithelial cells of epididymal corpus, and then added to the sperm surface during epididymal maturation in mouse, rat and macaque11,13,14. The protein has a long glycosylated carboxyl peptide tail contributing substantially to the sperm glycocalyx of nonhuman primates9,12. In human, our results of western blot and immunofluorescence indicated that DEFB126 also existed on the human sperm. In addition, DEFB126 had 17 possible glycosylation sites for glycosylation (Fig. 4a). As indicated by the specific bands, the protein at 30 kDa was greater than 10 kDa of its theoretical molecular weight (Fig. 4b), which might be ascribed to protein glycosylation. The two-nucleotide deletion in DEFB126 gene on both chromosomes (del/del) has been reported to result in a reading frame shift and generate a nonstop mRNA prone to degradation due to NSD surveillance mechanism8,33,34. Additionally, the level of protein product of nonstop mRNA containing a poly (A) might be reduced because of translation repression and protein destabilization by proteasome35. We found that the protein DEFB126 of sperm with del/del genotype was indeed unstable (Fig. 4b).
As a sensitive and high-throughput technology, lectin microarray has already been widely employed in profiling the surface glycans of a variety of human cells27,28. We applied the technology to exploring human sperm, and optimized its procedures such as fixation, staining, sperm concentration, etc. Specifically, a fixation step with the surface glycans well preserved was established for application after the sperm samples were collected. After fixation, the sperm could be stably stored at 4 °C for at least 6 months (Supplementary Fig. S2). The fixation not only made sperm lose motility, thus facilitating binding lectin microarray, but also provided the possibility of comparing the sperm samples collected at different time points in a consistent and reliable way.
Sperm are the most diverse cell type known. Different semen samples, even from the same individual at different time point, show highly variable characteristics, especially the sperm morphology, motility, vitality, and spontaneous acrosome reaction rate38. The mobile sperm enriched by centrifugation on percoll gradient presented enhanced fluorescent lectin binding39. It will be interesting to examine the relationship between the sperm surface glycosylation and these heterogeneous characteristics. And the lectin microarray is a suitable tool to compare the lectin binding profiling among the heterogeneous sperm. However, in the current study, the semen parameters, containing the sperm motility and viability, demonstrated no significant difference among sperm with different genotypes of DEFB126 (wt/wt, wt/del and del/del; Supplementary Table S1). On the other hand, it is reported that the spontaneous acrosome reaction rate in human spermatozoa is generally less than 15% under physiological conditions40. The binding signal of pissum sativum lectin (PSA), a well known lectin used to access acrosome reaction36,37, to the three groups (wt/wt, wt/del and del/del) also showed no significant difference (Supplementary Fig. S5). Nevertheless, to reduce the difference among individuals, 10 samples were analyzed on the lectin microarrays (Fig. 3), only the averaged data with standard deviations were presented.
We compared sperm with three genotypes of DEFB126, i.e., wt/wt, wt/del or del/del by the lectin microarray, finding that 6 lectins of Jacalin/AIA, GHA, ACL, MPL, VVL and ABA displayed significantly reduced binding ability in the sperm of del/del homozygotes (Fig. 5a). Of these lectins, ABA and MPL were further validated; the results were observed to be well consistent with those of lectin microarray (Fig. 6). The lower binding ability of ABA on sperm with del/del genotype was similar as described previously8, which further verified the reliability of the lectin microarray for exploring sperm surface glycome. Additionally, the 6 lectins recognized specifically β-galactose and/or N-acetylgalactosamine oligosaccharide. Especially, ABA and Jacalin lectins recognized the site specific for O-linked glycosylation (galactose-N-acetylgalactosamine-serine/threonine)12. The carboxyl tail of human DEFB126 has 17 predicted O-linked glycosylation sites, which suggested that the lower binding of those 6 lectins to sperm of del/del may be ascribed to the lower abundance of O-glycosylation of DEFB126, i.e., GalNAc residues. This demonstrated that the post-translational glycosylation of DEFB126 in human might be consistent with that in the previously reported macaque12. However, the Jacalin/ABA binding intensity was still high in sperm with del/del genotype, which may be ascribed to the compensation of the other glycans or glycosylated proteins in del/del sperm.
Sialic acids, the outmost glycocalyx of sperm, contribute mainly to the negative charge of sperm41. DEFB126 is endowed with negative charges due to its possession of sialic acid; treatment of DEFB126 with neuraminidase releases sialic acid and renders DEFB126 neutrality12. It is the negative charges on DEFB126 on the sperm surface that allow sperm to move through the cervical mucus, which is enriched in anionic glycosaminoglycans9. Upon capacitation, DEFB126 is released from the sperm surface9,42. If del/del mutation results the loss of DEFB126, the level of sialic acid has to be reduced. In the current study, however, the sialic acid specific lectins (MALII, SNA and SNA-I) demonstrated an almost invariable binding intensity with the sperm of the three genotypes via lectin microarray (Fig. 5b). This suggested that the reduced cervical mucus penetration ability of sperm with del/del was not caused by the loss of negative charge results from the lessened sialic acids. So far, there has been no definite proof that sperm glycocalyx is equivalent to the glycans of DEFB126. Free glycans and glycans of other glycoproteins can be part of sperm glycocalyx2. Compensation of the level of sialic acid can occur on the other sperm surface glycoproteins or glycans. In addition, the glycan structures of human DEFB126 have not been reported yet. However, if native DEFB126 could be obtained with a fair amount from human sperm, the glycan structures could be characterized by lectin blots and ultrasensitive mass spectrometer. These will give the precise glycosylation sites and glycan structures on DEFB126.
The impaired glycocalyx is associated with the reduced sperm fertility4,43. DEFB126 is a highly glycosylated protein and the carbohydrates of the protein contributes substantially to the sperm glycocalyx11. The homozygous two-nucleotide deletion in the DEFB126 gene causes impaired sperm function44. In the current study, however, the sperm with del/del presented normal semen parameters (Table 1), which suggested the routine examination in clinic could not fully assess the male fertility. In addition, qPCR, sequencing and western blotting can only identify the mutation types of genes and the quantity of proteins. But we observed that some sperm with del/del also had the DEFB126 protein by western blotting (Fig. 4b). Thus, none of these methods can demonstrate the status of protein’s post-translational modification. However, the 6 lectins, especially ABA and MPL (AUC > 0.7), could be employed to assess the quality of the sperm glycocalyx that may be defective because of the mutation in DEFB126. Such results suggested that these 6 lectins could have potential capability to serve as a biomarker individually or as a combination in diagnosing males with unexplained infertility due to DEFB126 mutation, which can offer a new insight into sperm infertility, especially into unexplained infertility.
Although it provided promising biomarkers for diagnosing the unexplained infertile patients with normal semen parameters, the current study had some limitations, which are to be addressed in our future studies. The cut-off value established with the data should be validated with an independent set of samples for increasing sensitivity and specificity. Moreover, it is necessary that prospective validation of the biomarkers be executed in a large set of samples before the test is applied to the clinic.
In conclusion, to globally profile the surface glycans of human sperm and explore the changes of glycans in sperm with del/del, we pioneered in optimizing the procedures of lectin microarray for detecting human sperm surface glycome. Through the technique, the sperm of three genotypes, i.e., wt/wt, wt/del or del/del were compared on the lectin microarray, 6 lectins identified to serve as potential biomarkers for subfertility diagnosis due to DEFB126 mutation. Our research on the defective glycocalyx of sperm provides insight into the detection of some unexplained male subfertility. In addition, the lectin microarray strategy can be employed to investigate the glycomic profiling of sperm related to glycocalyx changes in sperm capacitation, acrosome reaction, sperm-egg recognition, which will facilitate a deep insight into the glycocalyx-related functions of human sperm.
Methods
Sperm collection and preparation
Human semen samples were collected in Shanghai Ji Ai Genetics & IVF Institute. One hundred and twenty donors were recruited in this study. All the semen were evaluated for sperm concentration, total motility, viability and round cell concentration according to the fifth edition of WHO laboratory manual. The samples with normal semen parameters were included; i.e., they presented the normal concentration (≥15 × 106/ml), total motility (≥40%), viability (≥58%) and the low round cell concentration (≤1 × 106/ml). A small fraction (<300 μl) of each sample was used for DEFB126 genotyping. From the rest, the whole semen was centrifugated (500 g × 10 min) for collecting the sperm cells and washed with PBS, and then fixed with 2% paraformaldehyde containing 0.2% glutaraldehyde for 30 min, followed by twice washes with PBS, before stored at 4 °C for the subsequent lectin microarray and flow cytometry experiments. For other tests, the preparations of semen were described at the following each section in detail. The use of semen was allowed by the donors with written informed consent. This research was approved by the Institutional Review Committee of Fudan University. All experiments were performed in accordance with the relevant guidelines and regulations.
Preparation and analysis of Lectin microarray
Lectin microarray was prepared as previously described27,32. Ninety-one lectins were all purchased from EY Laboratories (San. Mateo, CA) and Vector Laboratories (Burlingame, CA), and other chemicals were purchased from Sigma-Aldrich (Shanghai, China). Lectin microarrays were prepared as we had reported previously27. The lectins were dissolved in PBS with 0.02% Tween-20, 25% glycerol and 0.05 μg/μl bovine serum albumin (BSA) at a final concentration of 1 μg/μl and then printed on OPPolymer Slide H slides (CapitalBio, Beijing, China) with SmartArrayTM-48 microarrayer (CapitalBio, Beijing, China). Each lectin was printed on blocks in triplicate with 18 × 16 arrangement, with 12 blocks printed on one slide. Afterwards, the slides were incubated at 4 °C overnight to ensure that lectins coated the surface. The prepared slides were stored at 4 °C for the further experiments.
Lectin microarrays were washed in 10 mM Tris Buffered Saline with 0.5% (v/v) Tween-20 (TBST) for 1 h, and in PBS with 0.5% Tween-20, then in PBS twice with gentle shaking, followed by air-drying at room temperature.
The fresh sperm were collected by centrifugation and labeled with 10 μM Carboxy-Fluorescein diacetate, Succinimidyl Ester (CFSE; Invitrogen, Carlsbad, CA) for 10min at room temperature (RT), and the fixed sperm were labeled with 20 μg/ml propidium iodide (PI; Sigma-Aldrich, Shanghai, China). Each block of lectin microarray was seeded with CFSE- or PI-labeled sperm in 200 μl PBS with 50 μM CaCl2 and 50 μM MnCl2, and then incubated in a wet box for 1 h at room temperature (RT) in the dark. Each sample was repeated four times in and between slides in a diagonal manner. The excess and unbound sperm were gently removed by submerging and inverting the slides in PBST. With the scanning condition set to 532 nm filter and 40% PMT value, the air-dried slides were scanned with a GenePix 4200A (Molecular Devices, Sunnyvale, CA) at 5 μm resolution.
Protein preparation and western blotting analysis
The liquefied semen was centrifuged to discard the seminal plasma, and the pellets were resuspended with 1 × SDS-PAGE loading buffer including β-mercaptoethanol in the ratio of 106 sperm to 100 μl loading buffer before they were boiled for 5 min. The supernatant proteins were stored at −80 °C with aliquots for the western blotting. In each sample, 10 μl proteins were separated by 12.5% SDS-PAGE and semi-dry blotted to PVDF (Polyvinylidene Fluoride) membranes (Millipore, Bedford, MA, USA). Blocked 2 h within 1 × NET, the membranes were incubated with primary rabbit polyclonal antibody to DEFB126 (1:1000; Santa Cruz, California, USA) overnight at 4 °C and then incubated with the HRP-labeled secondary antibody (1:10000; Cwbiotech, Beijing, China) for 1 h at RT. The bands, when washed thrice in TBST, were detected by ECL kit (GE Amersham, Pittsburgh, USA). The semi-quantification of target protein and reference protein were analyzed by ImageJ 1.48 gray scale scanning software.
Immunofluorescence and scanning confocal microscopy
The smears were fixed with 2% PFA containing 0.3% Tween-20 and 0.2% Triton X-100 for 30 min. The slides, washed thrice with PBS and blocked with 30% donkey serum including 2% BSA for 1 h at RT, were incubated with rabbit polyclonal antibody to DEFB126 (1:200) overnight at 4 °C, followed by an incubation with the donkey anti-rabbit IgG conjugated with Alexa Fluor 488 (1:200; Molecular Probes, California, USA) for 1 h at RT. The negative control was incubated with rabbit IgG in the same conditions as the rabbit polyclonal antibody to DEFB126. One drop of Dapi Fluoromount-G (SouthernBiotech, Birmingham, Alabama, USA) was added to the sperm slides to be air-dried for 5 min. The slides were examined under BX51 Fluorescence microscope (Olympus, Japan) and a laser confocal scanning microscope (Leica TCS SP5, Mannheim, Germany).
Genotype analysis of DEFB126 (rs11467417)
From the semen, the genomic DNA was extracted as a template in a real time PCR (qPCR) to identify DEFB126 genotype. The sequences of the genotyping primers were as follows: wt/wt (169-bp) forward primer 5′-AAGGGACTGCTGTGTTCCAG-3′, reverse primer, 5′-ACCAGTGGGAGAAACGGGCGT-3′; del/del (295-bp) forward primer 5′-CTTCGATGGCTCCTACGCG-3′, reverse primer 5′-GCTGTGGGCCTAGAACTGTC-3′. The qPCR cocktail solution contained 1.5 μl genomic DNA, 0.5 μl false-paired or right-paired primers, 5 μl Sharpvue 2 × universal qPCR Master Mix (Biovue Technology, Shanghai, China), and 3 μl nuclease-free ddH2O. The cycling conditions were as follows: 94 °C for 10 min, followed by 94 °C for 10 s and 60 °C for 1 min for 40 cycles; consequently, the melting curves were acquired from 60 °C to 94 °C, with an increasing rate 0.1%. The loci of interest were genotyped based on the melting temperature shift genotyping assay.
Lectin flow cytometry
The selected lectins were used to stain the fixed sperm by 2% paraformaldehyde containing 0.2% glutaraldehyde, 5 × 106 sperm re-suspended in PBS and incubated with 100 μg/ml of fluorescein isothiocyanate (FITC)-labeled lectins for 30 min at 37 °C in the dark. The sperm were washed and re-suspended with 500 μl PBS, to be analyzed in a Facs Calibur Flow cytometer using WinMID2.9 software.
Statistical analysis
The binding signals of the sperm were extracted via GenePix pro 6.0 from the lectin microarray images. The signal intensity to the local background noise ratio (SNR) was defined as F532 Mean/B532 Mean, and all the spots’ SNRs of lectin microarray were calculated and normalized. SNRs of the 12 replicate spots, four blocks repeated with triplicate spots on each block, were averaged for each lectin. The data of the sperm of different genotypes (wt/wt, wt/del or del/del) that bound with lectins was classified and averaged, respectively. The cut off of the positive lectin binding was set as SNR ≥ 2.
Data analysis and graphs were conducted by SPSS16.0 and GraphPad Prism 5 and all the data were described as the mean ± SEM. The significant differences of the SNRs and the Geo Mean among samples with wt/wt, wt/del or del/del genotype were determined by One-way analysis of variance (one-way ANOVA). Differences were considered as significant at p < 0.05. ROC analyses were performed with the ABA/MPL binding signal intensity plotted against del/del. The area under the ROC curves (AUC) were calculated to evaluate the subfertility of sperm.
Additional Information
How to cite this article: Xin, A. et al. Lectin binding of human sperm associates with DEFB126 mutation and serves as a potential biomarker for subfertility. Sci. Rep. 6, 20249; doi: 10.1038/srep20249 (2016).
Supplementary Material
Acknowledgments
This work was supported by the State Key Development Program for Basic Research of China (Grant No. 2009CB941700), the National Natural Science Foundation of China (No. 81270744, 81401252 and 31370813), the National High Technology Research and Development Program of China (No. 2012AA020103 and 2012AA020203), and MerckSerono China Research Fund for Fertility Experts. The authors thank Xuting Xu for data analysis.
Footnotes
Author Contributions H.S., S.T. and Y.Z. conceived and designed the experiments. A.X. and L.C. performed the experiments and drafted the manuscript. S.Z. and Y.S. prepared the lectin microarry. G.C., B.W., Y.Y., J.Z. and X.S. provided clinical samples. Y.W. and C.S. involved in data analysis. H.D., S.D., P.W. and Y.G. provided technical assistance. All authors have reviewed and approved the final manuscript.
References
- Diekman A. B. Glycoconjugates in sperm function and gamete interactions: how much sugar does it take to sweet-talk the egg? Cell. Mol. Life Sci. 60, 298–308 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroter S., Osterhoff C., McArdle W. & Ivell R. The glycocalyx of the sperm surface. Hum. Reprod. Update 5, 302–313 (1999). [DOI] [PubMed] [Google Scholar]
- Gatti J. L. et al. Post-testicular sperm environment and fertility. Anim. Reprod. Sci. 82-83, 321–339 (2004). [DOI] [PubMed] [Google Scholar]
- Kirchhoff C. & Schroter S. New insights into the origin, structure and role of CD52: a major component of the mammalian sperm glycocalyx. Cells Tissues Organs 168, 93–104 (2001). [DOI] [PubMed] [Google Scholar]
- Parry S. et al. The sperm agglutination antigen-1 (SAGA-1) glycoforms of CD52 are O-glycosylated. Glycobiology 17, 1120–1126 (2007). [DOI] [PubMed] [Google Scholar]
- Pang P. C. et al. Expression of bisecting type and Lewisx/Lewisy terminated N-glycans on human sperm. J. Biol. Chem. 282, 36593–36602 (2007). [DOI] [PubMed] [Google Scholar]
- Pang P. C. et al. Human sperm binding is mediated by the sialyl-Lewis(x) oligosaccharide on the zona pellucida. Science 333, 1761–1764 (2011). [DOI] [PubMed] [Google Scholar]
- Tollner T. L. et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci. Transl. Med. 3, 92ra65. [DOI] [PMC free article] [PubMed]
- Tollner T. L., Bevins C. L. & Cherr G. N. Multifunctional glycoprotein DEFB126–a curious story of defensin-clad spermatozoa. Nat. Rev. Urol. 9, 365–375 (2012). [DOI] [PubMed] [Google Scholar]
- Gomez-Torres M. J. et al. Characterization of the lectin binding pattern in human spermatozoa after swim-up selection. Histol. Histopathol. 27, 1621–1628 (2012). [DOI] [PubMed] [Google Scholar]
- Yudin A. I. et al. ESP13.2, a member of the beta-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol. Reprod. 69, 1118–1128 (2003). [DOI] [PubMed] [Google Scholar]
- Yudin A. I., Treece C. A., Tollner T. L., Overstreet J. W. & Cherr G. N. The carbohydrate structure of DEFB126, the major component of the cynomolgus Macaque sperm plasma membrane glycocalyx. J. Membr. Biol. 207, 119–129 (2005). [DOI] [PubMed] [Google Scholar]
- Yudin A. I. et al. Beta-defensin 22 is a major component of the mouse sperm glycocalyx. Reproduction 136, 753–765 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A. C., Jones R., Moisyadi S., Coadwell J. & Hall L. The novel epididymal secretory protein ESP13.2 in Macaca fascicularis. Biol. Reprod. 61, 965–972 (1999). [DOI] [PubMed] [Google Scholar]
- Yudin A. I. et al. Beta-defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies. Biol. Reprod. 73, 1243–1252 (2005). [DOI] [PubMed] [Google Scholar]
- Tollner T. L., Yudin A. I., Treece C. A., Overstreet J. W. & Cherr G. N. Macaque sperm coating protein DEFB126 facilitates sperm penetration of cervical mucus. Hum. Reprod. 23, 2523–2534 (2008). [DOI] [PubMed] [Google Scholar]
- Tollner T. L. et al. Beta-defensin 126 on the surface of macaque sperm mediates attachment of sperm to oviductal epithelia. Biol. Reprod. 78, 400–412 (2008). [DOI] [PubMed] [Google Scholar]
- Cummings R. D. Use of lectins in analysis of glycoconjugates. Methods Enzymol. 230, 66–86 (1994). [DOI] [PubMed] [Google Scholar]
- Kuno A. et al. Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling. Nat. Methods 2, 851–856 (2005). [DOI] [PubMed] [Google Scholar]
- Pilobello K. T., Krishnamoorthy L., Slawek D. & Mahal L. K. Development of a lectin microarray for the rapid analysis of protein glycopatterns. ChemBioChem 6, 985–989 (2005). [DOI] [PubMed] [Google Scholar]
- Angeloni S. et al. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 15, 31–41 (2005). [DOI] [PubMed] [Google Scholar]
- Zheng T., Peelen D. & Smith L. M. Lectin arrays for profiling cell surface carbohydrate expression. J. Am. Chem. Soc. 127, 9982–9983 (2005). [DOI] [PubMed] [Google Scholar]
- Hsu K. L., Pilobello K. T. & Mahal L. K. Analyzing the dynamic bacterial glycome with a lectin microarray approach. Nat. Chem. Biol. 2, 153–157 (2006). [DOI] [PubMed] [Google Scholar]
- Hsu K. L. & Mahal L. K. A lectin microarray approach for the rapid analysis of bacterial glycans. Nat. Protoc. 1, 543–549 (2006). [DOI] [PubMed] [Google Scholar]
- Amano K. et al. Engineering of mucin-type human glycoproteins in yeast cells. Proc. Natl. Acad. Sci. USA 105, 3232–3237 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy L., Bess J. W. Jr., Preston A. B., Nagashima K. & Mahal L. K. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat. Chem. Biol. 5, 244–250 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S. C. et al. Lectin microarrays identify cell-specific and functionally significant cell surface glycan markers. Glycobiology 18, 761–769 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno H. et al. A novel strategy for mammalian cell surface glycome profiling using lectin microarray. Glycobiology 17, 1138–1146 (2007). [DOI] [PubMed] [Google Scholar]
- Lee M. C. & Damjanov I. Lectin binding sites on human sperm and spermatogenic cells. Anat. Rec. 212, 282–287 (1985). [DOI] [PubMed] [Google Scholar]
- Wang G. et al. Mapping of the N-linked glycoproteome of human spermatozoa. J. Proteome Res. 12, 5750–5759 (2013). [DOI] [PubMed] [Google Scholar]
- Tomar A. K., Sooch B. S. & Yadav S. Computational analysis of Concanavalin A binding glycoproteins of human seminal plasma. Bioinformation 7, 69–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin A. J. et al. Comprehensive profiling of accessible surface glycans of mammalian sperm using a lectin microarray. Clin. Proteomics 11, 10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmeyer P. A. et al. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 295, 2258–2261 (2002). [DOI] [PubMed] [Google Scholar]
- van Hoof A., Frischmeyer P. A., Dietz H. C. & Parker R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295, 2262–2264 (2002). [DOI] [PubMed] [Google Scholar]
- Ito-Harashima S., Kuroha K., Tatematsu T. & Inada T. Translation of the poly(A) tail plays crucial roles in nonstop mRNA surveillance via translation repression and protein destabilization by proteasome in yeast. Genes Dev. 21, 519–524 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. Y. & Baker H. W. Calcium ionophore-induced acrosome reaction correlates with fertilization rates in vitro in patients with teratozoospermic semen. Hum. Reprod. 13, 905–910 (1998). [DOI] [PubMed] [Google Scholar]
- Zoppino F. C., Halon N. D., Bustos M. A., Pavarotti M. A. & Mayorga L. S. Recording and sorting live human sperm undergoing acrosome reaction. Fertil. Steril. 97, 1309–1315 (2012). [DOI] [PubMed] [Google Scholar]
- Ramon M. et al. Understanding sperm heterogeneity: biological and practical implications. Reprod Domest Anim 49 Suppl 4, 30–36 (2014). [DOI] [PubMed] [Google Scholar]
- De Maistre E., Bene M. C., Foliguet B., Touati F. & Faure G. C. Centrifugation on Percoll gradient enhances fluorescent lectin binding on human sperm: a flow cytometric analysis. Arch. Androl. 37, 179–187 (1996). [DOI] [PubMed] [Google Scholar]
- Rufas O., Gilman A., Fisch B. & Shalgi R. Spontaneous and follicular fluid-induced acrosome reaction in sperm samples from in vitro fertilizing and nonfertilizing normozoospermic patients. J. Assist. Reprod. Genet. 15, 84–89 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traving C. & Schauer R. Structure, function and metabolism of sialic acids. Cell. Mol. Life Sci. 54, 1330–1349 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollner T. L. et al. Release of DEFB126 from macaque sperm and completion of capacitation are triggered by conditions that simulate periovulatory oviductal fluid. Mol. Reprod. Dev. 76, 431–443 (2009). [DOI] [PubMed] [Google Scholar]
- Froman D. P. & Engel H. N. Jr. Alteration of the spermatozoal glycocalyx and its effect on duration of fertility in the fowl (Gallus domesticus). Biol. Reprod. 40, 615–621 (1989). [DOI] [PubMed] [Google Scholar]
- Tollner T. L. et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci. Transl. Med. 3, 92ra65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazgier M., Hoover D. M., Yang D., Lu W. & Lubkowski J. Human beta-defensins. Cell. Mol. Life Sci. 63, 1294–1313 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.