Abstract
Individuals vary in their social skills and motivation, the causes of which remain largely unknown. Here we investigated whether an individual’s propensity to interact with others measured within days after birth, and differences in infants’ early social environment, may predict a later social skill. Specifically, we tested whether neonatal imitation—newborns’ capacity to match modelled actions—and social experience in the first months of life predict gaze following (directing attention to locations where others look), in infant macaques (Macaca mulatta; n = 119). Facial gesture imitation in the first week of life predicted gaze following at 7 months of age. Imitators were better at gaze following than non-imitators, suggesting neonatal imitation may be an early marker predicting socio-cognitive functioning. In addition, infants with rich social environments outperformed infants with less socialization, suggesting early social experiences also support the development of infants’ gaze following competence. The present study offers compelling evidence that an individual difference present from birth predicts a functional social cognitive skill in later infancy. In addition, this foundational skill—gaze following—is plastic, and can be improved through social interactions, providing infants with a strong foundation for later social interaction and learning.
Social skills form the basis of the capacity to interact with others and to successfully integrate into society. Individual differences in adults’ social skill may be the result of two different yet interconnected processes: an individual’s natural potential to engage with others – related to individual differences in personality, intrinsic motivation, or genetic make-up; and the effect of the environment, either nurturing or suppressing this natural potential. While a retrospective analysis of the influences on social skills is valuable, prospective experimental studies of this issue can avoid sources of bias and confound. Here we investigated whether a newborn’s propensity to interact with others and the early social rearing environment predict a later socio-cognitive skill: gaze following (i.e., the ability to look where another individual is looking). We measured newborns’ social propensity with neonatal imitation (i.e., human and nonhuman primate (NHP) newborns’ ability to match modeled behaviours within days after birth1,2). We chose macaques for this study because humans and macaques exhibit similar social capacities across early infant development, including neonatal imitation and gaze following, with the added advantage that the rearing environment of macaques can be carefully controlled and manipulated.
Macaque newborns, like humans, engage in complex face-to-face interactions, including long bouts of mutual gaze3 and facial gesture imitation2,4. Both species exhibit striking individual differences in sociality from birth (for a review in humans, see5). For example, in humans and macaques, approximately half of newborns imitate and half do not6,7. While it is possible that this variability may be due to a transient cause, such as an infant’s state, a more intriguing possibility is that it may reflect a meaningful and stable individual difference. While this idea has been widely proposed8,9,10,11,12, it has yet to be thoroughly tested.
We hypothesized that individual differences in neonatal imitation may reflect individual differences in infants’ social cognitive skills, such as the ability to match another individual’s action with the infant’s own motor representation of that action. According to this hypothesis, observed actions activate one’s own action programs, thus facilitating action recognition, critical for early social interactions11. In monkeys, this system is functioning at birth13 and is expressed in neonatal imitation11. If this hypothesis is correct, neonatal imitation may positively predict later social skills8,9,10,11,12.
In support of this proposal, a handful of reports link neonatal imitation and other aspects of development (recent review:14). In humans, only one study examined neonatal imitation predictively and found it was associated with fewer looks away during an interaction at three months, potentially reflecting that imitators were more socially engaged7,15. In monkeys, neonatal imitators, compared to non-imitators, may better recognize social partners in the first week of life16 and exhibit more mature face viewing patterns at two to four weeks of age17. However, we know little about whether imitative skill predicts behaviour beyond the first month of life, or whether it predicts more advanced social skills.
One advanced social skill that emerges in the first year of life in human and nonhuman primates is the ability to follow another’s gaze into space18,19,20,21,22. Gaze following, like imitation, is a social skill that has been proposed to serve an important evolutionary function, allowing infants to use the gaze direction of older, more expert individuals to locate salient items, such as food, predators, and conspecifics23. By the middle of the first year of life, macaques follow the gaze of conspecifics21 and humans22, but their gaze following continues to improve into adulthood24,25,26, similar to humans (for a review:20).
Both neonatal imitation and gaze following require the interest and ability to track another individual’s behaviour27. In monkeys, neonatal imitators, compared to non-imitators, look more at the eye region of faces17, so imitators may be more likely to detect changes in such features. During face-to-face interactions, human and nonhuman primate newborns are sensitive to gaze engagement (e.g.28), a powerful cue for the development of social skills29. This link between early social skills and sensitivity to gaze may remain stable during development; however, the extent to which these skills are directly associated with one another remains untested.
As in neonatal imitation, there are interindividual differences in gaze following30. These individual differences may also be, in part, due to differences in infants’ early social experiences. In social species, including macaques and humans, the early social environment appears critical in the development of social skills31,32,33. While there is evidence of rudimentary gaze following in human newborns34, it continues to mature in the first year of life, during which time it may be influenced by social experience, such as through reinforcement learning35,36. That is, a rich social environment—especially one with joint attention interactions—provides opportunities for infants to learn links between others’ gaze and relevant environmental stimuli37,38. This hypothesis, however, is difficult to test in humans, as we have limited control over infants’ early social environments.
In the present study, our first goal was to explore whether imitation in the first week of life contributes to the development of a later social skill—gaze following—at 7 months, in infant macaques reared under controlled environmental conditions. Based on reports that individual differences in neonatal imitation may be associated with later social skills (e.g.17), we predicted that neonatal imitators would be more advanced in their gaze following behaviour than non-imitators. Our second goal was to explore whether early social experiences influence social skill development. To assess this, we compared infants with varying levels of social experience—high-socialization infants, housed with three to four of their peers—with low-socialization infants, housed individually with more limited peer interactions. We designed these environmental manipulations to mimic the variability in natural early social environments, with some infants receiving more opportunities for social interactions than others. We hypothesized that neonatal imitators, compared to non-imitators, would exhibit better gaze following due to their greater interest or skill in social interactions. We also hypothesized that high-socialization infants, compared to low-socialization infants, would exhibit better gaze following due to their increased exposure to social cues, enhancing their interest or skill in social interactions. Finally, we predicted imitation and social experience may interact, in one of two ways: imitators, who may be more socially motivated39, may show greater benefits of socially enriched early environments, compared to non-imitators, and therefore may better follow gaze. Alternatively, non-imitators, who may be initially less intrinsically social17, may benefit more from socially enriched early environments compared to imitators, and therefore may exhibit greater rearing-related improvements in gaze following.
Results
There was interindividual variability in neonatal imitation (see Supplemental Materials, Fig. S1). In the gaze following task, we analysed the proportion of infants’ correct responses against chance (0.50). Data were normally distributed with no outliers. All t tests were two-tailed and included Bonferroni corrections. We confirmed, with one-sample t tests, that infants followed gaze above chance for both head trials (M = 0.61, SD = 0.15), t(118) = 8.52, p < 0.001, d = 0.78, and head + torso trials (M = 0.66, SD = 0.15), t(118) = 11.59, p < 0.001, d = 1.06. For each sub-group of infants (imitators and non-imitators within both high- and low-socialization rearing), gaze following was also above chance, ps < 0.01, Supplemental Table 1. Out of 119 infants, 105 (82% of low-socialization infants and 89% of high-socialization infants) performed gaze following at rates above chance.
We tested our hypothesis that interindividual differences in gaze following would be predicted by neonatal imitation and rearing with a 2 × 2 × 2 mixed-design ANOVA with the within-subjects variable Trial type (head + torso-turn, head-turn), and the between-subjects variables Rearing (high- and low-socialization) and Imitator status (imitator, non-imitator). There was a main effect of Trial type, with a greater proportion of correct responses in head + torso trials (M = 0.66, SD = 0.15) compared to head only trials (M = 0.62, SD = 0.15), F(1,115) = 4.66, p = 0.033, ηp2 = 0.04. There was a main effect of Imitator status, with imitators exhibiting a greater proportion of correct responses (M = 0.66, SD = 0.10) compared to non-imitators (M = 0.61, SD = 0.11), F(1,115) = 6.68, p = 0.011, η2 = 0.06, Fig. 1. There was a main effect of Rearing, with a greater proportion of correct responses by high-socialization (M = 0.66, SD = 0.09) compared to low-socialization infants (M = 0.62, SD = 0.11), F(1,115) = 4.39, p = 0.038, η2 = 0.04, Fig. 1. There were no interactions, ps > 0.05.
Figure 1. Proportion of correct gaze following responses (chance = 0.50) for main effects of Rearing—high-socialization (striped bars) and low-socialization (solid bars) and Imitator status—imitator (light bars) and non-imitator (dark bars).
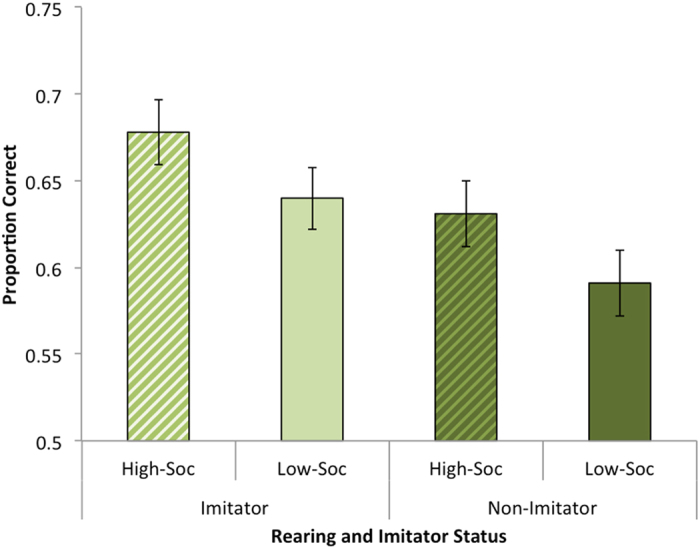
Error bars reflect standard error of the mean.
Discussion
We found support for our prediction that macaque neonates’ imitative capacity—to match facial gestures produced by a model—assessed in the first week of life, predicts a social skill in later infancy—gaze following at 7 months. The present study offers the first evidence (in any species, including humans), to our knowledge, that an individual difference present from birth modulates a social cognitive skill in later infancy. Neonatal imitation may, indeed, reflect a meaningful individual difference, as previously hypothesized8,9,10,11,12. This finding corroborates reports of higher sensitivity and responsivity to social cues in monkey neonatal lipsmacking (LPS; an affiliative facial gesture involving rapid opening and closing of the mouth) imitators, compared to non-imitators, who, in the first week of life may also better recognize social partners16, and, at one month, may attend more to the eye region of faces17. This finding is consistent with a report that, in human infants, neonatal imitators exhibit fewer looks away during a face-to-face interaction at 3 months8,15, perhaps because imitators were more socially engaged. However, the present findings are the first that suggest that imitators, compared to non-imitators, may possess more mature or functional social skills.
From a neurobiological perspective, imitative skills and joint attention activate different brain networks. The former relies on neural mechanisms mapping others’ actions (e.g., gestures) onto their own motor representation of that action, named the mirror mechanism13. The latter likely represents a building block for the development of more sophisticated mentalizing capacities (i.e., theory of mind) that, in adults, involves a network including the temporo-parietal junction and medial prefrontal cortex40. Although each system processes a different type of social information, both are part of the social brain. The mirror mechanism has been well described in monkeys and humans41. This mechanism emerges early in development and infants’ early capacity to imitate facial gestures probably relies upon it4,11,13. In fact, these systems may perform complementary, non-overlapping functions in service of social cognition42, acting together to support action understanding. For example, both the mirror mechanisms and the mentalizing system are engaged during joint actions43, while viewing or imagining social interactions44,45, and while viewing communicative gestures46.
The present study has implications for the development of social skills in human infants. Unlike in humans, we can experimentally manipulate the timing and nature of infant macaques’ early social experiences. In doing so, we found support for our prediction that infants reared in a high-socialization environment outperformed infants reared in a low-socialization environment. While both groups performed above chance, there were significant individual differences accounted for by infants’ early social environments. Our findings are consistent with the hypothesis that gaze following is learned through social exposure25,38 and that, in macaques, early social experiences may affect social skills4,33,47. On the one hand, this result is promising because it suggests there is some plasticity in this social cognitive skill. Particularly, in infants who show early deficits, there may be ways of supporting the development of this skill by providing them with additional social interaction opportunities. While we predicted that early imitative capacity might interact with early rearing, we instead found that all infants appeared to benefit from peer socialization, regardless of their initial imitative skill.
Notably, even infants who were non-imitators and did not receive enriched social interactions with peers—the most “at risk” group—nonetheless performed gaze following at above-chance levels. Of course, infants in the present study all had some peer and human caregiver social interactions, even if lower than in naturalistic contexts, which may have been sufficient for healthy social development. In contrast, infants raised in an environment with even less social stimulation may display insufficient or delayed gaze following due to their limited exposure to social stimuli, which may have downstream consequences given that in humans, gaze following is foundational for higher-level social development (e.g., joint attention;23,26, theory of mind48,49, social learning50). Through studies such as this one we can begin to understand the interdependence of different skills.
Many questions remain. The present study does not allow us to determine the precise factors within the social environment that may be supporting infants’ gaze following skills. For example, through reinforcement learning37 infants in the high-socialization condition had more opportunities to learn that gaze following provided useful information about their environment. In addition, early socialization may have altered infants’ social motivations38. If so, infants in the high-socialization condition, who had greater social experiences compared to the low-socialization infants, may have been more motivated to interact with social partners because they found such interactions more rewarding. In theory, social skill and intrinsic social motivation may influence one another bi-directionally, a challenge outside the scope of the present study, but perhaps relevant to understanding autism spectrum disorders (ASD)51.
Finally, the present study is limited in that we cannot determine the specific aspects of the model—movement of the eyes or head alone—that infants used to follow others’ gaze. In the present study, our models moved both their eyes and head together because, while adult monkeys follow gaze cues that include just the eyes, juvenile monkeys do not26. In addition, gaze following performance may have been better if the stimulus had been provided by a conspecific; however, this is not feasible to test with a live adult monkey model and previous studies have already demonstrated that orienting stimuli provided by a human experimenter are effective in triggering gaze shift responses in juvenile and adult monkeys26.
Social skills are foundational for successfully integrating into society, yet we still know little about the causes of individual differences in social skills52. In a prospective experiment with infant monkeys we explored the contributions of an individual’s natural potential to engage with others and the effect of the environment, nurturing or suppressing this potential. We found a positive association between infants’ neonatal imitation in the first week of life and gaze following ability at 7 months of age. This finding suggests that neonatal imitation assessments might compliment other screening tools for identifying infants at heightened risk for impaired social function17. At the same time, we found evidence that gaze following skills are plastic, positively influenced by early social experiences. This finding has clinical implications for populations at-risk for disorders, such as ASD, characterized by deficits in both imitation and gaze processing30,53,54,55,56. While we know of no published attempts to improve gaze following in high-risk infants, our findings suggest that such interventions might be worthwhile. Finally, we found evidence that the development of gaze following is resilient, developing even with limited opportunities for social interaction, and even among infants who exhibit low rates of neonatal imitation. In sum, the present findings provide support for the hypothesis that individual differences in neonatal imitation may reflect infants’ social cognitive skills, highlighting the importance of continued investigation into both early screening and potential interventions for at-risk infant populations.
Method
Subjects
Infant rhesus macaques (Macaca mulatta) participated in the neonatal imitation assessment between 1–8 days of age and in a gaze following assessment at approximately 7 months of age (M = 234 days, SD = 15). Subjects included singly housed surrogate-reared, low-socialization infants (n = 61; 28 females), and peer-reared, high-socialization infants (n = 58, 23 females). On the day of birth, infants were separated from their mothers and raised in a primate nursery. Infants were raised identically for the first five weeks. Once the youngest infant reached 37 days of age, infants were placed into groups. High-socialization infants were raised in groups of three to five peers. Low-socialization infants were individually housed, assigned to playgroups composed of three to four peers housed together two hours a day, five days a week. See Supplemental Materials for details.
Materials and Procedures
Neonatal Imitation Test
We tested infants three times a day, every other day, in the first week of life. Infants viewed live stimuli, including a lipsmacking gesture (LPS; rapid opening and closing of the mouth) and a control (CTRL) condition, consisting of a 15-cm diameter striped Disk, rotated back and forth 180°. Condition order was randomized between subjects. Each session began with a 40-second static baseline in which the monkey was faced by a human experimenter presenting a still face in the LPS condition and a still disk in the control condition. This baseline was followed by a 100-second stimulus period consisting of a 20-second dynamic stimulus presentation and a 20-second static period (still stimulus), repeated 3 times: dynamic-static-dynamic-static-dynamic. Sessions were videotaped and experimenters blind to the experimental condition coded facial gestures, offline. Infants were classified as imitators if they produced an increase in LPS (rate per sec) from the baseline (still face) to the stimulus period (LPS face) in the LPS condition (matching the model), to a greater extent than the increase in LPS from the baseline (still disk) to the stimulus period (disk rotating) in the control condition, averaged across days. Using this classification, 61 infants were imitators (29 high-socialization, 32 low-socialization) and 58 infants were non-imitators (29 high-socialization, 29 low-socialization). See Supplemental Materials for details.
Gaze Following Test
Using an experimental design adapted from30, infants were tested over four successive days, receiving 10 trials per day, for a total of 40 trials. A familiar caretaker handled the infants. An actor sat approximately two feet in front of the infant, and two evaluators sat approximately four feet behind the actor at opposite 45° angles. Thus, one evaluator was slightly to the left of the actor, and the other was slightly to the right of the actor. The actor sat at eye level with the infant and engaged in various attention getting behaviours to facilitate eye contact. Upon making eye contact, the actor looked either right or left, moving either the head or head + torso 90°, consistent with the direction of gaze, and held this position for approximately five seconds. Thus, there were four possible movements for the infant to observe: head left, head + torso left, head right, head + torso right. The direction of the actor’s eye gaze shift, and movement of torso or head were counterbalanced so the infant saw 10 of each. Head + torso trials contained larger and more obvious movement cues, while head-only trials were thought to be more challenging as they involved a subtler cue. Prior to the test session, one evaluator was assigned to call out the direction of the infant’s first gaze shift, after the actor’s movement. If a monkey did not shift his or her eyes for five seconds, then the eye movement was recorded as “straight ahead.” To ensure accuracy, the second evaluator either agreed or disagreed with the first evaluator’s statement of gaze direction. Upon disagreements or instances of the infant failing to attend to the actor’s movement, the trial was repeated until the evaluators agreed. We assessed infants’ performance by the proportion of correct responses—looking left or right, consistent with the model—out of the total number of left and right responses37,57,58.
Ethics Statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals and complied with the Animal Welfare Act. The Eunice Kennedy Shriver National Institute of Child Health and Development’s Animal Care and Use Committee approved this study.
Additional Information
How to cite this article: Simpson, E. A. et al. Neonatal imitation and early social experience predict gaze following abilities in infant monkeys. Sci. Rep. 6, 20233; doi: 10.1038/srep20233 (2016).
Supplementary Material
Acknowledgments
We thank Angela Ruggiero, Michelle Miller, and the staff and volunteers of the Laboratory of Comparative Ethology for help with data collection. Supported by the Division of Intramural Research, NICHD, and NICHD P01HD064653.
Footnotes
Author Contributions A.P. and P.F.F. designed the studies. A.P., E.A.S. and G.M. collected the data. E.A.S. and A.P. coded the neonatal imitation videos and analysed the data. G.M. and E.A.S. wrote the manuscript. All authors approved the final manuscript.
References
- Meltzoff A. N. & Moore M. K. Imitation of facial and manual gestures by human neonates. Science 198, 75–78 (1977). [DOI] [PubMed] [Google Scholar]
- Ferrari P. F. et al. Neonatal imitation in rhesus macaques. PLoS Biol. 4, e302 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P. F., Paukner A., Ionica C. & Suomi S. J. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr. Biol. 19, 1768–1772 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert R. E. et al. Early social experience affects neural activity to affiliative facial gestures in newborn nonhuman primates. Dev. Neurosci. 37, 243–252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J. & Fagen J. Individual differences in infancy: reliability, stability, prediction (Lawrence Erlbaum Associates, 1990). [Google Scholar]
- Ferrari P. F. et al. Interindividual differences in neonatal imitation and the development of action chains in rhesus macaques. Child Dev. 80, 1057–1068 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann M. Notes on individual differences and the assumed elusiveness of neonatal imitation in The imitative mind: development, evolution, and brain bases (eds Meltzoff A. N. & Prinz W.) 74–84 (Cambridge University Press, 2002).
- Heimann M., Nelson K. E. & Schaller J. Neonatal imitation of tongue protrusion and mouth opening: methodological aspects and evidence of early individual differences. Scand. J. Psychol. 30, 90–101 (1989). [DOI] [PubMed] [Google Scholar]
- Siller M. & Sigman M. From neonatal imitation to social cognition: social and cognitive pathways to developmental continuity in Social and moral development: emerging evidence on the toddler years (eds Leavitt L. A. & Hall D. M. B.) 143–164 (Johnson & Johnson Pediatric Institute, 2004). [Google Scholar]
- Maratos O. Neonatal, early and later imitation: same order phenomena? in The development of sensory, motor and cognitive capacities in early infancy: from perception to cognition (eds Simion F. & Butterworth G.) 145–160 (Psychology Press Ltd, 1998). [Google Scholar]
- Simpson E. A., Murray L., Paukner A. & Ferrari P. F. The mirror neuron system as revealed through neonatal imitation: presence from birth, predictive power, and evidence of plasticity. Phil. Trans. Roy. Soc. B. 369, 20130289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T., Oostenbroek J., Nielsen M. & Slaughter V. Is newborn imitation developmentally homologous to later social‐cognitive skills? Dev. Psychobiol. 55, 52–58 (2013). [DOI] [PubMed] [Google Scholar]
- Ferrari P. F. et al. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. J. Cogn. Neurosci. 24, 1165–1172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. A., Paukner A., Suomi S. J. & Ferrari P. F. Neonatal imitation and its sensorimotor mechanism in New frontiers in mirror neuron research (eds Ferrari P. F. & Rizzolatti G.) 296–314 (Oxford University Press, 2015). [Google Scholar]
- Heimann M. Neonatal imitation, gaze aversion, and mother-infant interaction. Infant Behav. Dev. 12, 495–505 (1989). [Google Scholar]
- Simpson E. A., Paukner A., Sclafani V., Suomi S. J. & Ferrari P. F. Lipsmacking imitation skill in newborn macaques is predictive of social partner discrimination. PLOS ONE 8, e82921 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A., Simpson E. A., Ferrari P. F., Mrozek T. & Suomi S. J. Neonatal imitation predicts how infants engage with faces. Dev. Sci. 17, 833–840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth G. & Jarrett N. What minds have in common is space: spatial mechanisms serving joint visual attention in infancy. Brit. J. Dev. Psychol. 9, 55–72 (1991). [Google Scholar]
- Rosati A. G. & Hare B. Looking past the model species: diversity in gaze-following skills across primates. Curr. Opin. Neurobiol. 19, 45–51 (2009). [DOI] [PubMed] [Google Scholar]
- Shepherd S. V. Following gaze: gaze-following behavior as a window into social cognition. Front. Integrative Neurosci. 4, 5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel C., Gutmann A., Pirow R. & Fischer J. Facial expressions modulate the ontogenetic trajectory of gaze‐following among monkeys. Dev. Sci. 13, 913–922 (2010). [DOI] [PubMed] [Google Scholar]
- Tomasello M., Hare B. & Fogleman T. The ontogeny of gaze following in chimpanzees, Pan troglodytes, and rhesus macaques, Macaca mulatta. Anim. Behav. 61, 335–343 (2001). [Google Scholar]
- Emery N. J., Lorincz E. N., Perrett D. I., Oram M. W. & Baker C. I. Gaze following and joint attention in rhesus monkeys (Macaca mulatta). J. Comp. Psychol. 111, 286–293 (1997). [DOI] [PubMed] [Google Scholar]
- Paukner A., Anderson J. R., Fogassi L. & Ferrari P. F. Do facial gestures, visibility or speed of movement influence gaze following responses in pigtail macaques? Primates 48, 241–244 (2007). [DOI] [PubMed] [Google Scholar]
- Ferrari P. F., Coudé G., Gallese V. & Fogassi L. Having access to others’ mind through gaze: the role of ontogenetic and learning processes in gaze-following behavior of macaques. Soc. Neurosci 3, 239–249 (2008). [DOI] [PubMed] [Google Scholar]
- Ferrari P. F., Kohler E., Fogassi L. & Gallese V. The ability to follow eye gaze and its emergence during development in macaque monkeys. Proc. Natl. Acad. Sci. USA 97, 13997–14002 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M., Nagell K., Tomasello M., Butterworth G. & Moore C. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monogr. Soc. Res. Child Dev. 63, 1–174 (1998). [PubMed] [Google Scholar]
- Farroni T., Massaccesi S., Pividori D. & Johnson M. H. Gaze following in newborns. Infancy 5, 39–60 (2004). [Google Scholar]
- Guellai B. & Streri A. Cues for early social skills: direct gaze modulates newborns’ recognition of talking faces. PloS ONE 6, e18610 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife M. & Bruner J. S. The capacity for joint visual attention in the infant. Nature 253, 265–266 (1975). [DOI] [PubMed] [Google Scholar]
- Alexander B. K. & Harlow H. F. Social behavior of juvenile rhesus monkeys subjected to different rearing conditions during the first six months of life. Zool. Jb. Physiol. Bd. 71, 489–508 (1965). [Google Scholar]
- Bowlby J. Attachment 2nd edition (Basic Books, 1982). [Google Scholar]
- Ruppenthal G. C., Harlow M. K., Eisele C. D., Harlow H. F. & Suomi S. J. Development of peer interactions of monkeys reared in a nuclear-family environment. Child Dev. 45, 670–682 (1974). [PubMed] [Google Scholar]
- Farroni T., Massaccesi S., Menon E. & Johnson M. H. Direct gaze modulates face recognition in young infants. Cognition 102, 396–404 (2007). [DOI] [PubMed] [Google Scholar]
- Moore C., Angelopoulos M. & Bennett P. The role of movement in the development of joint visual attention. Infant Behav. Dev. 20, 83–92 (1997). [Google Scholar]
- Triesch J., Teuscher C., Deák G. O. & Carlson E. Gaze following: why (not) learn it? Dev. Sci. 9, 125–147 (2006). [DOI] [PubMed] [Google Scholar]
- Corkum V. & Moore C. The origins of joint visual attention in infants. Dev. Psychol. 34, 28–38 (1998). [DOI] [PubMed] [Google Scholar]
- Gredebäck G., Fikke L. & Melinder A. The development of joint visual attention: a longitudinal study of gaze following during interactions with mothers and strangers. Dev. Sci. 13, 839–848 (2010). [DOI] [PubMed] [Google Scholar]
- Simpson E. A. et al. Inhaled oxytocin increases positive social behaviors in newborn macaques. Proc. Natl. Acad. Sci. USA 111, 6922–6927 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S. & Blakemore S. J. Functional connectivity during a social emotion task in adolescents and in adults. Eur. J. Neurosci. 2, 1294–1301 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G. & Fogassi L. The mirror mechanism: recent findings and perspectives. Phil. Trans. Roy. Soc. B. 369, 20130420 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta‐analysis. Human Brain Mapping 30, 829–858 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokal I., Gazzola V. & Keysers C. Acting together in and beyond the mirror neuron system. Neuroimage 47, 2046–2056 (2009). [DOI] [PubMed] [Google Scholar]
- Centelles L., Assaiante C., Nazarian B., Anton J. L. & Schmitz C. Recruitment of both the mirror and the mentalizing networks when observing social interactions depicted by point-lights: a neuroimaging study. PLoS ONE 6, e15749 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp K. et al. Imagining triadic interactions simultaneously activates mirror and mentalizing systems. NeuroImage 98, 314–323 (2014). [DOI] [PubMed] [Google Scholar]
- Mainieri A. G., Heim S., Straube B., Binkofski F. & Kircher T. Differential role of the Mentalizing and the Mirror Neuron system in the imitation of communicative gestures. NeuroImage 81, 294–305 (2013). [DOI] [PubMed] [Google Scholar]
- Harlow H. F., Rowland G. L. & Griffin G. A. The effect of total social deprivation on the development of monkey behavior. Psychiat. Res. Reports 19, 116–135 (1964). [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: an essay on autism and theory of mind. (MIT Press, 1995). [Google Scholar]
- Brooks R. & Meltzoff A. N. Connecting the dots from infancy to childhood: a longitudinal study connecting gaze following, language, and explicit theory of mind. J. Exp. Child Psychol. 130, 67–78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A. N., Kuhl P. K., Movellan J. & Sejnowski T. J. Foundations for a new science of learning. Science 325, 284–288 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E. S. & Schultz R. T. The social motivation theory of autism. Trends. Cogn. Sci. 16, 231–239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey E. A. Decoding the dyad: challenges in the study of individual differences in social behavior. Curr. Dir. Psychol. Sci. 24, 285–291 (2015). [Google Scholar]
- Emery N. J. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 24, 581–604 (2000). [DOI] [PubMed] [Google Scholar]
- Heimann M. & Ullsatdius E. In Imitation in infancy (eds Nadel J. & Butterworth G.) 235–253 (Cambridge University Press, 1999). [Google Scholar]
- Leekam S., Baron-Cohen S., Perrett D., Milders M. & Brown S. Eye-direction detection: a dissociation between geometric and joint attention skills in autism. Brit. J. Dev. Psychol. 15, 77–95 (1997). [Google Scholar]
- Nation K. & Penny S. Sensitivity to eye gaze in autism: is it normal? Is it automatic? Is it social? Dev. Psychopathol. 20, 79–97 (2008). [DOI] [PubMed] [Google Scholar]
- Morissette P., Ricard M. & Décarie T. G. Joint visual attention and pointing in infancy: a longitudinal study of comprehension. Brit. J. Dev. Psychol. 13, 163–175 (1995). [Google Scholar]
- Butterworth G. & Cochran E. Towards a mechanism of joint visual attention in human infancy. Int. J. Behav. Dev. 3, 253–272 (1980). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


