Abstract
Objective
To assess the role of Helicobacter pylori infection and interleukin 6 polymorphism -174 (rs1800795) in dyslipidemia.
Design
Case–control study comparing serum lipids between H. pylori positive and negative patients and controlling for IL-6 -174 polymorphism, age, sex and smoking.
Setting
3 hospitals performing outpatient endoscopies in the city of Oulu, Finland.
Participants
199 adult patients with dyspepsia symptoms fulfilling Rome criteria originating from ethnically Finnish population. Patients with an immunosuppressive disorder or malignant disease, treated H. pylori infection, immunosuppressive or anticoagulant medication, previous gastric surgery or ongoing antibiotic treatment were excluded.
Primary outcome measures
Association of H. pylori infection and serum lipid concentrations in the whole group or in genotype-based subgroups. The associations between peptic ulcer, gastric mucosal inflammation and serum lipid concentrations were assessed as secondary outcomes.
Results
The median high-density lipoprotein (HDL) serum concentration was significantly lower in the H. pylori positive group (0.81 mmol/L) than in the negative group (0.95 mmol/L; p<0.001). In the genotype subgroup analyses, a similar association between H. pylori infection and HDL serum levels was seen within the IL-6 -174 CC genotype group (HDL 0.72 vs 1.06 mmol/L, respectively; p<0.001), but no significant associations were seen in the GC or GG genotype groups. Additionally, patients with peptic ulcer demonstrated lower HDL levels (0.75 mmol/L) than H. pylori positive patients without ulcer (0.86 mmol/L; p=0.010).
Conclusions
H. pylori infection associated significantly with low serum levels of HDL in the IL-6 -174 CC genotype patients but not in the other genotypes. This suggests that the association between H. pylori infection and serum HDL could be transmitted through IL-6. We suggest that the role of IL-6 genotype should also be studied in relation to other associations between gastrointestinal microbiome and cardiovascular risk factors.
Keywords: GENETICS, INFECTIOUS DISEASES
Strengths and limitations of this study.
The associations of Helicobacter pylori and interleukin 6 -174 with dyslipidemia have not been studied together previously.
The study group is ethnically homogeneous, H. pylori positivity has been confirmed by multiple methods and we have data on ulcer status and mucosal inflammation.
The size of the study group is fairly small, the study group is composed of only patients with dyspepsia and we have no data on IL-6 serum levels, body mass index, diet, serum glucose levels or cholesterol medication.
Introduction
Helicobacter pylori-related gastritis is a major aetiological factor of peptic ulcer and gastric cancer. H. pylori infection has also been associated with atherogenic serum lipid changes in several studies during the past 20 years.1–15 H. pylori infection has also been associated with coronary heart disease, but the evidence is still equivocal.16 17
H. pylori infection causes a varied cytokine response including the release of interleukin 6.18 High IL-6 serum levels have been connected to changes in lipid metabolism and to coronary heart disease.19 A polymorphic allele, guanine/cytosine (G/C), at the IL-6 gene promoter at location -174 in the 5’ flanking region (rs1800795) has been previously associated with higher IL-6 serum levels20 and the effect has also been documented in Finnish populations.21 The relationship between IL-6 -174 polymorphisms and IL-6 serum levels seems to vary in different participant groups22 and to be modified by external factors such as exercise.23 The IL-6 -174 polymorphism has also been associated with lipid abnormalities24 and serum lipid changes during lifestyle interventions.25 26 IL-6 -174 has also been associated with the risk of coronary heart disease, but this association seems to vary between different participant groups.27 28
The objective of this study was to assess the role of H. pylori infection and IL-6 polymorphism -174 (rs1800795) in dyslipidemia. Thus, we have examined the serum lipid levels of patients with dyspepsia and compared the results between groups based on H. pylori infection, IL-6 -174 genotypes, the presence or absence of gastric or duodenal ulcers and histopathological findings.
Patients and methods
Our study group was collected among unselected consecutive adult patients with dyspepsia symptoms fulfilling Rome criteria. The patients were collected between years 1996 and 2000 from three outpatient endoscopies performing hospitals in the city of Oulu, Finland. Consecutive patients were enrolled until a certain amount of patients with ulcer (n=73) and H. pylori negative (n=84) and positive patients without ulcer(n=59) were achieved. Of the total 216 patients enrolled, 199 participated in serum lipid analyses. Of them, 57 were patients with ulcer, 84 H. pylori negative patients without ulcer and 58 H. pylori positive patients without ulcer. The patients were asked about the use of dyspepsia medicine (antacids, sucralfate, histamine 2 receptor antagonists and proton pump inhibitors) and smoking habits. All control and study participants originated from ethnically homogenous Finnish population. Patients with immunosuppressive or malignant diseases, treated H. pylori infections, immunosuppressive or anticoagulant medication, previous gastric surgery and patients receiving ongoing antibiotic treatment were excluded.29–31
We performed a case–control study with H. pylori positive patients as case patients and H. pylori negative patients as controls and controlled for the IL-6 -174 gene polymorphism, age, sex and smoking. Additionally, we tested if the presence of peptic ulcers or histological markers of inflammation would affect the possible associations. To detect a clinically significant difference in serum lipid concentrations (10%) between H. pylori positive and negative groups with a significance level of 0.05, power of 0.80 and expected SD of 24% of the mean value, one would need a sample size of 91 patients in each group. With the same criteria, we could detect 20% differences in serum lipid concentrations between subgroups as small as 23 patients.
Positive H. pylori status was based on a positive serology together with a positive bacterial culture, positive histology or a positive PCR test. Serology, bacterial culture and H. pylori PCR were performed as previously described.29 Serum total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglyceride serum concentrations were measured by routine methods in the clinical laboratory of Oulu University Hospital. The cut-off point of <1.00 mmol/L for low HDL was chosen because it is a common cut-off point in Finnish guidelines.32 The patients’ DNA was extracted from blood leucocytes as previously described.29 The restriction fragment length polymorphism method with an NlaIII restriction enzyme using previously published primers20 was used to detect the IL-6 -174 gene polymorphisms from the DNA samples. Endoscopies were performed by experienced endoscopists. Endoscopy findings, including the presence or absence of gastric or duodenal ulcer, were registered. Histopathological changes in the gastric mucosal biopsies were analysed according to the Sydney system33 by one pathologist (TJK). The presence of the pathogenic H. pylori gene variant, cagA, was detected as previously described.34
Medians and IQR are presented for variables with skewed distribution and means and SDs for normally distributed continuous variables. The significance of differences between two groups of normally distributed variables was estimated by the independent samples Student t test. The significance of differences between two groups in variables with a skewed distribution was assessed by the Mann-Whitney U test. The significance of differences between three groups in variables with a skewed distribution was assessed by the Kruskal-Wallis test. The significance of differences between discrete variables was assessed with crude and stepwise binary logarithmic regression models (with likelihood ratio criteria for stepwise models), which were also used to calculate ORs and 95% CIs. The significances of correlations were analysed with Spearman's test. A p value of <0.05 was considered statistically significant. Missing data were excluded pairwise. The data were analysed using the SPSS software V.19 (IBM, Armonk, New York, USA).
All patients gave their informed written consent.
Results
Of the whole group of 199 study participants, 114 were H. pylori positive and 85 were H. pylori negative. The HDL serum level was significantly lower in the H. pylori positive group (0.81 mmol/L; IQR 0.29 mmol/L) than in the H. pylori negative group (0.95 mmol/L; IQR 0.40 mmol/L; p<0.001). There were no significant differences in total cholesterol, LDL and triglyceride serum levels between H. pylori positive and negative patients (table 1).
Table 1.
Characteristics of the participant groups and differences between Helicobacter pylori positive and negative patients
| H. pylori negative | H. pylori positive | p Value* | |
|---|---|---|---|
| Patients, n (%) | 85 (42.7) | 114 (57.3) | – |
| Age in years, mean (SD) | 48.6 (14.0) | 57.0 (13.3) | <0.001 |
| Male, n (%) | 36 (42.4) | 46 (40.4) | 0.884 |
| Smokers, n (%) | 15 (17.6) | 35 (30.7) | 0.047 |
| On dyspepsia medication, n (%) | 12 (14.3) | 42 (36.8) | 0.001 |
| IL-6 -174 genotype, n (%) | |||
| GG | 21 (24.7) | 27 (23.7) | 0.410 |
| GC | 35 (41.2) | 57 (50.0) | |
| CC | 29 (34.1) | 30 (26.3) | |
| Serum total cholesterol mmol/L, mean (SD) | 5.56 (1.09) | 5.48 (1.23) | 0.596 |
| Serum HDL mmol/L, median (IQR) | 0.95 (0.40) | 0.81 (0.29) | <0.001 |
| Serum LDL mmol/L, mean (SD) | 4.08 (1.00) | 3.94 (0.93) | 0.309 |
| Serum triglycerides mmol/L, median (IQR) | 1.11 (0.70) | 1.14 (0.66) | 0.673 |
*Student's t test for age, total cholesterol and LDL, χ2 for sex, smoking status and genotypes, Mann-Whitney U for HDL and triglycerides.
HDL, high-density lipoprotein; IL-6, interleukin 6; LDL, low-density lipoprotein.
The IL-6 -174 polymorphisms were consistent with the Hardy-Weinberg equilibrium (GG 24.1%; GC 46.2%; CC 29.6%) and were not associated with H. pylori positivity (p=0.410). There was no significant difference in serum HDL between the genotypes (GG 0.79 mmol/L; IQR 0.34 mmol/L; GC 0.90 mmol/L; IQR 0.34 mmol/L; CC 0.86 mmol/L; IQR 0.43 mmol/L; p=0.227). To study the associations of multiple variables with low HDL serum levels (<1.00 mmol/L), we performed stepwise logistic regression analysis with H. pylori status, IL-6 -174 genotype (GG genotype as the reference), sex, age and smoking status as covariates. H. pylori was associated with low HDL with an OR of 3.659 (CI 1.905 to 7.026; p<0.001) and male sex was associated with low HDL with an OR of 2.766 (CI 1.382 to 5.536; p=0.004), while the IL-6 -174 genotype, age and smoking did not associate with HDL.
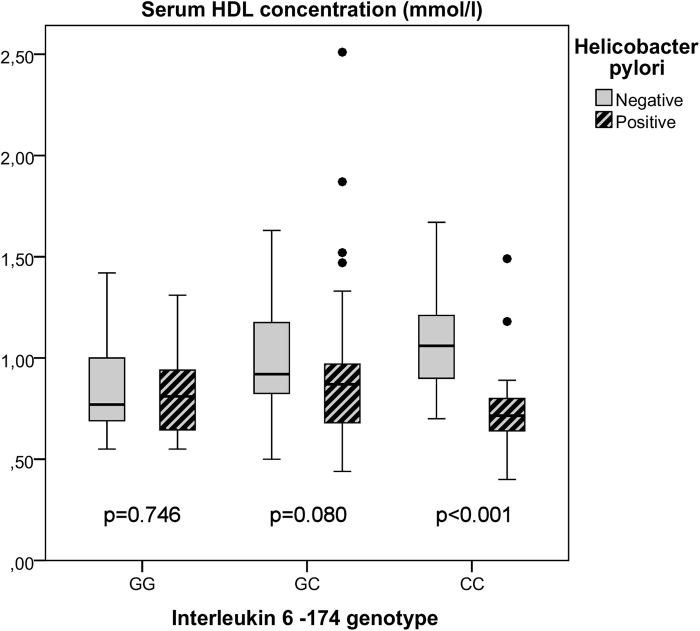
Additionally, we tested if the discovered association between H. pylori positivity and HDL serum levels would be different in the separate genotype groups (figure 1). A significant difference in the HDL serum concentrations between the H. pylori positive (0.72 mmol/L; IQR 0.17 mmol/L) and the H. pylori negative (1.06 mmol/L; IQR 0.33 mmol/L) patients was seen in the CC genotype group (p<0.001). No significant differences were seen between the H. pylori positive and negative patients in the GC (0.87 mmol/L; IQR 0.31 mmol/L vs 0.92 mmol/L, IQR 0.38 mmol/L, respectively, p=0.080) or GG genotype groups (0.81 mmol/L, IQR 0.31 mmol/L vs 0.77 mmol/L, IQR 0.35 mmol/L, respectively, p=0.746). H. pylori positivity was associated with low HDL in the CC genotype group (OR 32.190; CI 5.629 to 184.081; p<0.001) in a regression model. Also, sex (male OR 4.801; CI 1.038 to 22.200; p=0.045) was a significant factor, while age and smoking were omitted from the model as non-significant. In similar regression analyses of the GC and GG genotype patients, H. pylori positivity was not associated with low HDL levels.
Figure 1.
Box plot diagram (median line, quartile box and whiskers for the most extreme cases inside 1.5× IQR) of high-density lipoprotein (HDL) serum concentration in subgroups based on interleukin 6 -174 genotypes and Helicobacter pylori positivity.
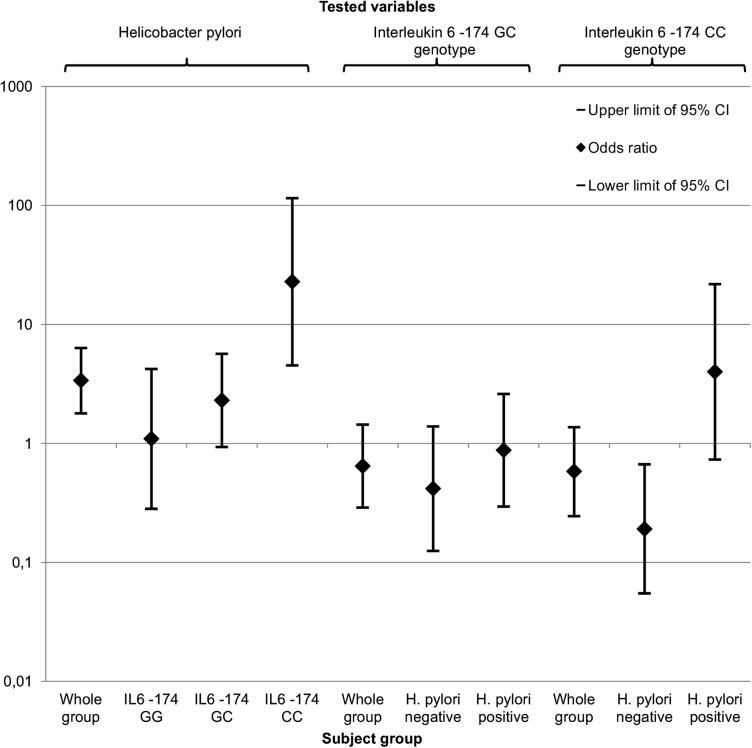
We also tested for associations between the IL-6 -174 genotypes and HDL in subgroups based on H. pylori positivity with similar regression models. In H. pylori negative patients, the IL-6 -174 genotype CC (versus reference genotype GG) was associated with high HDL serum levels (OR to low HDL: 0.191; CI 0.055 to 0.669; p=0.010), while the GC genotype did not associate with HDL. In the H. pylori positive patients, the IL-6 -174 genotype did not associate significantly with low HDL. We also performed crude regression analyses of the association between H. pylori, IL-6 -174 genotypes and HDL in different subgroups. The results have been compiled in figure 2.
Figure 2.
Diagram for crude ORs (diamond) and corresponding 95% CIs (whiskers) of associations with low (<1.0 mmol/L) high-density lipoprotein. Helicobacter pylori (compared with negative patients) and interleukin 6 (IL-6) -174 genotypes GC and CC (compared with GG) were tested separately in the whole groups and specified subgroups.
Of the 57 patients with ulcer, 1 was H. pylori negative and thus excluded from the following analyses. Of the rest, 40 had an active duodenal ulcer and 16 an active gastric ulcer. The ulcers were not associated with the IL-6 -174 genotypes, when compared with all patients without ulcer (p=0.958) or H. pylori positive patients without ulcer (p=0.883). The association between HDL and H. pylori remained significant after excluding patients with ulcer (HDL 0.96 mmol/L; IQR 0.40 mmol/L for H. pylori negative patients vs 0.86 mmol/L; IQR 0.31 mmol/L for positive patients; p=0.010). The HDL difference between H. pylori negative (1.06 mmol/L; IQR 0.33 mmol/L) and H. pylori positive (0.71 mmol/L; IQR 0.22 mmol) patients without ulcer is also significant (p<0.001) in the GG genotype subgroup, while the tests in CC and GC groups remained non-significant. The patients with ulcer had lower HDL serum levels (0.75 mmol/L; IQR 0.26 mmol/L) than the H. pylori positive patients without ulcer (0.86 mmol/L; IQR 0.31 mmol/L; p=0.010) and H. pylori negative patients without ulcer (p<0.001). The cagA analysis was successful on 107 participants (93.9%) of which 97 were cagA positive (90.7%). Among the participants with cagA data, 50/52 (96.2%) patients with ulcer were cagA positive and 47/55 (85.5%) patients without ulcer were cagA positive (p=0.094). The median HDL levels were 0.87 mmol/L (IQR 0.28 mmol/L) for cagA negative patients and 0.81 mmol/L (IQR 0.30 mmol/L) for cagA positive patients (p=0.683). The IL-6 -174 genotype frequencies did not associate with the Sydney system-based variables of histopathological findings.30 The HDL serum levels showed strong negative correlations with the antrum mononuclear cell score (r=−0.579), antrum neutrophil score (r=−0.636), antrum H. pylori score (r=−0.525), body mononuclear cell score (r=−0.685), body neutrophil score (r=−0.494) and body H. pylori score (r=−0.585; all p values<0.001) in the IL-6 -174 CC genotype patients but not in the GG or GC patients.
Discussion
We have demonstrated that H. pylori infection associates significantly with low serum HDL. In subgroup analyses based on the IL-6 -174 polymorphism, we documented that H. pylori positivity associated with low serum HDL only in the CC genotype patients. There was a similar but non-significant tendency in the GC genotype patients and no perceivable tendency in the GG genotypes. On the basis of the sample size and the specified significance and power levels, we conclude that the association of H. pylori infection on the HDL serum level is notable in the IL-6 -174 CC genotype patients, but in the other genotypes, especially in the GG genotype, the association is smaller and possibly clinically insignificant. In addition, the severity of the H. pylori associated disease seems to have a significance in dyslipidemia, which is demonstrated by the lower HDL concentrations in patients with peptic ulcer when compared with H. pylori positive patients without ulcer. CagA positivity was not significantly associated with HDL. However, cagA negative strains were rare, and a larger material would be necessary to assess reliably the association with HDL.
The associations of H. pylori and IL-6 -174 with dyslipidemia have not been previously studied together. Although the number of our study suspects was fairly small, the main results are statistically significant and on the basis of the specified significance and power levels consistent conclusions can be made. We did not have data on IL-6 serum levels. We also did not have data on the use of statin medication, but statin use was not common in Finland during the study period.35 Since there are no clear reasons, why the use of statins would be enriched in H. pylori negative patients, who had higher HDL levels, it is unlikely that the use of statin medication confounds our results. We did not have data on patients’ body mass index, diet or serum glucose, and thus we cannot assess the interplay between obesity, diet, glucose metabolism, H. pylori and the IL-6 -174 genotype on lipid metabolism. The study group consisted ethnically homogeneous Finnish patients suffering from dyspepsia. As always, the generalisation of the results to other kinds of populations should be done with caution.
H. pylori infection and peptic ulcers have been associated with increased IL-6 expression18 36 and high IL-6 serum levels have been associated with low HDL serum levels.37 IL-6 has been documented to reduce the activity of lipoprotein lipase38 and decreased lipoprotein lipase activity has been associated with a decrease in HDL,39 which offers a possible mechanism for the interaction between IL-6 and HDL. Previous studies have shown that exercise lowers IL-6 serum levels only in IL-6 -174 C allele carriers.23 Similarly, exercise and lifestyle interventions have been documented to increase HDL levels significantly more in the IL-6 -174 C allele carriers than in the G allele carriers.25 26 In line with these studies, our results suggest that the HDL levels are more prone to decrease by H. pylori infection in the IL-6 -174 C carriers than in the other genotypes. On the basis of these results, we hypothesise that the allele IL-6 -174 C carriers demonstrate variable IL-6 and HDL levels which are more dependent on external factors such as H. pylori infection, exercise or diet, and that allele G carriers demonstrate more constant and high levels of IL-6 and low levels of HDL.
The association between H. pylori infection and low HDL has been demonstrated previously in several studies.1 3 5 6 9–15 H. pylori eradication has also been documented to increase serum HDL levels.7 8 13 40 41 On the basis of our results, we hypothesise that the benefits of eradication, especially considering HDL levels, could be more prominent in the IL-6 -174 CC genotype patients than in the patients of other IL-6 -174 genotypes.
Recent observations have suggested that improvement of diet by whole grain supplement may induce concurrent intestinal microbiota changes, decreased IL-6 levels and associate with changes indicating improvement of metabolic function.42 These results suggest a role for IL-6 in systemic responses caused by dietary changes and their associated changes in the commensal mucosal flora. On the basis of our current findings, which indicate that the IL-6 -174 polymorphism modifies systemic effects induced by changes in the composition of gastric bacteria, we suggest that IL-6 polymorphisms should be investigated as a possible mechanism explaining associations between intestinal microbiome and the cardiovascular disease risk factors.
In conclusion, we have demonstrated an IL-6 -174 genotype dependent association between H. pylori infection and low serum HDL levels. Our results suggest that the association of H. pylori infection with low HDL levels could be transmitted through IL-6 and that the IL-6 -174 genotype might modify other associations between gastrointestinal microbial flora and cardiovascular disease risk factors.
Footnotes
Contributors: V-MP contributed to the study design, analysed the data, and drafted and revised the manuscript. O-PK collected part of the data and contributed to the revision of the manuscript. SEN collected part of the data and contributed to the revision of the manuscript. RAK designed the study, monitored the data collection and contributed to the revision of the manuscript. TJK designed the study, monitored the data collection, and drafted and revised the manuscript. All the authors approved the manuscript to be published.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: The regional ethics committee of the Northern Ostrobothnia Hospital District, Finland.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Murray LJ, Bamford KB, O'Reilly DP et al. Helicobacter pylori infection: relation with cardiovascular risk factors, ischaemic heart disease, and social class . Br Heart J 1995;74:497–501. 10.1136/hrt.74.5.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niemela S, Karttunen T, Korhonen T et al. Could Helicobacter pylori infection increase the risk of coronary heart disease by modifying serum lipid concentrations? Heart 1996;75:573–5. 10.1136/hrt.75.6.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Luis DA, Lahera M, Canton R et al. Association of Helicobacter pylori infection with cardiovascular and cerebrovascular disease in diabetic patients . Diabetes Care 1998;21:1129–32. 10.2337/diacare.21.7.1129 [DOI] [PubMed] [Google Scholar]
- 4.Laurila A, Bloigu A, Näyhä S et al. Association of Helicobacter pylori infection with elevated serum lipids . Atherosclerosis 1999;142:207–10. 10.1016/S0021-9150(98)00194-4 [DOI] [PubMed] [Google Scholar]
- 5.Hoffmeister A, Rothenbacher D, Bode G et al. Current infection with Helicobacter pylori, but not seropositivity to Chlamydia pneumoniae or cytomegalovirus, is associated with an atherogenic, modified lipid profile . Arterioscler Thromb Vasc Biol 2001;21:427–32. 10.1161/01.ATV.21.3.427 [DOI] [PubMed] [Google Scholar]
- 6.Takashima T, Adachi K, Kawamura A et al. Cardiovascular risk factors in participants with Helicobacter pylori infection . Helicobacter 2002;7:86–90. 10.1046/j.1083-4389.2002.00064.x [DOI] [PubMed] [Google Scholar]
- 7.Scharnagl H, Kist M, Grawitz AB et al. Effect of Helicobacter pylori eradication on high-density lipoprotein cholesterol . Am J Cardiol 2004;93:219–20. 10.1016/j.amjcard.2003.09.045 [DOI] [PubMed] [Google Scholar]
- 8.Kanbay M, Gür G, Yucel M et al. Does eradication of Helicobacter pylori infection help normalize serum lipid and CRP levels? Dig Dis Sci 2005;50:1228–31. 10.1007/s10620-005-2764-9 [DOI] [PubMed] [Google Scholar]
- 9.Sung KC, Rhee EJ, Ryu SH et al. Prevalence of Helicobacter pylori infection and its association with cardiovascular risk factors in Korean adults . Int J Cardiol 2005;102:411–17. 10.1016/j.ijcard.2004.05.040 [DOI] [PubMed] [Google Scholar]
- 10.Longo-Mbenza B, Nkondi Nsenga J, Vangu Ngoma D. Prevention of the metabolic syndrome insulin resistance and the atherosclerotic diseases in Africans infected by Helicobacter pylori infection and treated by antibiotics . Int J Cardiol 2007;121:229–38. 10.1016/j.ijcard.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 11.Gunji T, Matsuhashi N, Sato H et al. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population . Am J Gastroenterol 2008;103:3005–10. 10.1111/j.1572-0241.2008.02151.x [DOI] [PubMed] [Google Scholar]
- 12.Jia EZ, Zhao FJ, Hao B et al. Helicobacter pylori infection is associated with decreased serum levels of high density lipoprotein, but not with the severity of coronary atherosclerosis . Lipids Health Dis 2009;8:59 10.1186/1476-511X-8-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gen R, Demir M, Ataseven H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation . South Med J 2010;103:190–6. 10.1097/SMJ.0b013e3181cf373f [DOI] [PubMed] [Google Scholar]
- 14.Satoh H, Saijo Y, Yoshioka E et al. Helicobacter Pylori infection is a significant risk for modified lipid profile in Japanese male subjects . J Atheroscler Thromb 2010;17:1041–8. 10.5551/jat.5157 [DOI] [PubMed] [Google Scholar]
- 15.Moretti E, Gonnelli S, Campagna M et al. Influence of Helicobacter pylori infection on metabolic parameters and body composition of dyslipidemic patients . Intern Emerg Med 2014;9:767–72. 10.1007/s11739-013-1043-6 [DOI] [PubMed] [Google Scholar]
- 16.Kowalski M, Konturek PC, Pieniazek P et al. Prevalence of Helicobacter pylori infection in coronary artery disease and effect of its eradication an coronary lumen reduction after percutaneous coronary angioplasty . Dig Liver Dis 2001;33:222–9. 10.1016/S1590-8658(01)80711-8 [DOI] [PubMed] [Google Scholar]
- 17.Sharma V, Aggarwal A. Helicobacter pylori: does it add to risk of coronary artery disease . World J Cardiol 2015;7:19–25. 10.4330/wjc.v7.i1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai HF, Hsu PN. Interplay between Helicobacter pylori and immune cells in immune pathogenesis of gastric inflammation and mucosal pathology . Cell Mol Immunol 2010;7:255–9. 10.1038/cmi.2010.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danesh J, Kaptoge S, Mann AG et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review . PLoS Med 2008;5:e78 10.1371/journal.pmed.0050078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishman D, Faulds G, Jeffery R et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis . J Clin Invest 1998;102:1369–76. 10.1172/JCI2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raunio T, Nixdorf M, Knuuttila M et al. The extent of periodontal disease and the IL-6 -174 genotype as determinants of serum IL-6 level . J Clin Periodontol 2007;34:1025–30. 10.1111/j.1600-051X.2007.01151.x [DOI] [PubMed] [Google Scholar]
- 22.Potaczek DP, Undas A, Nowakowski T et al. Association between inflammatory markers and the interleukin-6 -174 G/C polymorphism is abolished in peripheral arterial occlusive disease . Int Angiol 2007;26:318–23. [PubMed] [Google Scholar]
- 23.Oberbach A, Lehmann S, Kirsch K et al. Long-term exercise training decreases interleukin-6 (IL-6) serum levels in subjects with impaired glucose tolerance: effect of the -174G/C variant in IL-6 gene . Eur J Endocrinol 2008;159:129–36. 10.1530/EJE-08-0220 [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Real JM, Broch M, Vendrell J et al. Interleukin-6 gene polymorphism and lipid abnormalities in healthy subjects . J Clin Endocrinol Metab 2000;85:1334–9. 10.1210/jcem.85.3.6555 [DOI] [PubMed] [Google Scholar]
- 25.Halverstadt A, Phares DA, Roth S et al. Interleukin-6 genotype is associated with high-density lipoprotein cholesterol responses to exercise training . Biochim Biophys Acta 2005;1734:143–51. 10.1016/j.bbalip.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Curti ML, Pires MM, Barros CR et al. Associations of the TNF-alpha -308 G/A, IL6 -174 G/C and AdipoQ 45 T/G polymorphisms with inflammatory and metabolic responses to lifestyle intervention in Brazilians at high cardiometabolic risk . Diabetol Metab Syndr 2012;4:49 10.1186/1758-5996-4-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphries SE, Luong LA, Ogg MS et al. The interleukin-6 -174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men . Eur Heart J 2001;22:2243–52. 10.1053/euhj.2001.2678 [DOI] [PubMed] [Google Scholar]
- 28.Basso F, Lowe GD, Rumley A et al. Interleukin-6 -174G>C polymorphism and risk of coronary heart disease in West of Scotland coronary prevention study (WOSCOPS) . Arterioscler Thromb Vasc Biol 2002;22:599–604. 10.1161/01.ATV.0000013283.84306.1A [DOI] [PubMed] [Google Scholar]
- 29.Koivurova OP, Karhukorpi JM, Joensuu ET et al. IL-1 RN 2/2 genotype and simultaneous carriage of genotypes IL-1 RN 2/2 and IL-1beta-511 T/T associated with oesophagitis in Helicobacter pylori-negative patients . Scand J Gastroenterol 2003;38:1217–22. 10.1080/00365520310006504 [DOI] [PubMed] [Google Scholar]
- 30.Pohjanen VM, Koivurova OP, Makinen JM et al. Interleukin 6 gene polymorphism -174 is associated with the diffuse type gastric carcinoma . Genes Chromosomes Cancer 2013;52:976–82. 10.1002/gcc.22093 [DOI] [PubMed] [Google Scholar]
- 31.Pohjanen VM, Koivurova OP, Huhta H et al. Toll-like receptor 4 wild type Homozygozity of polymorphisms+896 and+1196 is associated with high gastrin serum levels and peptic ulcer risk . PLoS ONE 2015;10:e0131553 10.1371/journal.pone.0131553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyslipidemia (online). Current Care Guidelines. http://www.kaypahoito.fi (accessed 07 Sep 2015).
- 33.Misiewicz JJ. The Sydney System: a new classification of gastritis. Introduction. J Gastroenterol Hepatol 1991;6:207–8. 10.1111/j.1440-1746.1991.tb01467.x [DOI] [PubMed] [Google Scholar]
- 34.Karhukorpi J, Yan Y, Kolho KL et al. cagA, vacA and iceA virulence genes of Helicobacter pylori isolates of children in Finland . Eur J Clin Microbiol Infect Dis 2000;19:790–3. 10.1007/s100960000366 [DOI] [PubMed] [Google Scholar]
- 35.Ruokoniemi P, Helin-Salmivaara A, Klaukka T et al. Shift of statin use towards the elderly in 1995–2005: a nation-wide register study in Finland . Br J Clin Pharmacol 2008;66:405–10. 10.1111/j.1365-2125.2008.03258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furukawa K, Takahashi T, Arai F et al. Enhanced mucosal expression of interleukin-6 mRNA but not of interleukin-8 mRNA at the margin of gastric ulcer in Helicobacter pylori-positive gastritis . J Gastroenterol 1998;33:625–33. 10.1007/s005350050148 [DOI] [PubMed] [Google Scholar]
- 37.Zuliani G, Volpato S, Blè A et al. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: the InChianti study . Atherosclerosis 2007;192:384–90. 10.1016/j.atherosclerosis.2006.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg AS, Nordan RP, McIntosh J et al. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia . Cancer Res 1992;52:4113–16. [PubMed] [Google Scholar]
- 39.Tsutsumi K. Lipoprotein lipase and atherosclerosis . Curr Vasc Pharmacol 2003;1:11–17. 10.2174/1570161033386673 [DOI] [PubMed] [Google Scholar]
- 40.de Luis DA, Garcia Avello A, Lasuncion MA et al. Improvement in lipid and haemostasis patterns after Helicobacter pylori infection eradication in type 1 diabetic patients . Clin Nutr 1999;18:227–31. 10.1054/clnu.1999.0028 [DOI] [PubMed] [Google Scholar]
- 41.Pellicano R, Oliaro E, Fagoonee S et al. Clinical and biochemical parameters related to cardiovascular disease after Helicobacter pylori eradication . Int Angiol 2009;28:469–73. [PubMed] [Google Scholar]
- 42.Martínez I, Lattimer JM, Hubach KL et al. Gut microbiome composition is linked to whole grain-induced immunological improvements . ISME J 2013;7:269–80. 10.1038/ismej.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]