Abstract
Lipoprotein lipase (LPL), a member of the triglyceride lipase gene family, is synthesised by parenchymal cells of the heart, skeletal muscle and adipose tissues before being transported to luminal surfaces of vascular endothelial cells to exert its main physiological function to hydrolyse plasma lipoproteins. LPL deficiency is a rare autosomal recessive disorder, resulting in severe hypertriglyceridaemia from birth. The effect of marked hypertriglyceridaemia on the immune function in children has not been described. We present a case of a neonate with LPL deficiency and grossly elevated plasma triglyceride levels, presenting with recurrent and recalcitrant perianal abscesses suggestive of underlying immunodeficiency. With reduced levels of plasma triglycerides, the recurrent perianal infections resolved. This case report reviews evidence for potential deleterious effects of hypertriglyceridaemia on immune function, however, underlying mechanisms are poorly understood. Whether hypertriglyceridaemia contributes to immune dysfunction in this context is unknown. If there is a pathophysiological link, this may have implications for hypertriglyceridaemia management.
Background
Lipoprotein lipase (LPL) deficiency, resulting from mutations in the gene encoding LPL, which is located on chromosome 8p22,1 results in marked hypertriglyceridaemia in infancy, with triglyceride levels up to 350 times the upper limit of normal.2 While the association between hypertriglyceridaemia and acute pancreatitis is well established,3 the potential effects of markedly elevated triglyceride levels on immune function in children have not been described. We report a case of a neonate diagnosed with LPL deficiency after presenting with recurrent and recalcitrant perianal abscesses, which resolved on correction of marked hypertriglyceridaemia. We hypothesise that hypertriglyceridaemia may contribute to immune dysfunction in individuals with severe hypertriglyceridaemia due to inherited disorders of lipid metabolism. Whether this association may have implications for those with milder hypertriglyceridaemia secondary to obesity and the metabolic syndrome has not been investigated.
Case presentation
A 4-week-old male neonate presented to our institution with frequent loose stools. He was the first child of healthy, non-consanguineous parents of Indian descent. He was born by elective caesarean section at 41 weeks gestation for breech presentation following an uneventful pregnancy. The birth and early neonatal course were unremarkable, and he was exclusively breastfed. While he remained systemically well and continued to breastfeed, the loose stools had resulted in perianal excoriation, for which his mother applied a barrier cream.
On clinical examination, he had a low-grade fever (38.0°C) and a tender, erythematous, fluctuant swelling to the right perineum with surrounding cellulitis. The abscess was incised and drained that day, with intraoperative findings including an internal opening within the anal canal with resultant fistula formation. A swab taken perioperatively cultured Escherichia coli and a Klebsiella spp. The immediate postoperative course was uneventful, and he received broad spectrum intravenous antibiotics (flucloxacillin, gentamicin and metronidazole) for a total of 3 days, followed by oral amoxycillin-clavulanic acid.
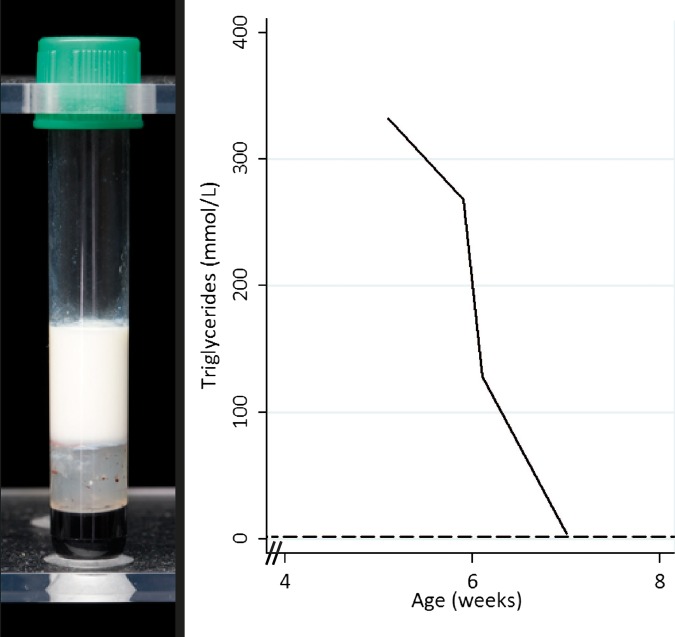
During the admission, blood samples were taken to assess inflammatory markers and perform an immunodeficiency screen. Gross lipaemia was identified on serial samples (figure 1A and table 1), with total plasma cholesterol of 40.2 mmol/L (reference interval 1.2 to 4.5 mmol/L) and triglycerides 332 mmol/L (reference limit <1.7 mmol/L), making interpretation of biochemistry difficult.
Figure 1.
Gross lipaemia in a blood sample collected prior to diagnosis (left), and elevated triglyceride levels at presentation with a rapid fall on appropriate dietary fat restriction (right).
Table 1.
Plasma lipid biochemical profile over time
| Normal ranges (mmol/L) | Chronological age (days) |
||||
|---|---|---|---|---|---|
| 36 days | 41 days | 43 days | 49 days | ||
| Cholesterol | 1.2–4.5 | 40.2 | 39.7 | 28.3 | 7.8 |
| Triglyceride | <1.7 | 332 | 268 | 128.6 | 4.2 |
| High-density lipoprotein (HDL) cholesterol | 0.9–2.0 | – | – | – | 0.7 |
| Low-density lipoprotein (LDL) cholesterol | Not available | – | – | – | 5.2 |
At this stage, breast-feeding was ceased and a fat-free formula started to provide daily energy requirements through the provision of carbohydrates and protein, resulting in a gradual fall in plasma lipid levels over the next month (figure 1B and table 1). While plasma lipid levels remained elevated, although decreasing, the baby continued to suffer from recurrent perianal abscesses, positive for Enterobacter spp, requiring surgical drainage and repeated courses of broad spectrum intravenous antibiotics.
Investigations
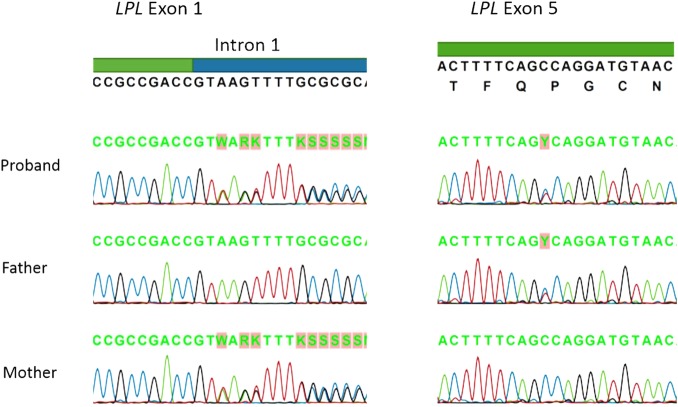
The diagnosis of LPL deficiency was suspected based on the presence of chylomicronaemia and confirmed by genetic testing, which showed compound heterozygous mutations in the LPL gene. Specifically, the baby was found to have a c.88+2dupT mutation in intron 1, which disrupts the intron 1 splice donor site,4 and a c.721C>T mutation in exon 5, which results in p.Pro214Ser missense mutation in a conserved region of the protein,4 a region thought to affect protein folding and stability.5 Parental testing revealed normal fasting plasma lipid profile in the father, in the presence of the exon 5 mutation, with mildly elevated cholesterol and triglyceride levels in the mother, in the presence of the intron 1 mutation (table 2). The patient and his mother had APOE genotype 3/2, whereas the father was 3/3. Sequence analysis for the proband and both parents is shown in figure 2.
Table 2.
Parental fasting lipid profiles (plasma)
| Normal ranges (mmol/L) | Father | Mother | |
|---|---|---|---|
| Cholesterol | <5.5 | 4.4 | 5.8 |
| Triglyceride | <1.7 | 1.5 | 2.9 |
| High-density lipoprotein (HDL) cholesterol | >1.0 | 0.9 | 1.1 |
| Low-density lipoprotein (LDL) cholesterol | <3.0 | 2.8 | 3.4 |
Figure 2.
LPL genetic sequence analysis for the proband and both parents. LPL, lipoprotein lipase.
Immune function studies were attempted during the period of hypertriglyceridaemia, however, the results were difficult to interpret in the presence of gross lipaemia. The presence of hypertriglyceridaemia required an additional step to wash the cells during lymphocyte subset characterisation, with results that were not reproducible. Similarly, the results from the neutrophil burst test were not reliable. It was not clear whether the lipaemia resulted in technical interference with the neutrophil burst assay, or whether elevated triglyceride levels had a direct effect on neutrophil function.
Differential diagnosis
Hypertriglyceridaemia presenting in the neonatal period is most commonly due to mutations in the LPL gene, which results in elevated levels of chylomicrons, while a number of other genes may be involved in genetic hypertriglyceridaemia presenting in adulthood, including APOE.6 While hypertriglyceridaemia presenting in the neonatal period is most commonly primary (genetic), the differential diagnosis of hypertriglyceridaemia in children and adolescents includes secondary causes such as hypothyroidism, obesity and the metabolic syndrome, cholestasis, nephrotic syndrome, glycogen storage disease type 1, poorly controlled diabetes and medications such as oral retinoids, the contraceptive pill and atypical antipsychotics.6 7
Outcome and follow-up
Following diagnosis of LPL deficiency, our patient has remained on a prescribed low-fat diet, with Monogen for the first year of life, followed by low-fat skimmed cows’ milk. His diet is carefully managed to provide a fat intake of 8–15% of total intake, in the form of Monogen, walnut oil and medium chain triglycerides oil. Following this diet, his plasma triglyceride levels have been stable, in the range of 10–20 mmol/L. He has had no further episodes of perianal abscesses nor of other deep-seated bacterial infections, and no episodes of pancreatitis. In the absence of ongoing clinical signs of immunodeficiency, immune function studies have not been repeated.
Discussion
This case describes an unusual clinical course for a neonate diagnosed with LPL deficiency. The child developed recurrent and recalcitrant perianal abscesses that were slow to respond to appropriate surgical and antimicrobial therapy. These abscesses occurred during a period of gross hypertriglyceridaemia, and ceased soon after triglyceride levels reached more modestly elevated levels. Coupled with the observation of an unsatisfactory neutrophil burst test in the presence of gross lipaemia, we hypothesise that the child's hypertriglyceridaemia may have contributed to immune dysfunction, possibly mediated through altered neutrophil function, particularly as neonatal perianal abscess has been reported as a presenting feature for autoimmune neutropaenia.8 However, it is also possible that hypertriglyceridaemia and subsequently LPL deficiency were detected in this case as incidental findings, following routine blood sampling in the context of unrelated neonatal perianal abscesses.
Most individuals with LPL deficiency present in the first decade of life, with abdominal pain, cutaneous xanthomas or hepatosplenomegaly.9 Only 25% of individuals with LPL deficiency present in the first year of life, with neonatal presentation a rare event.9 Clinical presentation with severe or recurrent infections has not been previously described.
Potential effects of hyperlipidaemia on immune function and susceptibility to infection have been previously described. Functional activity of monocytes,10 lymphocytes,11 antigen presenting cells12 and neutrophils13 14 are all impaired in the presence of elevated lipid levels. Further, mice with elevated lipid levels, including ApoE knockout mice, have impaired defences against several diverse pathogens, including Klebsiella pneumoniae, Listeria monocytogenes,15 Candida,16 Mycobacterium tuberculosis17 and hepatotoxic lymphocytic choriomeningitis virus,18 but not against Salmonella spp.19 The wide variety of pathogens affected suggests that lipaemia may result in immune dysfunction via several pathways, with evidence to support decreased phagocytic capacity of granulocytes,20 defective innate immunity21 and dysregulation of inflammatory cytokines22 in the presence of dyslipidaemia. This may have implications for treatment of infections, as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have been suggested to confer a survival benefit in influenza, even in the absence of dyslipidaemia.23 Further research is required to further characterise these pathways and determine whether the effects are likely to be seen in individuals with milder elevations of plasma lipids, such as those associated with obesity.
If immune dysfunction is associated with mild elevations of serum lipid levels, this may have implications for the management of hyperlipidaemia in children as well as in adults. It is unclear whether treatment of patients with elevated triglycerides, including those with familial LPL deficiency, has beneficial effects on immune function. In particular, it would be of interest to know whether alipogene tiparvovec (Glybera, AMT-011, AAVI-LPLS477X), a Ser447X variant of the human LPL gene in an adeno-associated virus vector, developed as an intramuscular gene therapy for the treatment of LPL deficiency,24 alters immune function in children with elevated lipid levels, including those with familial LPL deficiency. Further research in this area may assist in identifying children and adults at risk of immune dysfunction secondary to dyslipidaemia, and identify novel or existing therapeutic targets to modify this risk.
Learning points.
This is the first case to hypothesise that lipoprotein lipase deficiency may masquerade as immunodeficiency in the neonatal period, with treatment of hypertriglyceridaemia coinciding with the resolution of recurrent perianal abscesses.
Further research is required to establish the relationship between hypertriglyceridaemia and immune function.
Acknowledgments
The authors would like to acknowledge Dr Amanda J Hooper for her assistance with the genetic analyses.
Footnotes
Contributors: LSA, DKM and ACM were involved in the conception of the case report; JB was involved in the laboratory analysis of samples; LSA was involved in the drafting of the manuscript; JRB, DKM and ACM were involved in the critical revision of the manuscript for important intellectual content; all the authors were involved in the final approval of the version to be published and were also involved in the agreement to be accountable for all aspects of the work.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Li Y, He PP, Zhang DW et al. Lipoprotein lipase: from gene to atherosclerosis. Atherosclerosis 2014;237:597–608. 10.1016/j.atherosclerosis.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 2.Feoli-Fonseca JC, Levy E, Godard M et al. Familial lipoprotein lipase deficiency in infancy: clinical, biochemical, and molecular study. J Pediatr 1998;133:417–23. 10.1016/S0022-3476(98)70280-X [DOI] [PubMed] [Google Scholar]
- 3.Neal WA. 80.3 Disorders of lipoprotein metabolism and transport. In: Kliegman RM, Stanton BF, St Geme JW et al. eds. Nelson textbook of pediatrics. 19th edn Philadelphia, PA, USA: Elsevier Saunders, 2011:470–82. [Google Scholar]
- 4.Gupta N, Moore D, Hooper AJ et al. Pancreatitis in a child with lipemia due to novel lipoprotein lipase mutations. J Pediatr Gastroenterol Nutr 2010;50:457–9. 10.1097/MPG.0b013e3181b64407 [DOI] [PubMed] [Google Scholar]
- 5.Hata A, Ridinger DN, Sutherland SD et al. Missense mutations in exon 5 of the human lipoprotein lipase gene. Inactivation correlates with loss of dimerization. J Biol Chem 1992;267:20132–9. [PubMed] [Google Scholar]
- 6.Shah AS, Wilson DP. Primary hypertriglyceridemia in children and adolescents. J Clin Lipidol 2015;9(5 Suppl):S20–8. 10.1016/j.jacl.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Bordugo A, Carlin E, Demarini S et al. A neonate with a ‘milky’ blood. What can it be? Arch Dis Child Fetal Neonatal Ed 2014;99:F514 10.1136/archdischild-2014-305940 [DOI] [PubMed] [Google Scholar]
- 8.Lejkowski M, Maheshwari A, Calhoun DA et al. Persistent perianal abscess in early infancy as a presentation of autoimmune neutropenia. J Perinatol 2003;23:428–30. 10.1038/sj.jp.7210952 [DOI] [PubMed] [Google Scholar]
- 9.Brunzell JD. Familial lipoprotein lipase deficiency. In: Pagon RA, Adam MP, Ardinger HH, eds. Gene reviews [Internet]. Seattle, WA, USA: University of Washington, 2014. [PubMed] [Google Scholar]
- 10.Stragliotto E, Camera M, Postiglione A et al. Functionally abnormal monocytes in hypercholesterolemia. Arterioscler Thromb 1993;13:944–50. 10.1161/01.ATV.13.6.944 [DOI] [PubMed] [Google Scholar]
- 11.Lee HS, Park HJ, Nam JH et al. Immune and nutrition status in elderly Koreans with hyperLDL-cholesterolemia. J Nutr Sci Vitaminol (Tokyo) 2006;52:407–13. 10.3177/jnsv.52.407 [DOI] [PubMed] [Google Scholar]
- 12.Sen E, Chattopadhyay S, Bandopadhyay S et al. Macrophage heterogeneity, antigen presentation, and membrane fluidity: implications in visceral Leishmaniasis. Scand J Immunol 2001;53:111–20. 10.1046/j.1365-3083.2001.00856.x [DOI] [PubMed] [Google Scholar]
- 13.Antonaci S, Jirillo E, Garofalo AR et al. Cell-mediated immune response in patients with type IIa, type IIb and type IV primary hyperlipoproteinaemia. Cytobios 1988;54:181–9. [PubMed] [Google Scholar]
- 14.Efe H, Deger O, Kirci D et al. Decreased neutrophil antioxidative enzyme activities and increased lipid peroxidation in hyperlipoproteinemic human subjects. Clin Chem Acta 1999;279:155–65. 10.1016/S0009-8981(98)00178-8 [DOI] [PubMed] [Google Scholar]
- 15.Roselaar SE, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res 1998;39: 1740–3. [PubMed] [Google Scholar]
- 16.Vonk AG, De Bont N, Netea MG et al. Apolipoprotein-E-deficient mice exhibit an increased susceptibility to disseminated candidiasis. Med Mycol 2004;42: 341–8. 10.1080/13693780410001657135 [DOI] [PubMed] [Google Scholar]
- 17.Martens GW, Arikan MC, Lee J et al. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun 2008;76:3464–72. 10.1128/IAI.00037-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludewig B, Jaggi M, Dumrese T et al. Hypercholesterolemia exacerbates virus-induced immunopathologic liver disease via suppression of antiviral cytotoxic T cell responses. J Immunol 2001;166:3369–76. 10.4049/jimmunol.166.5.3369 [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, Joosten LA, Keuter M et al. Circulating lipoproteins are a crucial component of host defense against invasive Salmonella typhimurium infection. PLoS ONE 2009;4:e4237 10.1371/journal.pone.0004237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bont N, Netea MG, Demacker PN et al. Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae. Eur J Clin Invest 2000;30:818–22. 10.1046/j.1365-2362.2000.00715.x [DOI] [PubMed] [Google Scholar]
- 21.Lei L, Li H, Yan F et al. Hyperlipidemia impaired innate immune response to periodontal pathogen porphyromonas gingivalis in apolipoprotein E knockout mice. PLoS ONE 2013;8:e71849 10.1371/journal.pone.0071849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reboldi A, Dang EV, McDonald JG et al. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 2014;345:679–84. 10.1126/science.1254790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon A. Cholesterol metabolism and immunity. N Engl J Med 2014;371: 1933–5. 10.1056/NEJMcibr1412016 [DOI] [PubMed] [Google Scholar]
- 24.Burnett JR, Hooper AJ. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr Opin Mol Ther 2009; 11:681–91. [PubMed] [Google Scholar]