Abstract
Clostridium perfringens bacteraemia is a potentially fatal condition, and its early identification is paramount to maximise chances of survival. Prompt recognition of intravascular haemolysis, a known complication of C. perfringens bacteraemia, can help guide clinical decision-making before microbiology data becomes available. We present a novel finding of severe hypertension in a fatal case of Clostridial bacteraemia with massive haemolysis. A 58-year-old man with no known medical history presented to the emergency department with malaise, fever and hypertension. He developed abdominal pain and a hepatic abscess was identified on CT imaging. Within 4 h of presentation, he developed massive intravascular haemolysis, extreme hypertension, pulmonary oedema and respiratory failure. He died less than 8 h after presentation. His blood cultures subsequently grew C. perfringens. This case underscores the importance of early recognition of intravascular haemolysis complicating C. perfringens bacteraemia, and discusses the rare complication of hypertensive emergency in this setting.
Background
Infection due to Clostridium perfringens can have a range of clinical presentations, from asymptomatic disease to septic shock.1 Intravascular haemolysis, due to the organism's α-toxin, complicates approximately 7–15% of cases of Clostridial bacteraemia.2–4 These patients can experience a precipitous clinical decline with very poor survivability.1 5–7 A recent case review of 40 patients with bacteraemia complicated by intravascular haemolysis reported a mortality rate of 80%, with a median survival of 8 h from presentation.8 Of the eight patients who survived in this series, six underwent surgical or percutaneous interventions, suggesting that early identification and removal of the infectious source is critical to patient survival.
We present a case of massive intravascular haemolysis from fulminant Clostridial sepsis resulting from an underlying hepatic abscess. However, unlike previously reported cases of Clostridial sepsis in which patients presented as normotensive or hypotensive, this patient exhibited severe hypertension that persisted until the moment of cardiac arrest. We propose potential pathophysiological mechanisms to explain this rare presentation and give clinical recommendations to facilitate the early identification of infection and prevention of disease progression.
Case presentation
A 58-year-old Ethiopian man with no significant medical history presented to a university-affiliated county hospital emergency department (ED), with myalgias and fever for 2 days. He had not travelled recently and had lived in the USA for many years; he had no known recent infectious exposures. He had not been seen by a medical provider in over a decade. He presented to triage ambulatory and alert. His temperature was 38.9°C, heart rate 114 bpm and blood pressure 205/118 mm Hg. He was mildly tachypnoeic and his peripheral oxygen saturation (SpO2) was 99% on ambient air. General physical examination revealed mild abdominal tenderness on palpation of the bilateral upper quadrants. Initial venous lactate was 4.9 mmol/L (normal range 1.0 –1.8 mmol/L), white cell count (WCC) 14.2×109/L (normal range 4.5–10.0×109/L), haemoglobin (Hgb) 124 g/L (normal range 135–175 g/L), and haematocrit (Hct) 37% (normal range 38–50%). Basic metabolic and hepatic function panel results were delayed due to sample haemolysis despite repeat testing. International normalised ratio (INR) and partial thromboplastin time were normal. Urinalysis showed red urine; 3+blood was reported by chemical testing but no red blood cells (RBCs) were seen on microscopy. A chest radiograph showed mild streaky bibasilar opacities consistent with atelectasis. Antipyretics and 2 L intravenous normal saline were administered.
Two hours after his initial presentation, the patient reported worsening abdominal pain, and CT scan of his abdomen and pelvis was performed. This revealed a 5 cm gas-containing liver mass concerning for abscess, with pneumobilia and rupture of the gas into the subcapsular space. Vancomycin and piperacillin-tazobactam were administered intravenously. At that time, the patient's systolic blood pressure (SBP) had increased to 216 mm Hg and a nitroprusside infusion was begun. General surgery and interventional radiology teams were consulted for management of the hepatic abscess.
Three and 1½ hours after presentation, the patient developed acute respiratory distress and hypoxaemia with SpO2 72% on 9 L supplemental oxygen. A repeat chest radiograph showed interval development of extensive bilateral pulmonary infiltrates consistent with pulmonary oedema. Arterial blood gas performed with the patient breathing 15 L supplemental oxygen showed pH 7.06 (normal range 7.35–7.45), PaCO2 39 mm Hg (normal range 35–45 mm Hg), PaO2 49 mm Hg (normal range 80–100 mm Hg), HCO3 11 mmol/L (normal range 22–26 mmol/L) and base excess −18 mmol/L (normal base excess −2 to +2 mmol/L). An endotracheal tube was placed utilising delayed-sequence intubation, and mechanical ventilation was initiated.9 Frothy pink sputum was evident in the endotracheal tube. On repeat laboratory evaluation, arterial lactate was 9.7 mmol/L, WCC was 31×109/L, Hgb could not be calculated due to haemolysis and Hct was 10%. Repeat INR was 3.1 (normal range 0.8–1.3). Three units of packed RBCs and 1 unit of fresh frozen plasma were transfused. A metabolic panel revealed a potassium of 7.6 mmol/L (normal range 3.7–5.2 mmol/L). An ECG showed peaked T-waves and widened QRS complexes, and management of hyperkalaemia was initiated. Intravenous clindamycin was added to the antibiotic regimen for suspected Clostridial infection. The SBP remained over 220 mm Hg throughout these events.
Investigations
Key investigations in this case were the patient's laboratory studies, urinalysis and CT scan. When the patient initially presented with malaise, myalgias and fever in the absence of localising complaints, a basic infectious evaluation was initiated. This included a CBC, urinalysis, blood cultures and chest radiograph. This evaluation did not initially reveal a specific source.
From the time of the first laboratory assays, however, there was evidence of intravascular haemolysis. This could be visualised with the naked eye, as seen in figure 1, and also caused multiple laboratory studies to be uninterpretable (this was especially problematic with the basic chemistry and hepatic function panels). The urine was red and the urine dipstick test for blood (which measures peroxidase-like activity of haemoglobin) was markedly positive without RBCs evident on microscopy. Together, these findings suggested massive haemolysis and frank haemoglobinuria.
Figure 1.
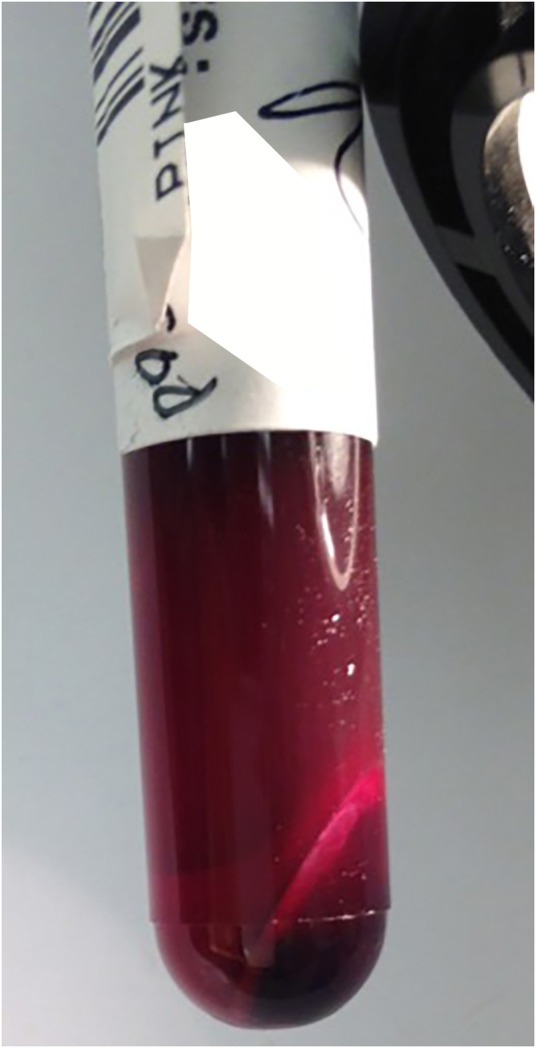
Haemolysed blood with an extremely low packed cell volume.
Two hours after presentation, the patient reported worsening abdominal pain and upper quadrant tenderness to palpation. Given concern for infection, this prompted the treating team to obtain an abdominal CT scan. This study showed a large gas-forming hepatic abscess (figure 2), the likely cause of the patient's illness.
Figure 2.
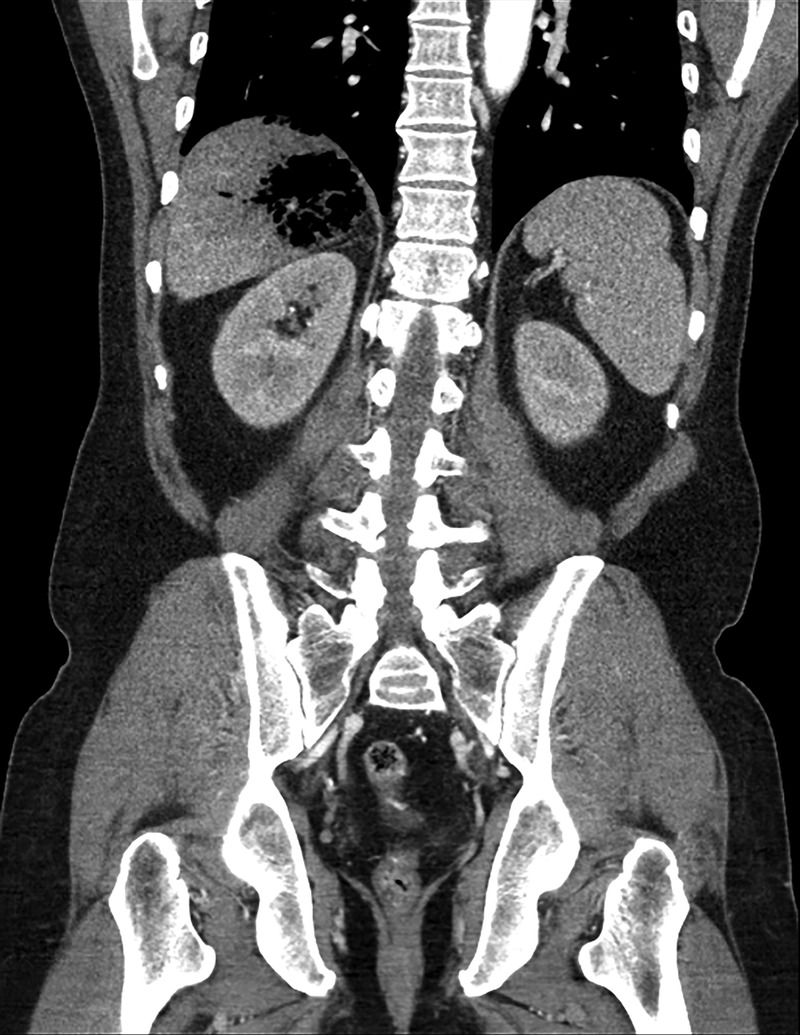
Subcapsular gas-containing liver abscess, with rupture of gas into the subcapsular space.
Differential diagnosis
The aetiology of liver abscesses is extremely variable and is dependent on clinical context; many pyogenic hepatic abscesses are polymicrobial.10 11 Comorbid immunosuppression, diabetes mellitus or malignancy, as well as a history of recent gastrointestinal procedures, are key determinants of the causative organism(s) for a specific patient. The differential diagnosis for the cause of liver abscess in this patient would include Klebsiella pneumoniae, Escherichia coli, Streptococcal spp including the Streptococcus milleri group, Enterococcus, Bacterioides or Clostridium spp.10 11 Less common potential causes of hepatic abscess in an Ethiopian immigrant include Mycobacterium tuberculosis, Burkholderia pseudomallei (though primarily found in Southeast Asia and Australia) and Entamoeba histolytica amoebiasis, but the fact that this patient had no travel to endemic areas within the past several years makes the latter two much less likely.
The differential diagnosis of haemolysis in the setting of infection includes haemolysis due to the infectious organism itself (such as in parasitic infections), RBC damage due to bacterial products, disseminated intravascular coagulopathy (DIC), immune-mediated haemolysis and haemolysis due to oxidative stress in patients with underlying RBC disorders such as sickle cell disease or G6PD deficiency.12 Given that the patient had not recently travelled to endemic regions, parasitic infection was very unlikely in this case. While both C. perfringens and Haemophilus influenzae type B can cause haemolysis through toxins released by the bacterium (α-toxin in the case of C. perfringens), the haemolysis associated with C. perfringens is usually much more severe than that seen with Haemophilus influenzae. The extreme degree of haemolysis in this case (visualised on blood draw and on examination of the urine, as well as with the precipitous drop in Hgb) was out of proportion to that which is expected with even severe DIC—one clue that this could be a Clostridial infection. Although the patient did not have known G6PD deficiency or sickle cell disease, these were in the differential diagnosis because he had not seen a medical provider in many years.
Another major feature of this case was the severe hypertension that persisted up to the time of cardiac arrest. The differential diagnosis for hypertensive emergency includes acute-on-chronic hypertension (either essential or secondary), drug toxicity, acute withdrawal of antihypertensive medications such as clonidine, or brain injury such as trauma or stroke. As this patient had not been evaluated by a physician in many years, it is possible that he was very hypertensive at baseline without his knowledge. However, the vast majority of patients who develop multiorgan dysfunction syndrome and death in the setting of acute infection are hypotensive due to septic shock. The fact that this patient's blood pressure remained elevated until the time of his death is very unusual, and in fact has not been reported in any similar cases in the literature. This patient's hypertensive emergency in the setting of severe sepsis may have been due to the nitric oxide (NO)-scavenging properties of cell-free haemoglobin, and therefore served as a marker of the tremendous degree of haemolysis.
In this patient with a gas-containing liver abscess, severe sepsis and acute massive haemolysis with associated hypertensive emergency, a key element of the overall differential diagnosis of the case was the severity and rapidity of the patient's intravascular haemolysis. While the presence of haemolysis itself does not help to narrow the differential diagnosis of pyogenic liver abscess, C. perfringens is unique in the extreme nature of its associated haemolysis. Additionally, this is a known enteric pathogen and is a recognised cause of gas-containing hepatic abscesses.
Treatment
Initial treatment in this case focused on management of severe sepsis. Beyond performing a thorough evaluation for the source of infection, key elements of severe sepsis care include early administration of empiric antibiotics, consideration of mechanical source control as appropriate, fluid resuscitation and haemodynamic management, and other organ support. In this case, piperacillin-tazobactam was chosen initially for broad spectrum coverage of intra-abdominal infection, with clindamycin added subsequently for anaerobic coverage and toxin control due to the emerging suspicion for C. perfringens bacteraemia. Appropriate treatment of C. perfringens includes antibiotic coverage with penicillin G plus clindamycin.13 Various experts have suggested that other β-lactams such as piperacillin-tazobactam, ticarcillin-clavulanate or carbapenems are acceptable alternatives to penicillin G, given the high sensitivity of the organism to this class of antibiotics.14 In every case, however, the addition of clindamycin to a β-lactam is essential for inhibition of toxin production and is a key component of antibiotic therapy.13 14 One reason piperacillin-tazobactam was used in this case, rather than penicillin G, was to treat other potential aetiologies of hepatic abscess until the culture data became available. The choice of this regimen is consistent with recommendations by the Infectious Disease Society of America (IDSA).15 In addition to early initiation of antibiotic therapy, General Surgery and Interventional Radiology were consulted regarding mechanical source control. However, the patient was deemed too clinically unstable to tolerate interventions at the time.
In addition to therapies focused on the primary infection, this patient required treatment of his massive haemolysis as well as management of hypertensive emergency. For ongoing haemolysis, in addition to the transfusion of packed RBCs to correct anaemia, it is critical to monitor for and treat complications of associated hyperkalaemia. This patient did have severe hyperkalaemia, and was treated with calcium gluconate, sodium bicarbonate, albuterol, insulin and dextrose. He exhibited evidence of hypertensive emergency, with end-organ dysfunction, and thus blood pressure control was attempted with nitroprusside infusion.
Outcome and follow-up
Six hours after presentation, the patient was transported from the ED to the intensive care unit (ICU), in critical condition. Transfusion of packed RBCs was continued and the Hct improved to 26%. He remained critically hypertensive, with severe metabolic acidosis and acute hypoxaemic respiratory failure. Shortly after transfer, he developed cardiac arrest due to pulseless electrical activity (PEA). The ICU team initiated advanced cardiac life support and continued management of hyperkalaemia, severe acidaemia and anaemia. Cardiopulmonary resuscitation efforts were eventually terminated for refractory PEA and the patient's death was pronounced 7.5 h after his initial presentation to the ED. Four hours after he died, the microbiology laboratory reported that C. perfringens was identified in all four blood culture bottles collected at presentation. A postmortem examination was declined by the patient's family.
Discussion
Hepatic abscess due to C. perfringens with associated bacteraemia and massive haemolysis is a rare and life-threatening condition that requires early identification, immediate administration of appropriate antibiotics and surgical or percutaneous source control. The bacteria's rapid doubling time of 7 min can lead to mortality rates as high as 90% within hours of presentation.1 5 16 In this case, our patient's death within 8 hours of arrival at the hospital is consistent with the rapid time to death in other cases of C. perfringens bacteraemia reported in the literature.8 As culture results are typically not available in this early time frame, it is important for clinicians to recognise clinical features that suggest Clostridial infection so that appropriate management can be initiated prior to microbiological confirmation.
C. perfringens produces α-toxin with phospholipase C lecithinase activity, resulting in RBC phospholipid membrane destruction.3 8 16 In this case, repeated haemolysed labs and the presence of cell-free Hgb on urinalysis within the first hours of evaluation were indicators of massive intravascular haemolysis, and were therefore potential clues to the presence of an infection with Clostridium spp. These features were most readily apparent to Laboratory Medicine team members working with the specimens, and the timely communication of these findings to clinicians was critical in this case.
Additionally, source control in the case of C. perfringens hepatic abscess with associated bacteraemia should include mechanical debridement of the abscess. In a review of C. perfringens bacteraemia complicated by intravascular haemolysis, 75% of patients who survived (8 survivors out of 40 total cases) underwent surgical or percutaneous interventions—corroborating other reports of patients with C. perfringens bacteraemia that have suggested that early identification and control of the infectious source is critical to patient survival.1 8 17 18
Many case reports of Clostridial infection with associated intravascular haemolysis describe patients with underlying comorbidities such as malignancy, immunocompromise or diabetes mellitus.2 3 5 18–21 It is unknown if this patient had any of these conditions, as he had not been seen by a medical provider in many years. Additionally, there was insufficient evidence to evaluate for them during this patient's brief clinical course.
This case is unique in that this patient remained severely hypertensive throughout his course. All case studies we identified documenting critical C. perfringens infection describe patients who were normotensive or hypotensive on presentation.1 3 5 8 18–23 The majority of patients who had similar rapidly-progressive courses resulting in death due to overwhelming infection experienced distributive shock and hypotension. To our knowledge, this is the first documented case of C. perfringens infection with acute hypertensive emergency.
Studies of massive intravascular haemolysis in animal models and other clinical settings demonstrate that the release of cell-free Hgb reduces NO bioavailability, leading to dysfunction of NO-mediated regulation of vascular tone, and resulting in unopposed vasoconstriction.24–28 Additionally, Hgb is a potent colloid, causing an intravascular shift of extracellular fluid and worsening systemic hypertension. In this case, we suspect that, despite the patient's severe sepsis, he avoided hypotension through these mechanisms, until his increased afterload resulted in eventual cardiopulmonary failure.
Learning points.
Massive intravascular haemolysis is a known complication of Clostridium perfringens bacteraemia, and is characterised by precipitous clinical decline and a high case fatality rate. Early clinical findings include vital sign abnormalities consistent with the systemic inflammatory response syndrome and signs of gas-containing areas of infection. Classic comorbidities include malignancy, immunocompromised status and diabetes mellitus.
Laboratory findings of severe haemolysis include haemoglobinuria with absent red blood cells on urinalysis, rapidly falling haematocrit levels and repeatedly haemolysed blood samples.
The prompt recognition of clinical clues and early diagnosis is paramount to expedite rapid surgical and/or percutaneous intervention, which has been associated with improved survival in C. perfringens infection.
The differential diagnosis for pyogenic liver abscess, which is frequently polymicrobial, includes Clostridium spp in addition to Klebsiella, Streptococcus, Enterococcus, Escherichia coli, Bacterioides and other organisms.
Acknowledgments
The authors would like to thank Dr. Timothy Eoin West for contributing significant editorial assistance to this manuscript.
Footnotes
Contributors: AGL and KER drafted the manuscript, performed the literature review and designed the intellectual framework of this case. AGL and KER contributed equally to this work. MH and JRH drafted the manuscript, provided significant edits and offered critical review for this case.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Simon TG, Bradley J, Jones A et al. Massive intravascular hemolysis from Clostridium perfringens septicemia: a review. J Intensive Care Med 2014;29:327–33. 10.1177/0885066613498043 [DOI] [PubMed] [Google Scholar]
- 2.Rechner PM, Agger WA, Mruz K et al. Clinical features of Clostridial bacteremia: a review from a rural area. Clin Infect Dis 2001;33:349–53. 10.1086/321883 [DOI] [PubMed] [Google Scholar]
- 3.Kapoor JR, Monteiro B, Tanoue L et al. Massive intravascular hemolysis and a rapidly fatal outcome. Chest 2007;132:2016–19. 10.1378/chest.07-0853 [DOI] [PubMed] [Google Scholar]
- 4.Anas AA, Wiersinga WJ, de Vos AF et al. Recent insights into the pathogenesis of bacterial sepsis. Neth J Med 2010;68:147–52. [PubMed] [Google Scholar]
- 5.Kurasawa M, Nishikido T, Koike J et al. Gas-forming liver abscess associated with rapid hemolysis in a diabetic patient. World J Diabetes 2014;5:224–9. 10.4239/wjd.v5.i2.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zapata EP, Penide AS, Guillén AB et al. Hemólisis masiva intravascular secundaria a sepsis por Clostridium perfringens. Rev Esp Anestesiol Reanim 2010;57:314–16. 10.1016/S0034-9356(10)70234-6 [DOI] [PubMed] [Google Scholar]
- 7.Blanco M, Ferreirós C. Hémolyse massive par Clostridium perfringens: diagnostic rapide chez une patiente présentant un sepsis sévère. Ann Biol Clin (Paris) 2007;65:181–4. [PubMed] [Google Scholar]
- 8.Van Bunderen CC, Bomers MK, Wesdorp E et al. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med 2010;68:343–6. [PubMed] [Google Scholar]
- 9.Weingart SD, Trueger NS, Wong N et al. Delayed sequence intubation: a prospective observational study. Ann Emerg Med 2015;65:349–55. 10.1016/j.annemergmed.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Chen CH, Chiu KL et al. Clinical outcome and prognostic factors of patients with pyogenic liver abscess requiring intensive care. Crit Care Med 2008;36:1184–8. 10.1097/CCM.0b013e31816a0a06 [DOI] [PubMed] [Google Scholar]
- 11.Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol 2004;2:1032–8. 10.1016/S1542-3565(04)00459-8 [DOI] [PubMed] [Google Scholar]
- 12.Berkowitz FE. Hemolysis and infection: categories and mechanisms of their interrelationship. Rev Infect Dis 1991;13:1151–62. 10.1093/clinids/13.6.1151 [DOI] [PubMed] [Google Scholar]
- 13.Stevens DL, Bisno AL, Chambers HF et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis 2014;59:e10–52. 10.1093/cid/ciu444 [DOI] [PubMed] [Google Scholar]
- 14.Stevens DL, Aldape MJ, Bryant AE. Life-threatening Clostridial infections. Anaerobe 2012;18:254–9. 10.1016/j.anaerobe.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Solomkin JS, Mazuski JE, Bradley JS et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the surgical infection society and the infectious diseases society of America. Clin Infect Dis 2010;50:133–64. 10.1086/649554 [DOI] [PubMed] [Google Scholar]
- 16.Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev 1990;3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran G, Bothma P, Brodbeck A. Intravascular haemolysis and septicaemia due to Clostridium perfringens liver abscess. Anaesth Intensive Care 2010;38:942–5. [DOI] [PubMed] [Google Scholar]
- 18.Cochrane J, Bland L, Noble M. Intravascular hemolysis and septicemia due to Clostridium perfringens emphysematous cholecystitis and hepatic abscesses. Case Rep Med 2015;2015:523402 10.1155/2015/523402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sathiyamoorthy G, Kohlitz P, Maskey M et al. Bubbles in the liver; Clostridium perfringens liver abscess and septicemia. Chest 2012;142:274A 10.1378/chest.138821622871746 [DOI] [Google Scholar]
- 20.Law ST, Lee MK. A middle-aged lady with a pyogenic liver abscess caused by Clostridium perfringens. World J Hepatol 2012;4:252–5. 10.4254/wjh.v4.i8.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng H, Lam SM, Shum HP et al. Clostridium perfringens liver abscess with massive haemolysis. Hong Kong Med J 2010;16:310–12. [PubMed] [Google Scholar]
- 22.Juntermanns B, Radunz S, Heuer M et al. Fulminant septic shock due to Clostridium perfringens skin and soft tissue infection eight years after liver transplantation. Ann Transplant 2011;16:143–6. 10.12659/AOT.882009 [DOI] [PubMed] [Google Scholar]
- 23.Cécilia R, Baptiste V, Benjamin C et al. Acute hemolysis in the emergency department: think about Clostridium perfringens!. Case Rep Emerg Med 2013;2013:948071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo TW, Lampah DA, Tjitra E et al. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis 2009;200:1522–9. 10.1086/644641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandrim VC, Montenegro MF, Palei AC et al. Increased circulating cell-free hemoglobin levels reduce nitric oxide bioavailability in preeclampsia. Free Radic Biol Med 2010;49:493–500. 10.1016/j.freeradbiomed.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 26.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med 2004;36:707–17. 10.1016/j.freeradbiomed.2003.11.032 [DOI] [PubMed] [Google Scholar]
- 27.Olsson MG, Centlow M, Rutardóttir S et al. Increased levels of cell-free hemoglobin, oxidation markers, and the antioxidative heme scavenger alpha(1)-microglobulin in preeclampsia. Free Radic Biol Med 2010;48:284–91. 10.1016/j.freeradbiomed.2009.10.052 [DOI] [PubMed] [Google Scholar]
- 28.Hess JR, Macdonald VW, Brinkley WW. Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J Appl Physiol 1993;74:1769–78. [DOI] [PubMed] [Google Scholar]


