Abstract
Dermatomyositis and polymyositis are the major idiopathic inflammatory myopathies in adults. They are associated with an elevated risk of malignancy. However, renal tumours have rarely been described in dermatomyositis patients. We report the case of a 27-year-old Caucasian man with chromophobe renal cell cancer (ChRCC) and antinuclear matrix protein (NXP-2)-associated dermatomyositis. To the best of our knowledge, there are no previous reports of ChRCC presenting with dermatomyositis.
Background
Up to 30% of idiopathic inflammatory myopathies cases are associated with an underlying malignancy,1 but the majority of these are gynaecological (ovarian), pulmonary, gastrointestinal (pancreatic, stomach and colorectal) and non-Hodgkin's lymphoma in origin among patients with dermatomyositis (DM).2 Renal tumours have only rarely been described in patients with DM and, to the best of our knowledge, there are no previous reports of chromophobe renal cell cancer (ChRCC) presenting with dermatomyositis.3–7
Case presentation
A 27-year-old Caucasian man presented with a 7-month history of myalgia, muscle weakness and dysphagia. Initial work up 1 month after the onset of symptoms revealed a markedly elevated creatine phosphokinase (CPK) at 26 000 U/L, an irritable myopathy on electromyography (EMG) and a non-diagnostic muscle biopsy. He was given a presumptive diagnosis of inflammatory myopathy and was started on prednisone 60 mg/day. CT of the abdomen and pelvis performed for cancer screening revealed a 2 cm right renal mass. The patient was referred to urology and underwent a partial nephrectomy for a stage T1a, grade 1 ChRCC 6 weeks after presentation. Following nephrectomy, the patient became weaker with worsening myalgias. He was continued on steroids and received five courses of monthly intravenous immunoglobulin (IVIG). IVIG infusion provided little benefit and was replaced with methotrexate 25 mg/week, and the patient was referred to our hospital.
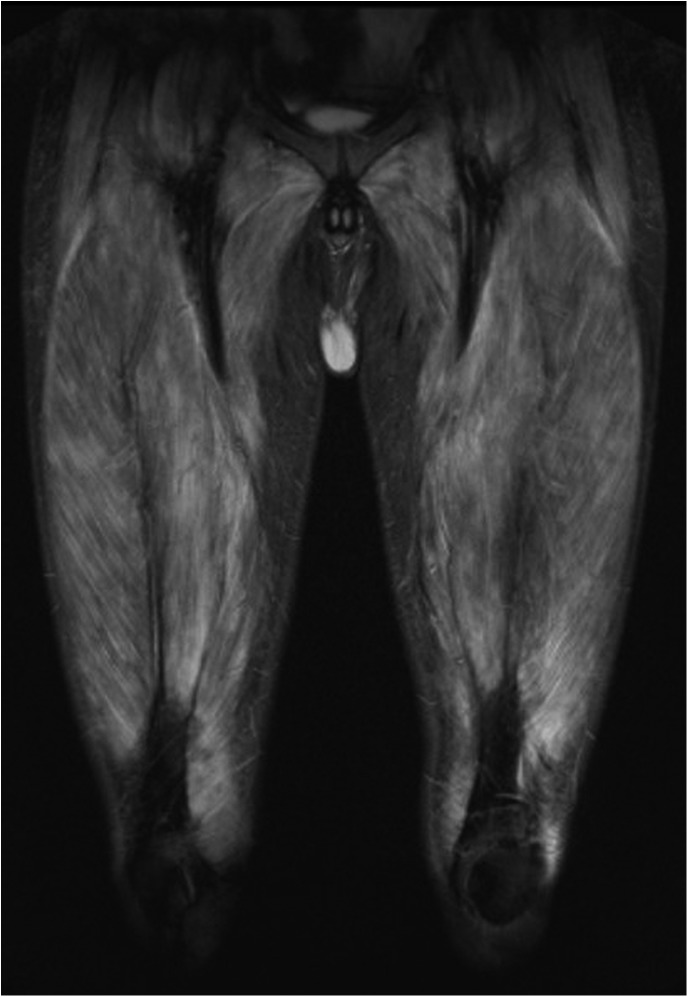
On admission he had severe laryngeal weakness. Examination demonstrated 2/5 deltoid and 3/5 hip flexor strength with preserved distal strength. Mild cuticular and periungual erythaema was noted with no facial rash, no Gottron's papules and no mechanic's hands. CPK was normal at 169 U/L, but aldolase was elevated at 16.0 U/L (normal <8.1 U/L). EMG revealed an irritable myopathy and MRI of the lower extremities showed extensive bilateral oedema and enhancement consistent with myositis (figure 1). Malignancy work up included MRI of the abdomen, CT of the chest/abdomen/pelvis, positron emission tomography CT (PET-CT) and urine cytology. There was no evidence of malignancy recurrence. Antinuclear antibody was positive at 1:320, but anti-Jo1, signal recognition particle, double-stranded DNA, Sm, ribonucleoprotein, antineutrophil cytoplasmic antibody and a paraneoplastic panel were negative. Immunoprecipitation initially showed no evidence of myositis-specific antibodies. Muscle biopsy of the right rectus femoris revealed myophagocytosis with perifascicular myofibre atrophy, degeneration and regeneration consistent with DM (figure 2). A final diagnosis of DM sine dermatitis was given based on weakness, elevated muscle enzymes, EMG, MRI and characteristic muscle biopsy without classic rash.8 9
Figure 1.
Coronal short τ inversion recovery MRI showing extensive oedema in thigh muscles.
Figure 2.
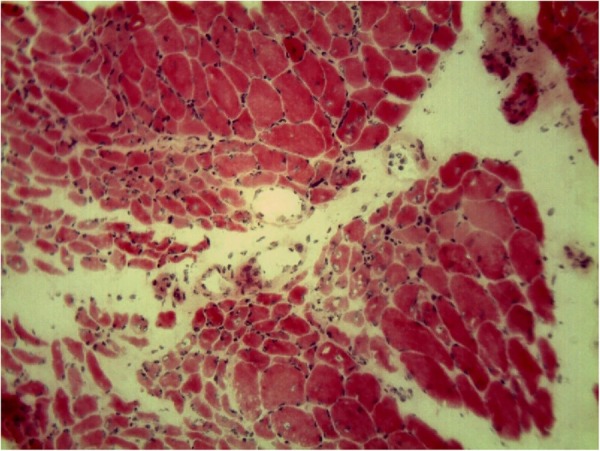
H&E staining showing smaller muscle fibres at the periphery of the fascicles (perifascicular atrophy).
The patient was again treated with 2 g/kg of IVIG over 5 days with no improvement, followed by plasmapheresis and mycophenolate mofetil 1500 mg two times a day. Over the next several months, because of persistent symptoms, mycophenolate mofetil was replaced with azathioprine 150 mg/day with substantial improvement in muscle symptoms. Over the next 4 years, the patient continued to have weakness and intermittent flares requiring treatment with IVIG or intravenous corticosteroids. Repeat testing for myositis-specific autoantibodies revealed antibodies to nuclear matrix protein NXP-2 antigen.
Two years later, the patient experienced a flare with worsening proximal muscle weakness, typical Gottron's papules over his metacarpophalangeal joints as well as a heliotrope rash. Manual muscle strength was graded 3/5 in proximal upper and lower extremities, and the aldolase level was elevated at 13.9 U/L. The patient also complained of fevers occurring every other day with temperatures as high as 101°F. Whole body MRI revealed severe acute myopathy with intramuscular and fascial oedema involving nearly all of the chest, neck, abdominal, pelvic, proximal upper extremity and bilateral lower extremity musculature, as well as diffuse subcutaneous oedema of the entire body. A repeat malignancy work up including PET-CT was negative. The patient underwent treatment with anakinra, but because of side effects it was discontinued. He is currently being treated with 15 mg/day prednisone.
Discussion
The young age and close temporality of cancer and DM in our patient support a positive association. However, he continued to have progressive muscle symptoms despite tumour resection. A parallel course between DM activity and concomitant malignant disease has been reported in as many as two-thirds of patients in some studies, suggesting the DM in these cases is a paraneoplastic phenomenon.10 In all previously reported cases of renal cell carcinoma-associated DM, clinical improvement was observed after surgery.3 5–7 It is unknown whether a lack of improvement in DM with cancer treatment, as in this case, indicates that the two processes are unrelated or rather that malignancy triggered autoimmunity has become self-perpetuating.
The patient described is also notable for his initial lack of classic rash, with onset of pathognomonic rash 6 years after the initial presentation. In DM, cutaneous manifestations typically precede, accompany or may occur without muscle weakness, as in amyopathic DM. DM muscle involvement without classic rash, or ‘dermatomyositis sine dermatitis’, is a diagnosis based on muscle biopsy showing evidence of DM rather than polymyositis. This entity is only occasionally described in the literature and has been reported in malignancy associated myositis.7 9 Long-term follow-up studies are necessary to better understand the incidence of late-onset development of typical skin findings and prognosis in DM sine dermatitis patients.
Learning points.
Patients with renal cell carcinoma including chromophobe renal cell cancer may present with dermatomyositis (DM) symptoms.
Adult-onset DM, particularly in the presence of antinuclear matrix protein antibodies, is associated with an elevated risk of malignancy and deserves a thorough work up for cancer.
The clinical course of DM could be unparalleled with malignancy.
The typical skin findings in DM could appear several years after the onset of muscle symptoms. Therefore, the finding of perifascicular atrophy on the muscle biopsy in a patient presenting with symmetric proximal weakness should raise the suspicion of DM rather than polymyositis.
Acknowledgments
The authors would like to thank Andrea Corse and Hristelina Ilieva from the Johns Hopkins Department of Neurology for generous assistance with reviewing pathology slides and preparing figures.
Footnotes
Contributors: MDG and LC-S managed the patient. MDG and AHL wrote the case report, collected the references and drafted the manuscript. MDG and LC-S critically revised the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Madan V, Chinoy H, Griffiths CE et al. Defining cancer risk in dermatomyositis. Part I. Clin Exp Dermatol 2009;34:451–5. 10.1111/j.1365-2230.2009.03216.x [DOI] [PubMed] [Google Scholar]
- 2.Hill CL, Zhang Y, Sigurgeirsson B et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 2001;357:96–100. 10.1016/S0140-6736(00)03540-6 [DOI] [PubMed] [Google Scholar]
- 3.Nevins E, Zayat AS, Browning AJ et al. Renal cell carcinoma-associated adult dermatomyositis treated laparoscopic nephrectomy. Urol Ann 2013;5:299–301. 10.4103/0974-7796.120302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benages Pamies J, Cecchini Rosell L, Sanfeliu Cortes F et al. Dermatomyositis and small cell tumor of the kidney. Actas Urol Esp 1997;21:67–70. [PubMed] [Google Scholar]
- 5.Schaefer O, Lohrmann C, Harder J et al. Treatment of renal cell carcinoma associated dermatomyositis with renal arterial embolization and percutaneous radiofrequency heat ablation. J Vasc Interv Radiol 2004;15:97–9. 10.1097/01.RVI.0000114833.75873.25 [DOI] [PubMed] [Google Scholar]
- 6.Shinohara N, Harabayashi T, Suzuki S et al. Advanced renal pelvic carcinoma associated with dermatomyositis. Int J Urol 2005;12:906–8. 10.1111/j.1442-2042.2005.01178.x [DOI] [PubMed] [Google Scholar]
- 7.Szwebel TA, Perrot S, Kierzek G et al. Paraneoplasic dermatomyositis sine dermatitis associated with a tumor of the renal excretion system. J Clin Neuromuscul Dis 2008;10:35–6. 10.1097/CND.0b013e3181828ce3 [DOI] [PubMed] [Google Scholar]
- 8.Hoogendijk JE, Amato AA, Lecky BR et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord 2004;14:337–45. 10.1016/j.nmd.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 9.Whitmore SE, Watson R, Rosenshein NB et al. Dermatomyositis sine myositis: association with malignancy. J Rheumatol 1996;23:101–5. [PubMed] [Google Scholar]
- 10.Andras C, Ponyi A, Constantin T et al. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol 2008;35:438–44. [PubMed] [Google Scholar]