Abstract
In Japan, the 7-valent pneumococcal conjugate vaccine (PCV7) was introduced to the nation's routine immunization program in April 2013 and was replaced by the 13-valent pneumococcal conjugate vaccine (PCV13) in November 2013. Distribution of serotypes and macrolide resistance genotypes was investigated for a total of 1097 (975 children, 122 adults) and 960 (873 children, 87 adults) clinical isolates of Streptococcus pneumoniae from noninvasive infections in Hokkaido (northern main island of Japan) in the routine immunization periods for PCV7 and PCV13 (April–October 2013 and November 2013–November 2014, respectively). Serotype was determined by sequential multiplex PCR and additional genetic analyses. Macrolide resistance genes erm(B) and mef(A/E) were detected by multiplex PCR. Although the most prevalent serotypes in children were 23A and 6C in the PCV7 period, after replacement with PCV13, 19A became the most common, followed by 6C, 15A and 23A. Among adults, serotype 3 was consistently the most frequent throughout the study periods. Compared with values from the pre-PCV7 routine immunization period, PCV7 serotypes decreased from 48.3 to 3.3% in the PCV13 period among children, while the rates of non-PCV13 serotypes (particularly 15A, 23A, 11A, 10A and 35B) increased from 39.7 to 75.1% (p < 0.001). In the PCV13 period, erm(B), mef(A/E) and both of these genes were detected in 75.8, 31.6 and 11.3% of all isolates, respectively. Serotype 19A accounted for 76.9% of the isolates with both the macrolide resistance genes, and emerging non-PCV13 serotypes 15A, 15C and 23A mostly harboured erm(B).
Keywords: Macrolide resistance genotypes, pneumococcal conjugate vaccines, pneumococcal diseases, serotypes, surveillance
Introduction
Streptococcus pneumoniae (pneumococcus) is a major cause of community-acquired infections, such as pneumonia, meningitis, septicemia and otitis media worldwide, particularly in younger children and the elderly. Among at least 95 capsular serotypes classified for S. pneumoniae, several serotypes are more commonly associated with infections in humans and have been targeted by pneumococcal vaccines. To date, S. pneumoniae has been increasingly become resistant to antibiotics represented by β-lactams and macrolides worldwide [1]. Particularly after the introduction of the 7-valent pneumococcal vaccine (PCV7) in the United States since 2000, the increase of isolates resistant to erythromycin (macrolides) has been noted [2], [3]. Furthermore, isolates that are not susceptible to at least one antimicrobial increased among nonvaccine serotypes after the introduction of PCV7 and the 13-valent pneumococcal conjugate vaccine (PCV13) in Ireland [4].
The PCV7 vaccine has been available for infant vaccination programs in the United States and many other countries since the 2000s. Since 2010, a new 13-valent pneumococcal conjugate vaccine (PCV13), which contains PCV7 serotypes and six additional serotypes (1, 3, 5, 6A, 7F and 19A), has been introduced worldwide instead of PCV7. However, non-PCV13 serotypes associated with multidrug resistance, such as 15A and 35B, were found to emerge in the PCV13 period [5].
In Japan, PCV7 was first introduced in February 2010 as a voluntary vaccination in children <5 years of age, and the estimated vaccination coverage rate gradually increased up to 80–90% in 2012 [6]. Since April 2013, PCV7 had been implemented into the routine immunization program and was replaced by the PCV13 in November 2013. After the introduction of voluntary PCV7 vaccination for children in Japan, a decrease in the incidence of invasive and noninvasive pneumococcal infections due to PCV7 serotypes has been observed in children and unvaccinated adults [6], [7]. Regarding vaccination for adults, a 23-valent pneumococcal polysaccharide vaccine (PPSV23), which contains PCV13 serotypes (except for 6A) and 12 other serotypes (2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F and 33F), was approved for routine vaccination in October 2014, while PCV13 had been available for voluntary use since June 2014 for adults ≤65 years of age.
The purpose of the present study was to analyse the changes in serotypes and its relationship to macrolide resistance genotypes among noninvasive or colonization isolates of S. pneumoniae in northern Japan during the PCV7 and PCV13 routine immunization periods and compare it with previous data of the PCV7 voluntary immunization period in 2011.
Materials and Methods
Bacterial isolates
A total of 2057 S. pneumoniae isolates from noninvasive infections in outpatients among children (<16 years old) and adults (≥16 years old) who visited various hospitals and clinics throughout Hokkaido (northern main island of Japan) were analysed. Among all the isolates, 1097 (975 children, 122 adults), and 960 (873 children, 87 adults) isolates were collected in the PCV7 period (April 2013–October 2013) and the PCV13 period (November 2013–November 2014), respectively. Prevalence of serotypes and macrolide resistance genotypes in the present study was compared with those of our previous study in 2011 (PCV7 voluntary immunization period, 998 and 63 isolates from children and adults, respectively) [8]. Bacterial isolation from clinical specimens and species identification were carried out by conventional methods in the Sapporo Clinical Laboratory, Japan, and only one isolate per patient was analysed. The pneumococci in this study were isolated from nasal discharges (90.3%), ear discharges (4.7%) and pharynx or sputum (4.4%). Isolates were preserved in Microbank (Pro-Lab Diagnostics, Richmond Hill, Canada) at −80°C until analysed.
Serotyping and macrolide resistance genotypes
Serotypes were determined by sequential multiplex PCR (SM-PCR) with a series of combinations of serotype-specific primer pairs targeted at capsular polysaccharide biosynthesis (cps) locus (Table 1), using a modified version of the scheme described by Pai et al. [9]. The scheme in the present study was designed to determine serotypes of Japanese isolates more conveniently, based on serotype prevalence in our previous report [8]. The cpsA was detected in each time of multiplex PCR (M-PCR) as described previously [9]. For untypeable isolates after the total six reactions of SM-PCR, uniplex PCR or M-PCR was performed to assign serotypes 2, 21, 24, 7C, 7F, 8, 17F, 20 and 31 using primers described previously [8], [9], [10]. M-PCR combined with mutagenic PCR and restriction fragment length polymorphism was used for discrimination of serotypes 6A, 6B, 6C and 6D as described previously [8]. Genotype 6E was detected by M-PCR as described previously for all the isolates assigned to 6A and 6B [11]. The macrolide resistance genes erm(B) and mef(A/E) were detected by M-PCR using primers described previously [12], [13]. Serotypes 15B and 15C were identified by PCR and direct sequencing of partial wciZ as previously described [8], after detection of 15B/C by SM-PCR.
Table 1.
Sequential multiplex PCR scheme to determine Streptococcus pneumoniae serotypes designed for Japanese isolates
| Reaction no. | Serotypes assigned in each reaction |
|---|---|
| 1 | 19A, 3, 22F, 6A/B/C/D |
| 2 | 15B/C, 23A, 10A, 11A |
| 3 | 23F, 33F, 15A, 38 |
| 4 | 19F, 16F, 18, 35B |
| 5 | 1, 34, 35F |
| 6 | 4, 14, 12F, 9V |
Statistical analysis
Statistical analysis was performed by SPSS 19.0 (IBM, Armonk, NY, USA). A two-tailed chi-square test or Fisher's accurate probability methods (for small group sizes) was used to examine the significance in the changes of serotypes during the study periods. A p value of <0.05 was considered statistically significant.
Results
Prevalence of serotypes
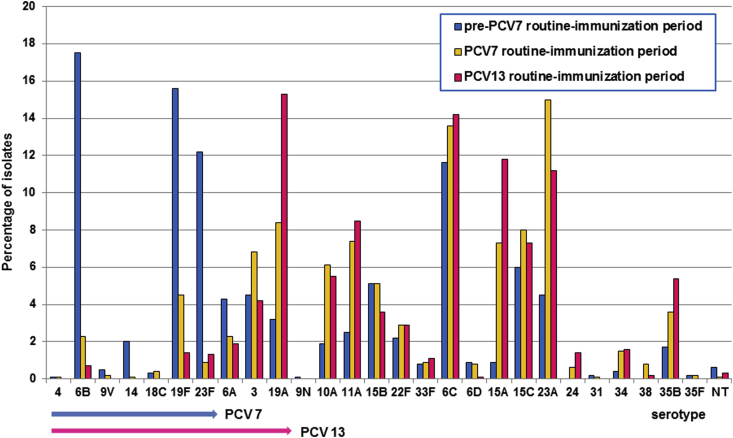
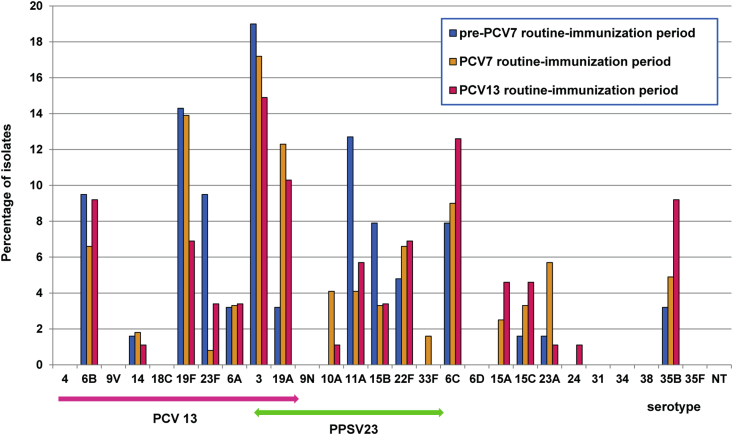
Among the total of 2057 isolates, serotype was determined for 2053 isolates (99.8%) by SM-PCR. Detection rates of individual serotypes in children and adults in the three periods (pre-PCV7, PCV7 and PCV13 routine immunization periods) are shown in Fig. 1, Fig. 2, respectively, and frequencies of serotypes are summarized in Supplementary Table S1. In the PCV7 period, the common serotypes were 23A (15.0%), followed by 6C (13.6%), 19A (8.4%), 15C (8.0%) and 15A (7.3%) in children and were 3 (17.2%), 19F (13.9%) and 19A (12.3%) in adults. In the PCV13 period, the prevailing serotypes were 19A (15.3%), 6C (14.2%), 15A (11.8%) and 23A (11.2%) in children and were 3 (14.9%), 6C (12.6%), 19A (10.3%), 6B and 35B (each 9.2%) in adults.
Fig. 1.
Serotype distribution of Streptococcus pneumoniae among children in the pre-PCV7, PCV7 and PCV13 routine immunization periods. Serotypes included in PCV7 and PCV13 are indicated by blue and red arrows, respectively. NT, nontypeable; PCV, pneumococcal conjugate vaccine.
Fig. 2.
Serotype distribution of Streptococcus pneumoniae among adults in the pre-PCV7, PCV7 and PCV13 routine immunization periods (see Fig. 1). Green arrow indicates serotypes included in PPSV23. PCV, pneumococcal conjugate vaccine.
Compared to our previous data in 2011 (pre-PCV7 period) to the PCV13 period in 2013–2014, a significant increase was observed for serotype 19A (from 3.2 to 15.3%, p < 0.001), 15A (from 0.9 to 11.8%, p < 0.001), 23A (from 4.5 to 11.2%, p < 0.001) and 11A (from 2.5 to 8.5%, p < 0.001); similarly, an increase was also evident for serotypes 10A (from 1.9 to 5.5%) and 35B (from 1.7 to 5.4%) in children. In contrast, PCV7 serotypes decreased from 48.3% in 2011 to 3.3% in 2013–2014 (p < 0.001).
Among isolates from adults, serotype 3 was consistently the most frequent throughout the study periods. Compared with 2011, PCV13 serotypes 19F and 23F, and PPSV23 serotypes 11A and 15B decreased in the PCV13 period, while an increase was observed for non-PCV13 serotypes 6C (7.9 to 12.6%, p = 0.434), 15A (0 to 4.6%, p = 0.143), 15C (1.6 to 4.6%, p = 0.405), and 35B (3.2 to 9.2%, p = 0.197). PCV13 serotypes 1, 5 and 7F were not detected in any periods.
Overall, from 2011 (pre-PCV7 routine immunization) to 2013–2014 (PCV13 period), PCV7- and PCV13-associated serotypes in children decreased from 48.3 to 3.3% and 60.3 to 24.9%, respectively (p < 0.001). Similarly in adults, decrease of the PCV7, PCV13 and PPSV23 types was found (34.9 to 20.7%, 60.3 to 49.4% and 85.7 to 63.2%, respectively). Consequently, the percentage of non-PCV13 serotypes in the all ages increased from 39.7% in 2011 to 72.9% in the PCV13 period (p < 0.001).
Prevalence of macrolide resistance genotypes
Macrolide resistance genotypes in the three different periods are summarized in Table 2. During the PCV13 period, the macrolide resistance genes erm(B), mef(A/E) and both of these genes were detected in 74.8, 32.3 and 11.5% of isolates in children, respectively, and similar rates were evident in adults. Compared with the pre-PCV7-routine immunization period, the proportion of mef(A/E) decreased from 40.0 to 31.6% in all ages in the PCV13 period. In contrast, the rate of isolates carrying erm(B) increased from 66.4 to 75.1%.
Table 2.
Comparison of macrolide resistance genotypes of Streptococcus pneumoniae isolates in three periods by age
| Macrolide resistance genotype | Noninvasive S. pneumoniae isolates, n (%), for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-PCV7 routine immunization period (previous study in 2011) |
PCV7 routine immunization period (April–October 2013) |
PCV13 routine immunization period (November 2013–November 2014 |
|||||||
| All ages (n = 1061) | <16 years (n = 998) | ≥16 years (n = 63) | All ages (n = 1097) | <16 years (n = 975) | ≥16 years (n = 122) | All ages (n = 960) | <16 years (n = 873) | ≥16 years (n = 87) | |
| erm(B)+ | 704 (66.4) | 671 (67.2) | 33 (52.4) | 807 (73.6) | 711 (73.0) | 96 (78.7) | 721 (75.1) | 653 (74.8) | 68 (78.2) |
| mef (A/E)+ | 424 (40.0) | 396 (39.7) | 28 (44.4) | 307 (28.0) | 278 (28.5) | 29 (23.8) | 303 (31.6) | 282 (32.3) | 21 (24.1) |
| erm(B)+ and mef(A/E)+ | 113 (10.7) | 109 (10.9) | 4 (6.3) | 79 (7.2) | 70 (7.2) | 9 (7.4) | 108 (11.3) | 100 (11.5) | 8 (9.2) |
| erm(B)+ and mef(A/E)− | 591 (55.8) | 562 (56.3) | 29 (46.0) | 728 (66.4) | 641 (65.7) | 87 (71.3) | 613 (63.9) | 553 (63.3) | 60 (69.0) |
| erm(B)− and mef(A/E)+ | 311 (29.3) | 287 (28.8) | 24 (38.1) | 228 (20.8) | 207 (21.3) | 20 (16.4) | 195 (20.3) | 182 (20.8) | 13 (14.9) |
| erm(B)− and mef(A/E)− | 46 (4.3) | 40 (4.0) | 6 (9.5) | 62 (5.7) | 56 (5.7) | 6 (4.9) | 44 (4.6) | 38 (4.4) | 6 (6.9) |
PCV, pneumococcal conjugate vaccine.
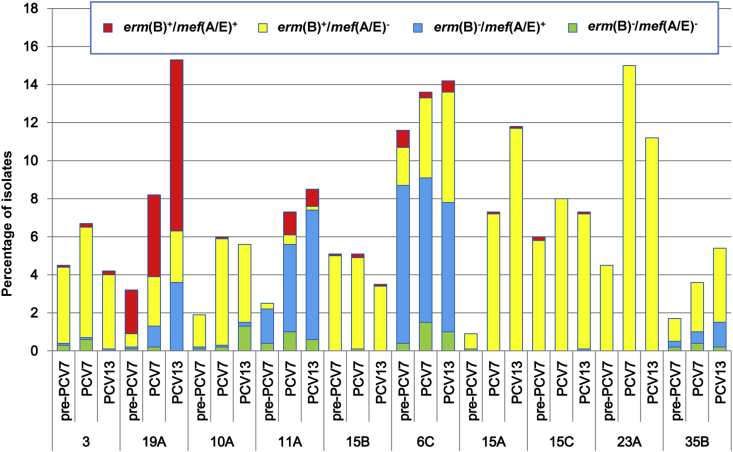
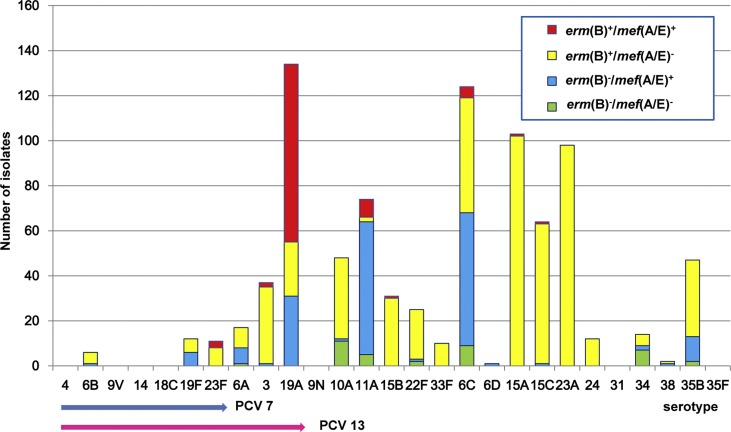
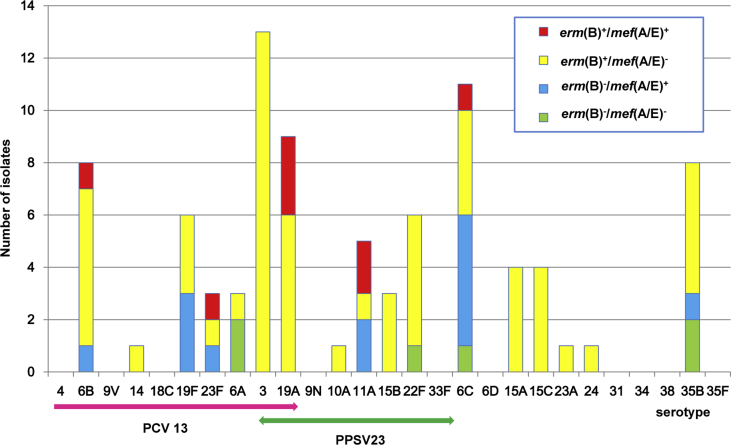
The distribution of macrolide resistance genotypes in individual serotypes during the PCV13 period in children and adults is presented in Supplementary Figs S1 (Supplementary Table S2) and S2 (Supplementary Table S3), respectively, and comparison of the genotypes among non-PCV7 serotypes in children is shown in Fig. 3. During the PCV13 period, erm(B) was carried by all isolates of non-PCV13 serotypes 15A, 15B, 23A, 24 and 33F in children, accounting for 27.9% (244/873). Most of serogroup 15 (serotypes 15A, 15B and 15C) isolates harboured erm(B) (99.5%, 197/198), while only one isolate harboured mef(A/E) solely. Presence of mef(A/E) solely was found at highest rate in 11A (79.7%, 59/74), followed by 6C (47.6%, 59/124) and 35B (23.4%, 11/47). All the isolates of serotypes 6B, 19F, 23F, 3, 19A, 22F and 38 carried erm(B) and/or mef(A/E) genes. Isolates harbouring both the genes (100 isolates) mostly belonged to serotypes 19A (79%), 11A (8%) and 6C (5%). Among adults, all the isolates of the serotype 3 harboured erm(B) solely in the PCV13 period (Supplementary Table S3).
Fig. 3.
Comparison of macrolide resistance genotypes in non-PCV7 serotypes in three study periods among children. PCV, pneumococcal conjugate vaccine.
Discussion
In Japan, after the introduction of PCV7, a distinct reduction of incidence was observed for pediatric meningitis due to pneumococcus (decrease by 80%, from 2009 to 2012) [14], and nonmeningitis invasive pneumococcal disease (IPD) (decrease by 52% from 2008–2010 to 2012) [7]. Accordingly, patients with IPD due to PCV7 serotypes also decreased from 73.3% in 2010 to 14.7% in 2012 [6]. Similarly, the coverage rate of PCV7 serotypes for pediatric noninvasive isolates was reported to have decreased significantly, from 49.3% (2008–2009) to 23.4% (2011–2012) in the Tokai region (the Pacific Ocean side of central Honshu Island), Japan [15]. However, the trend of pneumococcal serotypes after replacement by PCV13 (i.e. since November 2013) had not been clarified.
Our present study, conducted in Hokkaido, Japan, elucidated a change in serotypes from the pre-PCV7 routine immunization period (2011) until the initial 1-year period of PCV13 (November 2013–November 2014), revealing evident decrease of PCV7- and PCV13-associated serotypes in both children and adults. In contrast, non-PCV13 serotypes increased significantly when the pre-PCV7 period and the PCV13 period are compared. Similar findings have been reported in studies in the the United States [16], [17]. However, it was noteworthy in the present study that serotype 19A, which is included in PCV13, exhibited increase in the PCV13 period compared with the PCV7 period. Serotype 19A is known to have emerged as the predominant cause of both IPD and non-IPD globally after the introduction of PCV7, but its prevalence in pneumococcal infections among children has decreased by replacement of PCV13 [4], [18], [19], [20]. While increase of serotype 19A stopped immediately after introduction of PCV13 in the United States [16], incidence of serotype 19A in IPD of adults decreased 2.5 years after the PCV13 national immunization in children in Israel [21]. The PCV13 period of our present study was the initial 1-year period after switching to PCV13 from PCV7, i.e. the early PCV13 period. Therefore, the serotype distribution in this period is considered to be still influenced by routine immunization of PCV7, causing a high incidence of serotype 19A. However, 19A may be predicted to decrease shortly because the use of PCV13 has been becoming established. In contrast, a slight decrease of other PCV13 serotypes, such as 3 and 6A, was observed in the PCV13 period among children in the present study. This finding suggests that these serotypes might be prevented by PCV13 more effectively than 19A; otherwise they might not spread selectively by the use of PCV7.
Serotype 19A has been recognized as a multidrug-resistant type in the United States [22], [23], [24] and Europe during the PCV7 era [25], [26]. Also in Japan, as demonstrated in the present study, serotype 19A emerged as a dominant serotype after PCV7 introduction, showing the highest rate of isolates with both erm(B) and mef(A/E) among all the serotypes. Similarly, a high prevalence of these macrolide resistance genes in serotype 19A pneumococci was described in the United States, Europe and Asia (Taiwan) [27], [28], [29], [30], furthermore, macrolide-resistant pneumococci carrying both resistance genes were associated with multidrug resistance [31], [32]. Thus, it is possible that such drug resistance traits of serotype 19A may be one of the reasons for persistence of this serotype even after introduction of PCV13.
In the present study, after implementation of routine immunization of PCV13 in Japan, 72.9% of pneumococcal isolates from non-IPD were revealed to belong to nonvaccine serotypes, represented by 6C, 15A, 23A, 11A, 10A and 35B, among which 6C, 11A, 15A and 35B appeared to have increased gradually since the introduction of PCV7 in children. These nonvaccine serotypes have been also described as being the common types among isolates from IPDs, non-IPDs, and carriage after introduction of PCV13 as well as PCV7 in other countries [16], [20], [33], [34], [35], [36]. Particularly serotypes 15A, 6C and 35B were reported to be major multidrug resistance serotypes in the United States [37]. Of note, in European countries, non-PCV13 serotypes 11A and 35B among IPD isolates were documented to be statistically associated with the risk of death [38]. Consequently, ongoing increase of nonvaccine serotypes is a matter of concern after the use of pneumococcal vaccines worldwide.
The macrolide resistance genes erm(B) and/or mef(A/E) were detected at a high rate (>95%) among invasive and noninvasive pneumococci in Japan [8], [39], a finding also shown in the present study. In the United States, macrolide resistance mediated by erm(B) and/or mef(A/B) was found to increase after the introduction of PCV7, mainly as a result in the increase of serotype 19A [29], [40], while the highest erm(B)-positive rate (100%) was seen in serotype 15A in Hong Kong [36]. Our present study also showed that the non-PCV13 types 15A, 15C and 23A among children were mostly positive for erm(B) (99.6%). Compared to our previous study in 2011, the erm(B)-positive rate in non-IPD isolates in the PCV13 period increased, associated with an increasing trend of non-PCV7 serotypes, despite the high proportion of erm(B) in the PCV7 serotypes, such as 6B, 19F and 23F, which has decreased by PCV7. Therefore, the high rate of erm(B) has still persisted after the use of pneumococcal vaccines.
In conclusion, our results in northern Japan revealed the increase of non-PCV7/PCV13 serotypes among non-IPD isolates after implementation of PCVs in Japan, associated with high prevalence of erm(B). Our only exceptional finding was an increase of serotype 19A in the early PCV13 period. Further continuous surveillance on serotypes and antimicrobial resistance is thus necessary for 19A and several non-PCV13 serotypes, such as 6C, 10A, 11A, 15A, 23A and 35B.
Acknowledgement
Supported by a Grant-in-Aid for Scientific Research (KAKENHI) (26893212) from the Japan Society for Promotion of Science (JSPS).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.nmni.2015.11.001.
Conflict of Interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Reinert R.R. The antimicrobial resistance profile of Streptococcus pneumoniae. Clin Microbiol Infect. 2009;15(Suppl. 3):7–11. doi: 10.1111/j.1469-0691.2009.02724.x. [DOI] [PubMed] [Google Scholar]
- 2.Link-Gelles R., Thomas A., Lynfield R., Petit S., Schaffner W., Harrison L. Geographic and temporal trends in antimicrobial nonsusceptibility in Streptococcus pneumoniae in the post-vaccine era in the United States. J Infect Dis. 2013;208:1266–1273. doi: 10.1093/infdis/jit315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z., Nachamkin I., Edelstein P.H., Lautenbach E., Metlay J.P. Serotype emergence and genotype distribution among macrolide-resistant invasive Streptococcus pneumoniae isolates in the postconjugate vaccine (PCV-7) era. Antimicrob Agents Chemother. 2012;56:743–750. doi: 10.1128/AAC.05122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElligott M., Vickers I., Cafferkey M., Cunney R., Humphreys H. Non-invasive pneumococcal serotypes and antimicrobial susceptibilities in a paediatric hospital in the era of conjugate vaccines. Vaccine. 2014;32:3495–3500. doi: 10.1016/j.vaccine.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 5.Golden A.R., Adam H.J., Gilmour M.W., Baxter M.R., Martin I., Nichol K.A. Assessment of multidrug resistance, clonality and virulence in non-PCV-13 Streptococcus pneumoniae serotypes in Canada, 2011–13. J Antimicrob Chemother. 2015;70:1960–1964. doi: 10.1093/jac/dkv061. [DOI] [PubMed] [Google Scholar]
- 6.Chiba N., Morozumi M., Shouji M., Wajima T., Iwata S., Ubukata K. Changes in capsule and drug resistance of pneumococci after introduction of PCV7, Japan, 2010–2013. Emerg Infect Dis. 2014;20:1132–1139. doi: 10.3201/eid2007.131485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oishi K., Tamura K., Akeda Y. Global control of pneumococcal infections by pneumococcal vaccines. Trop Med Health. 2014;42(2 Suppl.):83–86. doi: 10.2149/tmh.2014-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchiya M., Urushibara N., Ghosh S., Kuwahara O., Morimoto S., Ito M. Serotype distribution and susceptibility to penicillin and erythromycin among noninvasive or colonization isolates of Streptococcus pneumoniae in northern Japan: a cross-sectional study in the pre-PCV7 routine immunization period. Microb Drug Resist. 2014;20:456–465. doi: 10.1089/mdr.2013.0196. [DOI] [PubMed] [Google Scholar]
- 9.Pai R., Gertz R.E., Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol. 2006;44:124–131. doi: 10.1128/JCM.44.1.124-131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Gloria Carvalho M., Pimenta F.C., Jackson D., Roundtree A., Ahmad Y., Millar E.V. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol. 2010;48:1611–1618. doi: 10.1128/JCM.02243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawaguchiya M., Urushibara N., Kobayashi N. High prevalence of genotype 6E (putative serotype 6E) among noninvasive/colonization isolates of Streptococcus pneumoniae in northern Japan. Microb Drug Resist. 2015;21:209–214. doi: 10.1089/mdr.2014.0181. [DOI] [PubMed] [Google Scholar]
- 12.Klaassen C.H., Mouton J.W. Molecular detection of the macrolide efflux gene: to discriminate or not to discriminate between mef(A) and mef(E) Antimicrob Agents Chemother. 2005;49:1271–1278. doi: 10.1128/AAC.49.4.1271-1278.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagai K., Shibasaki Y., Hasegawa K., Davies T.A., Jacobs M.R., Ubukata K. Evaluation of PCR primers to screen for Streptococcus pneumoniae isolates and beta-lactam resistance, and to detect common macrolide resistance determinants. J Antimicrob Chemother. 2001;48:915–918. doi: 10.1093/jac/48.6.915. [DOI] [PubMed] [Google Scholar]
- 14.Shinjoh M., Iwata S., Yagihashi T., Sato Y., Akita H., Takahashi T. Recent trends in pediatric bacterial meningitis in Japan—a country where Haemophilus influenzae type b and Streptococcus pneumoniae conjugated vaccines have just been introduced. J Infect Chemother. 2014;20:477–483. doi: 10.1016/j.jiac.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Okade H., Funatsu T., Eto M., Furuya Y., Mizunaga S., Nomura N. Impact of the pneumococcal conjugate vaccine on serotype distribution and susceptibility trends of pediatric non-invasive Streptococcus pneumoniae isolates in Tokai, Japan over a 5-year period. J Infect Chemother. 2014;20:423–428. doi: 10.1016/j.jiac.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Richter S.S., Heilmann K.P., Dohrn C.L., Riahi F., Diekema D.J., Doern G.V. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011. Emerg Infect Dis. 2013;19:1074–1083. doi: 10.3201/eid1907.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin M.R., Zhu Y., Moore M.R., Whitney C.G., Grijalva C.G. US hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–163. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gounder P.P., Bruce M.G., Bruden D.J., Singleton R.J., Rudolph K., Hurlburt D.A. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae—Alaska, 2008–2012. J Infect Dis. 2014;209:1251–1258. doi: 10.1093/infdis/jit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olarte L., Hulten K.G., Lamberth L., Mason E.O., Jr., Kaplan S.L. Impact of the 13-valent pneumococcal conjugate vaccine on chronic sinusitis associated with Streptococcus pneumoniae in children. Pediatr Infect Dis J. 2014;33:1033–1036. doi: 10.1097/INF.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 20.Zuccotti G., Mameli C., Daprai L., Garlaschi M.L., Dilillo D., Bedogni G. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine. 2014;32:527–534. doi: 10.1016/j.vaccine.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Regev-Yochay G., Paran Y., Bishara J., Oren I., Chowers M., Tziba Y. Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: a nationwide surveillance study. Vaccine. 2015;33:1135–1142. doi: 10.1016/j.vaccine.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Reinert R., Jacobs M.R., Kaplan S.L. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine. 2010;28:4249–4259. doi: 10.1016/j.vaccine.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs M.R., Good C.E., Beall B., Bajaksouzian S., Windau A.R., Whitney C.G. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. J Clin Microbiol. 2008;46:982–990. doi: 10.1128/JCM.02321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pichichero M.E., Casey J.R. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298:1772–1778. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 25.Setchanova L.P., Alexandrova A., Dacheva D., Mitov I., Kaneva R., Mitev V. Dominance of multidrug-resistant Denmark(14)-32 (ST230) clone among Streptococcus pneumoniae serotype 19A isolates causing pneumococcal disease in Bulgaria from 1992 to 2013. Microb Drug Resist. 2015;21:35–42. doi: 10.1089/mdr.2014.0076. [DOI] [PubMed] [Google Scholar]
- 26.Ardanuy C., Rolo D., Fenoll A., Tarrago D., Calatayud L., Linares J. Emergence of a multidrug-resistant clone (ST320) among invasive serotype 19A pneumococci in Spain. J Antimicrob Chemother. 2009;64:507–510. doi: 10.1093/jac/dkp210. [DOI] [PubMed] [Google Scholar]
- 27.Tothpal A., Kardos S., Laub K., Nagy K., Tirczka T., van der Linden M. Radical serotype rearrangement of carried pneumococci in the first 3 years after intensive vaccination started in Hungary. Eur J Pediatr. 2015;174:373–381. doi: 10.1007/s00431-014-2408-1. [DOI] [PubMed] [Google Scholar]
- 28.Picazo J., Duenas J., Ramirez A., Perez A.R., Padilla E., Herrero S. Incidence of pediatric invasive pneumococcal disease in the island of Majorca (2008–2010), an area with non-universal vaccination, and estimations of serotype and children population coverage by available conjugate vaccines. BMC Infect Dis. 2013;13:503. doi: 10.1186/1471-2334-13-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins S.G., Farrell D.J. Increase in pneumococcus macrolide resistance, United States. Emerg Infect Dis. 2009;15:1260–1264. doi: 10.3201/eid1508.081187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safari D., Kuo L.C., Huang Y.T., Liao C.H., Sheng W.H., Hsueh P.R. Increase in the rate of azithromycin-resistant Streptococcus pneumoniae isolates carrying the erm(B) and mef(A) genes in Taiwan, 2006–2010. BMC Infect Dis. 2014;14:704. doi: 10.1186/s12879-014-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrell D.J., Jenkins S.G., Brown S.D., Patel M., Lavin B.S., Klugman K.P. Emergence and spread of Streptococcus pneumoniae with erm(B) and mef(A) resistance. Emerg Infect Dis. 2005;11:851–858. doi: 10.3201/eid1106.050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins S.G., Brown S.D., Farrell D.J. Trends in antibacterial resistance among Streptococcus pneumoniae isolated in the USA: update from PROTEKT US years 1–4. Ann Clin Microbiol Antimicrob. 2008;7:1. doi: 10.1186/1476-0711-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grivea I.N., Priftis K.N., Giotas A., Kotzia D., Tsantouli A.G., Douros K. Dynamics of pneumococcal carriage among day-care center attendees during the transition from the 7-valent to the higher-valent pneumococcal conjugate vaccines in Greece. Vaccine. 2014;32:6513–6520. doi: 10.1016/j.vaccine.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Benfield T., Skovgaard M., Schonheyder H.C., Knudsen J.D., Bangsborg J., Ostergaard C. Serotype distribution in non-bacteremic pneumococcal pneumonia: association with disease severity and implications for pneumococcal conjugate vaccines. PLoS One. 2013;8:e72743. doi: 10.1371/journal.pone.0072743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keck J.W., Wenger J.D., Bruden D.L., Rudolph K.M., Hurlburt D.A., Hennessy T.W. PCV7-induced changes in pneumococcal carriage and invasive disease burden in Alaskan children. Vaccine. 2014;32:6478–6484. doi: 10.1016/j.vaccine.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 36.Liyanapathirana V., Nelson E.A., Ang I., Subramanian R., Ma H., Ip M. Emergence of serogroup 15 Streptococcus pneumoniae of diverse genetic backgrounds following the introduction of pneumococcal conjugate vaccines in Hong Kong. Diagn Microbiol Infect Dis. 2015;81:66–70. doi: 10.1016/j.diagmicrobio.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Richter S.S., Diekema D.J., Heilmann K.P., Dohrn C.L., Riahi F., Doern G.V. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob Agents Chemother. 2014;58:6484–6489. doi: 10.1128/AAC.03344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro-Torne A., Dias J.G., Hruba F., Lopalco P.L., Pastore-Celentano L., Gauci A.J. Risk factors for death from invasive pneumococcal disease, Europe, 2010. Emerg Infect Dis. 2015;21:417–425. doi: 10.3201/eid2103.140634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugita G., Hotomi M., Sugita R., Kono M., Togawa A., Yamauchi K. Genetic characteristics of Haemophilus influenzae and Streptococcus pneumoniae isolated from children with conjunctivitis–otitis media syndrome. J Infect Chemother. 2014;20:493–497. doi: 10.1016/j.jiac.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Hawkins P.A., Chochua S., Jackson D., Beall B., McGee L. Mobile elements and chromosomal changes associated with MLS resistance phenotypes of invasive pneumococci recovered in the United States. Microb Drug Resist. 2015;21:121–129. doi: 10.1089/mdr.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.