Abstract
Introduction
Atrial fibrillation (AF) is the most common clinically relevant arrhythmia and it is strongly associated with stroke. Left atrial appendage (LAA) is considered to be the most often source of thrombotic material. In recent decades a number surgical, percutaneous and hybrid approaches for LAA occlusion have been described revealing very different level of success and showing a variety of challenges associated with this matter. We present the first Polish experience with the stand-alone totally thoracoscopic LAA exclusion using novel clipping system.
Material and methods
Four patients (one male) in mean age of 74 (± 13) years with long-standing persistent and chronic AF were admitted for totally thoracoscopic LAA exclusion. All patients had significant comorbidities and the history of the oral anticoagulation intolerance or suboptimal/unstable level (CHA2DS2-VASC > 5, HAS_BLED > 3). Three procedures were performed through totally thoracoscopic access. In one patient due to massive adhesions in the left pleura we performed minithoracotomy in fourth left intercostal space. In two months follow-up we observed no mortality, no strokes and no bleedings.
Results
In all patient total exclusion of LAA with no residual remnant was confirmed. The “skin-to-skin” procedural time took on average 40, minimum 20 minutes. Patients were extubated directly or within two hours after procedure. All patients were discharged early in a good condition.
Conclusions
Our initial first experience with the novel totally thoracoscopic clipping system for stand-alone LAA exclusion is very promising showing very high efficacy and good safety profile.
Keywords: LAA occlusion, atrial fibrillation, totally thoracoscopic
Abstract
Wstęp
Migotanie przedsionków to najczęściej występująca arytmia w populacji ludzkiej. Wiąże się ze zwiększonym ryzykiem wystąpienia udaru mózgu. Główne miejsce powstawania materiału zakrzepowo-zatorowego stanowi uszko lewego przedsionka (left atrial appendage – LAA). Na przestrzeni lat rozwinięto szereg chirurgicznych, przezskórnych i hybrydowych metod zamykania LAA. Ze względu na specyfikę tego obszaru terapie te wiążą się nadal jednak z szeregiem wyzwań i ograniczeń. W pracy przedstawiono pierwsze polskie doświadczenia z izolowanym całkowicie torakoskopowym zamknięciem LAA z użyciem nowego sytemu opartego na mechanizmie klipsowania.
Materiał i metody
Czterech pacjentów (1 mężczyzna) w średnim wieku 74 (± 13) lat z przetrwałym, długo trwającym i utrwalonym migotaniem przedsionków zostało zakwalifikowanych do całkowicie torakoskopowego zamknięcia LAA. U wszystkich pacjentów występowały liczne obciążenia dodatkowe oraz nietolerancja lub niestabilny poziom antykoagulacji doustnej (CHA2DS2-VASC > 5, HAS_BLED > 3). U trzech pacjentów wykonano całkowicie torakoskopowe zamknięcie uszka lewego przedsionka. U jednego pacjenta z powodu masywnych zrostów w lewej opłucnej niezbędne było wykonanie minitorakotomii lewostronnej w czwartym międzyżebrzu.
Wyniki
U wszystkich pacjentów przebieg śródoperacyjny oraz zamknięcie LAA przebiegało bez powikłań, z potwierdzonym całkowitym wykluczeniem jego światła. Średni czas operacji wyniósł 40 minut, najkrótszy to 20 minut. Pacjenci byli ekstubowani bezpośrednio po lub w przeciągu 2 godziny od zabiegu. Pacjenci zostali wypisani w dobrym stanie do domu we wczesnych dobach. W dwumiesięcznej obserwacji nie wystąpiły zgony, udary ani krwawienia.
Wnioski
Pierwsze polskie doświadczenia z całkowicie torakoskopowym systemem do zamykania uszka lewego przedsionka wskazują na wysoką skuteczność oraz bezpieczeństwo tej metody leczenia u pacjentów wysokiego ryzyka udaru mózgu.
Introduction
Atrial fibrillation (AF) is the most common clinically relevant arrhythmia, and it is strongly associated with stroke. The left atrial appendage (LAA) is the most frequent source of thrombotic material leading to cerebrovascular events [1]. Oral anticoagulation remains the standard of care of patients with AF. Current guidelines recommend such therapy when CHA2DS2-VASc exceeds 1 [2, 3]. Recent publications indicate that LAA closure might not be inferior to oral anticoagulation in patients in whom it is not recommended [4]. Current guidelines of the European Society of Cardiology reflect this position, indicating that LAA closure in this group of patients (elevated CHA2DS2-VASc and/or HAS-BLED with contraindications for oral anticoagulation) may be recommended [2, 3]. In recent decades a number of surgical, percutaneous and hybrid approaches for LAA occlusion have been described, revealing very different levels of success and showing a variety of challenges associated with this issue.
We present the first Polish experience with the new clipping system for totally thoracoscopic LAA exclusion.
Material and methods
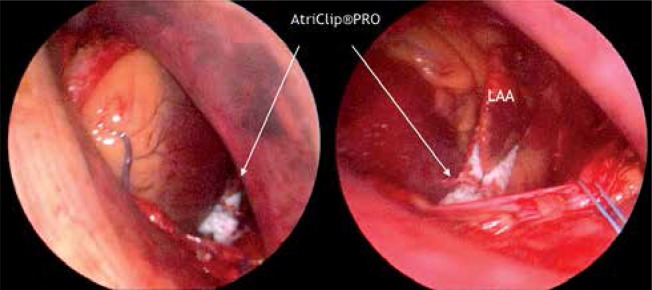
The device for surgical totally thoracoscopic left atrial appendage occlusion AtriClip PRO LAA Exclusion System (AtriClip, AtriCure, Dayton, OH, USA) consists of an automatically closing clip placed in a deployment loop on a disposable holder with head articulation of 60 degrees side-to-side and up/down (Fig. 1). The several novel features of the system in comparison to previous ones, such as its length, maneuverability and releasing system, enable it to be used in a totally thoracoscopic fashion. The AtriClip PRO has parallel titanium crossbars that equalize the force over the tissue trabeculations of the LAA during deployment, ensuring a sealed line at the base of the LAA orifice, as was confirmed in preclinical and clinical studies [5]. The clip can be opened and closed repeatedly before final deployment.
Fig. 1.
AtriClip PRO LAA Exclusion System (courtesy of AtriClip, AtriCure, Dayton, OH, USA)
Four patients (one man, three women) at mean age of 74 (± 13) years with long-standing persistent and chronic atrial fibrillation were admitted to our department for totally thoracoscopic left atrial appendage (LAA) exclusion in order to reduce the risk of stroke and complications of inadequate anticoagulation. All patients had significant comorbidities (Table I) and a history of oral anticoagulation intolerance or a suboptimal/unstable level. The mean left ventricle ejection fraction was 48% (± 11%), and the mean left atrial diameter was 47 mm (± 2 mm). The minimal CHA2DS2-VASC score was 5 and the HAS-BLED score was over 3, in one patient reaching 7 points (Table II). All patients prior to the operation underwent transesophageal echocardiography (TEE) to rule out thrombus in the LAA.
Tab. I.
Patient characteristics
| Age, mean ± SD, years | 74 ± 13 |
| Arterial hypertension, no | 3 |
| Diabetes mellitus, no | 3 |
| Stable coronary artery disease, no | 2 |
| Chronic obstructive pulmonary disease, no | 1 |
| Chronic renal disease, no | 3 |
| Cardiomyopathy, no | 1 |
| Stroke/transient ischemic attack, no | 1 |
| Pacemaker/implantable cardioverter-defibrillator, no | 1 |
| Chronic liver failure, no | 1 |
| Coagulopathy, no | 2 |
| Morbid obesity (BMI > 30), no | 2 |
| Mean left atrium diameter, mm | 47 ± 2 |
| Mean left ventricle ejection fraction,% | 48 ± 11 |
Tab. II.
Risk scores
| No. | CHA2DS2-VASC Score | HAS-BLED Score |
|---|---|---|
| 1 | 5 | 4 |
| 2 | 5 | 3 |
| 3 | 7 | 7 |
| 4 | 8 | 3 |
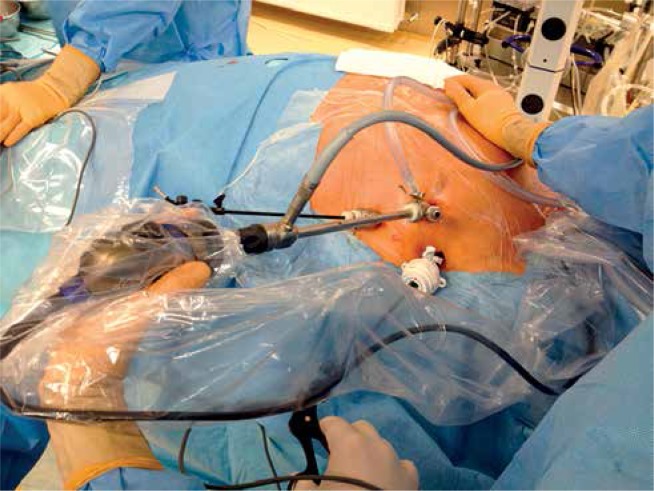
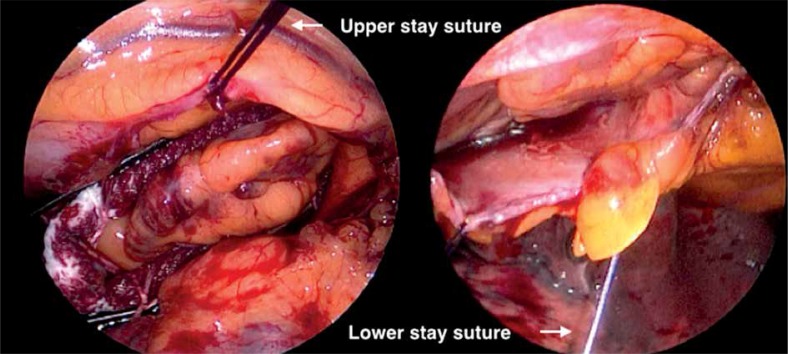
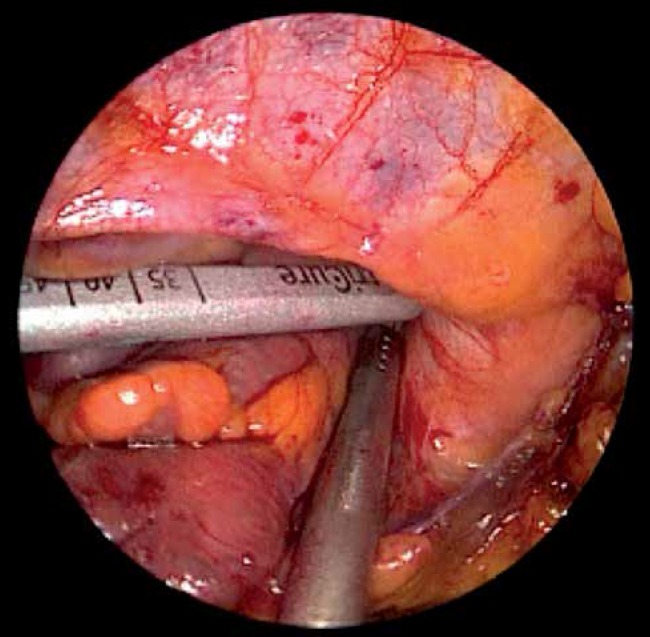
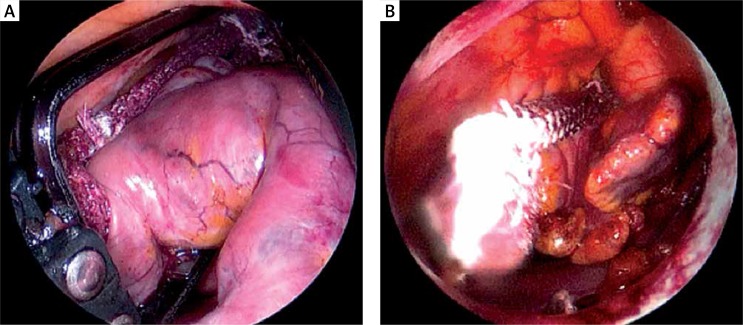
The patients were placed in the supine position and were intubated under general anesthesia with a double lumen intratracheal tube. Transesophageal echocardiography probe was introduced. Single right lung ventilation was started. Subsequently, 3 thoracoscopic ports were placed – one through the fourth intercostal space in the anterior axillary line (for the endoscopic camera), and two through the third and sixth intercostal spaces at the midaxillary line into the left pleura (working ports) (Fig. 2). Working space was created with CO2 insufflation. Pericardiectomy was performed parallel to the phrenic nerve to visualize the LAA, beneath the nerve in 3 patients and above in one patient. Stay sutures were placed on the lower edge of the pericardium for better access to the LAA. If necessary one stay suture on the upper edge of the pericardium was added (Fig. 3). The diameter of the base of the LAA was measured with a dedicated selection guide (Fig. 4). The AtriClip PRO was introduced through the incision in the sixth intercostal space enlarged to 2-3 cm. Under TEE control it was deployed at the base of the left atrial appendage with special care in order not to leave any residual stump (Fig. 5). The possibility of repeated opening of the clip before the final deployment enabled correction of its position if the intraoperative echocardiography showed incomplete LAA closure or an LAA remnant. Full LAA exclusion was confirmed with TEE visualization (Fig. 6). In one patient due to massive adhesions in the left pleura totally thoracoscopic access was not possible. We performed minithoracotomy in the fourth left intercostal space laterally to the mammary line. By gentle dissection, the direct visualization of the LAA was easily achieved. The AtriClip PRO was deployed through minithoracotomy without further complications (Fig. 7). The skin-to-skin procedural time took on average 40, minimum 20 minutes.
Fig. 2.
Thoracoscopic ports placement on the left side of the chest
Fig. 3.
Stay sutures and access to left atrial appendage
Fig. 4.
Measurement of the base of the left atrial appendage in order to choose the proper size of AtriClip PRO
Fig. 5.
Fully deployed AtriClip PRO
Fig. 6.
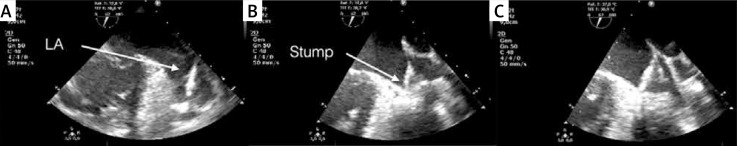
Intraoperative transesophageal echocardiographic monitoring. Left atrial appendage (A). Incomplete occlusion of the left atrial appendage (LAA) orifice (B). Full LAA exclusion after repositioning (C)
Fig. 7.
Minithoracotomy. AtriClip PRO at the base of left atrial appendage (LAA)
Results
The perioperative period was uneventful. Two first patients were extubated two hours after the procedure. The two next patients were extubated directly after the procedure in the operating room. Except for one patient with extreme coagulopathy due to chronic liver failure chest tubes were removed on the first postoperative day. Three patients were discharged home on the second or third postoperative day in a good condition. One patient due to many extracardiac comorbidities required longer stay without any additional complications and was discharged in a good condition on the sixth postoperative day.
During two months of follow-up we observed no bleeding events, no strokes, and no mortality. Echocardiography monitoring showed good and stable clip position. Further imaging with computed tomography is planned.
Discussion
The recommendations for the LAA exclusion have been widened over recent years, but still there are many questions to be answered. In previous decades cardiac surgery was the only source of knowledge in this area; it revealed a number of challenges associated with this apparently easy task of closing or excluding a small appendage within the left atrium. In recent years a few percutaneous and hybrid technologies have been introduced, showing good efficacy, but also confirmed the “old” difficulties, mainly associated with the auricle's fragility, and variable anatomy of the LAA orifice, causing difficulties with device matching and thrombogenic potential of implanted devices [4, 6]. The possible complications include LAA tear, pericardial effusion or tamponade requiring drainage or surgery, acute stroke due to air or thrombus, device embolization or infection requiring its removal and incomplete closure or a leak to the LAA after the procedure [4, 7, 8]. An overlooked issue is long exposure to X-ray radiation and high volume contrast infusion during the percutaneous procedure, whose long-term influence remains unexamined. The problem mainly associated with surgical approaches was the late recanalization, while incomplete closures were more typical for percutaneous techniques and were strictly associated with LAA anatomy, which is currently one of the most important parameters in qualifying a patient for such a procedure [5, 9, 10]. The game remains risky since it has been proven that incomplete LAA closure increases the risk of stroke to a higher level than leaving it open [5]. Also a stump of LAA bigger than 1 cm is considered to be thrombogenic [11].
Another major question arose regarding generally the clinical relevance of such a procedure in large cohorts. To date, only one large trial has allowed the conclusion that effective LAA exclusion prevents stroke, although the level of total occlusion in this trial was far from ideal [4].
The above presented AtriClip PRO system used in terms of the first Polish experience is a promising surgical technique for minimally invasive LAA exclusion for primary and secondary stroke prevention in patients with persistent atrial fibrillation. The published data show that up to 100% efficacy with total LAA exclusion, both in short-term and observation over a few years, is possible [9–12]. Furthermore, apart from the excellent efficacy, no early or late complications such as bleeding, pericardial effusion, device malposition, or infection within 3 years have been revealed and, what is of utmost importance, no stroke was observed during this period although the mean CHADS2-VASC score was 4 (about 10% yearly stroke risk) and half of the patient group was off oral anticoagulation therapy. No bleeding incident occurred during this observation [13].
In the technologies used to date, the anatomy of the left atrial appendage has been a crucial factor influencing the results, but also one contraindicating the percutaneous procedure in some situations. The simplicity of the AtriClip system makes it totally independent from the LAA anatomy. It is also important that during the procedure the clip can be repeatedly reopened and repositioned under the simultaneous transesophageal control in case of any suspicion of incomplete occlusion or remnant, in order to achieve the optimal result. The problem of recanalization or residual leak has been recently raised for surgical LAA closure within the left atrium. In none of our cases was the LAA remnant (stump) bigger than 1 cm or a leakage to the excluded LAA observed, which was an important finding taking into account that their presence significantly increases the risk of stroke (even more than leaving the LAA open) and which may be some limitation of other technologies [14, 15]. Also in a prospective study of an epicardial device for LAA closure there was no remnant or residual leak in long-term follow-up [13]. What should be emphasized is that the implantation of the AtriClip system does not require X-ray radiation or intravenous contrast administration, which might be a significant advantage since very many patients qualified for LAA exclusion are multimorbid, with impaired renal function. The access site is reduced to three 1-2 cm incisions on the left side of the chest. The procedural skin-to-skin time reaches less than 40 minutes and patients are extubated at once after the operation. Patients can be discharged from the hospital on the second or third postoperative day. From the practical point of view it is important that the implantation setup does not require an X-ray arm or hybrid operating room, although close cooperation with an experienced echocardiographer is recommended.
Limitations of the procedure are few, but still its invasiveness should be mentioned. A history of surgery within the pericardium and/or left pleural cavity as well as the presence of thrombus within the LAA are contraindications for totally thoracoscopic LAA clipping. The advantages of the procedure are presented in Table III. Due to the balance between the advantages and disadvantages of surgical thoracoscopic and percutaneous approaches (similarly to the field of persistent atrial fibrillation ablation) a prospective randomized trial may be recommended.
Tab. III.
Advantages of the procedure
| 1 | Very high safety profile |
| 2 | Up to 100% efficacy in total LAA exclusion including long-term follow-up |
| 3 | Independence from LAA anatomy |
| 4 | Totally thoracoscopic, beating heart procedure |
| 5 | Very short (mean 40, minimum in our hands 20 minutes) procedure |
| 6 | Extubation “on-table” |
| 7 | Short 2-3 day hospital stay |
LAA – left atrial appendage
Additionally, electrical isolation of the LAA from the left atrium accompanying the implementation of the AtriClip was described. In a few cases it was associated with the cure of atrial fibrillation [16].
Conclusions
It may be concluded that stand-alone LAA exclusion/occlusion opens new very interesting, promising but also challenging perspectives. Cardiac surgeons in cooperation with cardiologists should further participate in this progress since they also have good tools. The PROTECT AF trial has guided the way towards the new indication and therapeutic option, and further clinical research has to be undertaken.
Our first experience with the new totally thoracoscopic LAA clipping system is very promising, showing very high efficacy and a good safety profile. Further studies are warranted.
Biography

Disclosure
Authors report no conflict of interest.
References
- 1.Frost L, Engholm G, Johnsen S, Moller H, Husted S. Incident stroke after discharge from the hospital with a diagnosis of atrial fibrillation. Am J Med. 2000;108:36–40. doi: 10.1016/s0002-9343(99)00415-5. [DOI] [PubMed] [Google Scholar]
- 2.European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 3.European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery ESC Committee for Practice Guidelines. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 4.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P, PROTECT AF Investigators Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 5.Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924–929. doi: 10.1016/j.jacc.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 6.Bartus K, Han FT, Bednarek J, Myc J, Kapelak B, Sadowski J, Lelakowski J, Bartus S, Yakubov SJ, Lee RJ. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013;62:108–118. doi: 10.1016/j.jacc.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DR, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Han FT, Bartus K, Lakkireddy D, Rojas F, Bednarek J, Kapelak B, Bartus M, Sadowski J, Badhwar N, Earnest M, Valderrabano M, Lee RJ. The effects of LAA ligation on LAA electrical activity. Heart Rhythm. 2014;11:864–870. doi: 10.1016/j.hrthm.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ailawadi G, Gerdisch MW, Harvey RL, Hooker RL, Damiano RJ, Jr, Salamon T, Mack MJ. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–1009. doi: 10.1016/j.jtcvs.2011.07.052. 1009.e1001. [DOI] [PubMed] [Google Scholar]
- 10.Salzberg SP, Plass A, Emmert MY, Desbiolles L, Alkadhi H, Grunenfelder J, Genoni M. Left atrial appendage clip occlusion: early clinical results. J Thorac Cardiovasc Surg. 2010;139:1269–1274. doi: 10.1016/j.jtcvs.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Contractor T, Khasnis A. Left Atrial Appendage Closure in Atrial Fibrillation: A World without Anticoagulation? Cardiol Res Pract. 2011;2011:752808. doi: 10.4061/2011/752808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salzberg SP, Gillinov AM, Anyanwu A, Castillo J, Filsoufi F, Adams DH. Surgical left atrial appendage occlusion: evaluation of a novel device with magnetic resonance imaging. Eur J Cardiothorac Surg. 2008;34:766–770. doi: 10.1016/j.ejcts.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 13.Emmert MY, Puippe G, Baumüller S, Alkadhi H, Landmesser U, Plass A, Bettex D, Scherman J, Grünenfelder J, Genoni M, Falk V, Salzberg SP. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: first long-term results from a prospective device trial. Eur J Cardiothorac Surg. 2014;45:126–131. doi: 10.1093/ejcts/ezt204. [DOI] [PubMed] [Google Scholar]
- 14.Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Gallinghouse GJ, Burkhardt JD, Cesarani F, Scaglione M, Natale A, Gaita F. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Pillarisetti J, Reddy YM, Gunda S, Swarup V, Lee R, Rasekh A, Horton R, Massumi A, Cheng J, Bartus K, Badhwar N, Han F, Atkins D, Bommana S, Earnest M, Nath J, Ferrell R, Bormann S, Dawn B, Di Biase L, Mansour M, Natale A, Lakkireddy D. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: Understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm. 2015;12:1501–1507. doi: 10.1016/j.hrthm.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, Gallinghouse GJ, Bailey SM, Zagrodzky JD, Santangeli P, Hao S, Hongo R, Beheiry S, Themistoclakis S, Bonso A, Rossillo A, Corrado A, Raviele A, Al-Ahmad A, Wang P, Cummings JE, Schweikert RA, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Lewis WR, Natale A. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]