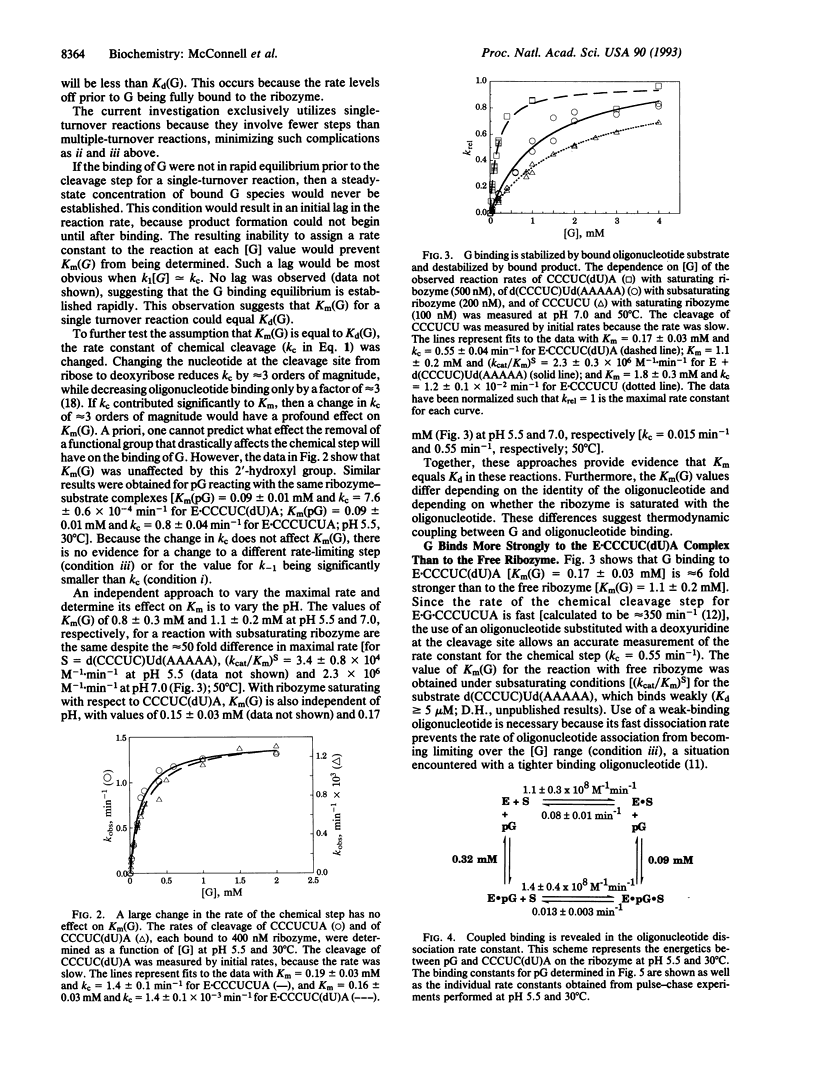
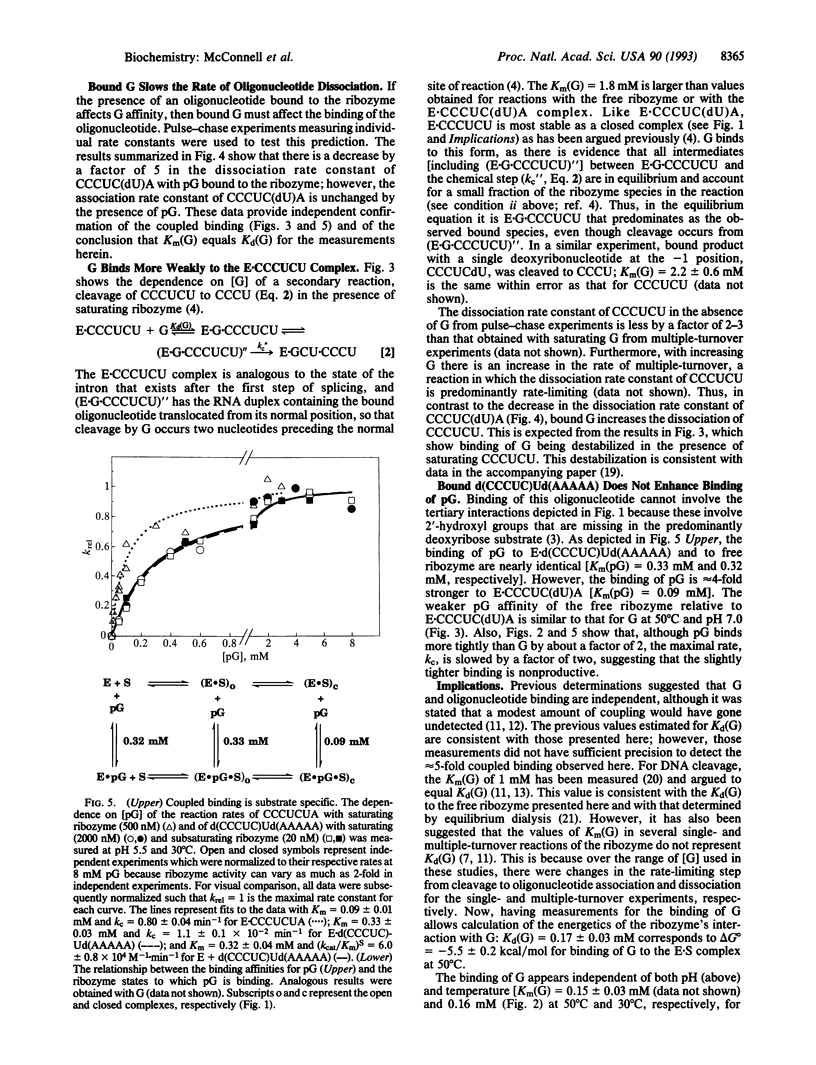
Abstract
The L-21 Sca I ribozyme derived from the group I intron of Tetrahymena thermophila pre-rRNA catalyzes an endonuclease reaction analogous to the first step of self-splicing. Guanosine (G) is bound by the ribozyme, and its 3'-hydroxyl group acts as the nucleophile. Here, we provide evidence that Km for G in several single-turnover reactions is equal to the equilibrium dissociation constant for G. This evidence includes the observation that removal of the 2'-hydroxyl group at the cleavage site of the oligoribonucleotide substrate [from CCCUCUA to CCCUC(dU)A] decreases the rate of cleavage approximately 1000-fold but has no effect on either the Km for G (0.17 mM) or for guanosine 5'-monophosphate (pG) (0.09 mM). In the course of this study, it was observed that Km for G or pG was lower by a factor of 5 for reactions with the ribozyme-CCCUC(dU)A complex compared with the free ribozyme, indicating a modest amount of thermodynamic coupled binding of the two substrates. The decrease in the rate of oligonucleotide dissociation upon addition of saturating pG provides independent support for this coupling. Coupling is lost with a substrate that cannot make the normal tertiary interactions with the ribozyme, providing evidence that coupled binding requires docking of the substrate into the catalytic core. Surprisingly, the binding of product CCCUCU and G is slightly anticooperative, indicating that the cleaved pA is important for coupling with substrate. Coupled binding suggests a splicing model in which the intron binds G tightly to promote the first step of reaction, after which its binding is an order of magnitude weaker, thereby facilitating the second step.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Been M. D., Perrotta A. T. Group I intron self-splicing with adenosine: evidence for a single nucleoside-binding site. Science. 1991 Apr 19;252(5004):434–437. doi: 10.1126/science.2017681. [DOI] [PubMed] [Google Scholar]
- Bevilacqua P. C., Johnson K. A., Turner D. H. Cooperative and anticooperative binding to a ribozyme. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8357–8361. doi: 10.1073/pnas.90.18.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua P. C., Kierzek R., Johnson K. A., Turner D. H. Dynamics of ribozyme binding of substrate revealed by fluorescence-detected stopped-flow methods. Science. 1992 Nov 20;258(5086):1355–1358. doi: 10.1126/science.1455230. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Herschlag D., Piccirilli J. A., Pyle A. M. RNA catalysis by a group I ribozyme. Developing a model for transition state stabilization. J Biol Chem. 1992 Sep 5;267(25):17479–17482. [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- Herschlag D., Cech T. R. Catalysis of RNA cleavage by the Tetrahymena thermophila ribozyme. 1. Kinetic description of the reaction of an RNA substrate complementary to the active site. Biochemistry. 1990 Nov 6;29(44):10159–10171. doi: 10.1021/bi00496a003. [DOI] [PubMed] [Google Scholar]
- Herschlag D., Cech T. R. Catalysis of RNA cleavage by the Tetrahymena thermophila ribozyme. 2. Kinetic description of the reaction of an RNA substrate that forms a mismatch at the active site. Biochemistry. 1990 Nov 6;29(44):10172–10180. doi: 10.1021/bi00496a004. [DOI] [PubMed] [Google Scholar]
- Herschlag D., Cech T. R. DNA cleavage catalysed by the ribozyme from Tetrahymena. Nature. 1990 Mar 29;344(6265):405–409. doi: 10.1038/344405a0. [DOI] [PubMed] [Google Scholar]
- Herschlag D. Evidence for processivity and two-step binding of the RNA substrate from studies of J1/2 mutants of the Tetrahymena ribozyme. Biochemistry. 1992 Feb 11;31(5):1386–1399. doi: 10.1021/bi00120a015. [DOI] [PubMed] [Google Scholar]
- Herschlag D., Piccirilli J. A., Cech T. R. Ribozyme-catalyzed and nonenzymatic reactions of phosphate diesters: rate effects upon substitution of sulfur for a nonbridging phosphoryl oxygen atom. Biochemistry. 1991 May 21;30(20):4844–4854. doi: 10.1021/bi00234a003. [DOI] [PubMed] [Google Scholar]
- Kruger K., Grabowski P. J., Zaug A. J., Sands J., Gottschling D. E., Cech T. R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982 Nov;31(1):147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- Legault P., Herschlag D., Celander D. W., Cech T. R. Mutations at the guanosine-binding site of the Tetrahymena ribozyme also affect site-specific hydrolysis. Nucleic Acids Res. 1992 Dec 25;20(24):6613–6619. doi: 10.1093/nar/20.24.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Hanna M., Green R., Bartel D. P., Szostak J. W. The guanosine binding site of the Tetrahymena ribozyme. Nature. 1989 Nov 23;342(6248):391–395. doi: 10.1038/342391a0. [DOI] [PubMed] [Google Scholar]
- Michel F., Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990 Dec 5;216(3):585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- Moran S., Kierzek R., Turner D. H. Binding of guanosine and 3' splice site analogues to a group I ribozyme: interactions with functional groups of guanosine and with additional nucleotides. Biochemistry. 1993 May 18;32(19):5247–5256. doi: 10.1021/bi00070a037. [DOI] [PubMed] [Google Scholar]
- Pyle A. M., Murphy F. L., Cech T. R. RNA substrate binding site in the catalytic core of the Tetrahymena ribozyme. Nature. 1992 Jul 9;358(6382):123–128. doi: 10.1038/358123a0. [DOI] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Ogilvie K. K., Pon R. T. Prevention of chain cleavage in the chemical synthesis of 2'-silylated oligoribonucleotides. Nucleic Acids Res. 1989 May 11;17(9):3501–3517. doi: 10.1093/nar/17.9.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., Illangesekare M., Christian E. An axial binding site in the Tetrahymena precursor RNA. J Mol Biol. 1991 Dec 20;222(4):995–1012. doi: 10.1016/0022-2836(91)90590-3. [DOI] [PubMed] [Google Scholar]
- Young B., Herschlag D., Cech T. R. Mutations in a nonconserved sequence of the Tetrahymena ribozyme increase activity and specificity. Cell. 1991 Nov 29;67(5):1007–1019. doi: 10.1016/0092-8674(91)90373-7. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Grosshans C. A., Cech T. R. Sequence-specific endoribonuclease activity of the Tetrahymena ribozyme: enhanced cleavage of certain oligonucleotide substrates that form mismatched ribozyme-substrate complexes. Biochemistry. 1988 Dec 13;27(25):8924–8931. doi: 10.1021/bi00425a008. [DOI] [PubMed] [Google Scholar]



