Abstract
Aging is associated with decreased neurogenesis in the hippocampus and diminished hippocampus-dependent cognitive functions. Expression of bone morphogenetic protein 4 (BMP4) increases with age by more than 10-fold in the mouse dentate gyrus while levels of the BMP inhibitor, noggin, decrease. This results in a profound 30-fold increase in phosphorylated-SMAD1/5/8, the effector of canonical BMP signaling. Just as observed in mice, a profound increase in expression of BMP4 is observed in the dentate gyrus of humans with no known cognitive abnormalities. Inhibition of BMP signaling either by overexpression of noggin or transgenic manipulation not only increases neurogenesis in aging mice, but remarkably, is associated with a rescue of cognitive deficits to levels comparable to young mice. Additive benefits are observed when combining inhibition of BMP signaling and environmental enrichment. These findings indicate that increased BMP signaling contributes significantly to impairments in neurogenesis and to cognitive decline associated with aging, and identify this pathway as a potential druggable target for reversing age-related changes in cognition.
Keywords: Aging, dentate gyrus, environmental enrichment, neural stem cell, novel object recognition
1. Introduction
Many cognitive functions are well preserved into old age, but impairments in memory and the rapid use of novel information are common (Bishop, et al., 2010). The dentate gyrus (DG) of the hippocampus is crucial in the integrative learning and memory functions that typically decline during aging. Increasing evidence suggests that newly generated neurons in the subgranular zone (SGZ) of the DG are vital for this cognitive functioning (Clelland, et al., 2009,Deng, et al., 2010,Marin-Burgin and Schinder, 2012,Ming and Song, 2011,Yassa and Stark, 2011,Zhang, et al., 2008), and neurogenesis declines with age in parallel with cognitive impairments (Kuhn, et al., 1996). Age-related deficits in neurogenesis and cognition can be improved with long-term exposure to running or an enriched environment (Kempermann, et al., 2002,Kronenberg, et al., 2006,van Praag, et al., 2005). Though most enhancements in neurogenesis by environmental enrichment (EE) are linked to exercise (Kobilo, et al., 2011,Mustroph, et al., 2012), EE without running has been shown to increase neurotrophic factor release and enhance synaptogenesis (Birch, et al., 2013,Zhao, et al., 2014). Further, a combination of running and EE has additive effects on neurogenesis (Fabel, et al., 2009). These studies suggest that signaling pathways that are activated by exposure to exercise or to EE can reverse the age-associated decline in neural stem/progenitor cell (NSC/NPC) proliferation in the SGZ.
Bone morphogenetic protein (BMP) signaling is one of the many pathways that are regulated by exposure to running or EE (Gobeske, et al., 2009). BMP signaling regulates NSC fate in both the developing and adult brain (Bond, et al., 2012). In the hippocampus, BMP signaling is regulated by changes in both BMP ligands and inhibitors (Bond, et al., 2014,Gobeske, et al., 2009,Mira, et al., 2010). BMP4 is the primary BMP family member in the adult hippocampus and noggin is its main inhibitor (Lim, et al., 2000,Mikawa and Sato, 2011,Mikawa, et al., 2006). BMP signaling promotes quiescence of hippocampal NSC/NPC, and inhibition of BMP signaling recruits both NSC and NPC back into cell cycle with accelerated generation of new neurons (Bond, et al., 2014). The pro-neurogenic effect of running correlates with increased expression of noggin, decreased expression of BMP4, and an overall decrease of BMP signaling in the DG (Gobeske, et al., 2009). These observations suggest a possible role for BMPs in the neurogenic deficits present in the aging brain.
Here we identify the BMP signaling pathway as a key regulator of age-related cognitive decline and demonstrate that inhibiting this pathway improves neurogenesis and hippocampus-dependent cognitive function in mice. We show that there are significant age-related increases in BMP4 expression in human and mouse hippocampus suggesting that increased BMP signaling may underlie age-related changes in neurogenesis and cognition in humans as well as mice.
2. Materials & Methods
2.1. Animals
Male C57BL/6 mice were housed in groups of 3-5 per cage, except during enrichment exposures, with food and water ad libitum in a facility with constant temperature, humidity and a 14/10-hour light/dark cycle. Experimental protocols were approved by Northwestern University's CCM and by IACUC.
2.2. Generation of Transgenic Mice and Tamoxifen Administration
Generation of BMPRII floxed mutant mice (BMPRIIfx/fx) and breeding to Ascl1-CreERTM; BMPRIIflx; RosazsGreen/+ was described previously (Beppu, et al., 2005,Bond, et al., 2014,Kim, et al., 2011). The transgenic mice were maintained with heterozygous floxed or heterozygous wild-type BMPRII and recombined cells expressed zsGreen (ZG) fluorescent reporter. Male and female mice were used and no sex-related differences in neurogenesis were observed. 4-hydroxy-tamoxifen (Sigma Aldrich #T5648, 30mg/ml solution in 10% ethanol and 90% corn oil) was administered at 10-12 months of age via intraperitoneal injections using an insulin syringe according to animal weight (180mg/kg). Five consecutive daily injections were given per animal to ensure efficient recombination, and the animals were sacrificed on the specified day after the day of initial injection. All animal procedures followed NIH guidelines and were approved by the Animal Care and Use Committee of Northwestern University.
2.3. Enrichment exposure
Up to 15 mice were housed together in special "enrichment" cages made of clear plastic (74x53x25cm) with a wire cage top providing food and water ad libitum and allowing extensive exploratory climbing. In addition to social enhancement, enrichment objects provided mice with topological and kinesthetic diversity, and were substituted piecewise with novel objects every 5-7 days. Running wheels were excluded from enrichment cages. Additional bedding material and igloo/shepherd-shack housing were also supplied (Gobeske, et al., 2009).
2.4. Protein Isolation and Western Blotting
The DG was mechanically dissociated and lysed with T-PER Protein Extraction Reagent (Pierce) with 1x Halt Protease+Phosphotase inhibitor cocktail (Thermo Scientific). Protein samples were boiled for 10 minutes in strong denaturing conditions and 7-10µg of sample was loaded into 4% – 20% sodium dodecyl sulfate – polyacrylamide gels (Bio-Rad). Proteins were transferred onto polyvinylidene difluoride membranes at 4°C for 1 h, which were then blocked in Tris-buffered saline with 0.1% Tween-20 with 5% nonfat dry milk or 5% bovine serum albumin for 1 h at room temperature. Primary antibodies were diluted in blocking solution and incubated with membranes overnight at 4°C. The membranes wer e incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000, Santa Cruz Biotechnology) and developed using SuperSignal West Pico enhanced chemiluminescent reagent (Thermo Scientific). Primary antibodies include: BMP4 (mouse IgG1, 1:1000, Origene), Noggin (rabbit polyclonal, 1:4000, Chemicon), phospho-Smad1/5/8 (rabbit polyclonal, 1:1000, Chemicon), Smad1/5/8 (rabbit polyclonal, 1:1000, Cell Signaling). Specificity of the BMP4 antibody has been demonstrated previously (Masuhara, et al., 1995), confirming no cross reactivity with TGFβ1 and BMP2, a BMP family member that shares over 90% identity with BMP4 (Feng, et al., 1994). Densitometric analysis was performed using ImageJ software and intensity was measured relative to Gapdh unless noted.
2.5. Immunohistochemistry
Mice were sacrificed with CO2 inhalation, perfused with HBSS (Lonza) and fixed with 4% paraformaldehyde (PFA) in 1X PBS. Brains were fixed for 2 hours or overnight in 4% PFA and then cryoprotected in 30% sucrose solution overnight. Each brain was either frozen and sectioned into 40µm sections on a microtome (HM 450, Microm) or embedded in Tissue-Tek OCT embedding compound (Sakura), frozen on dry ice and sliced into 20µm sections on Leica CM3050S cryostat. Tissue samples were blocked in 1X PBS with 10% normal donkey serum or 10% normal goat serum and stained with primary antibody overnight in 1X PBS with 1% bovine serum albumin (BSA) and 0.25% Triton X-100. Sections were washed with 1X PBS and treated with secondary Alexa Fluor-conjugated antibodies (Invitrogen, 1:200) and DAPI nuclear stain (Invitrogen) for 1h at room temperature. Slides were washed with 1X PBS and mounted with Prolong Gold anti-fade reagent (Invitrogen). Primary antibodies used include: GFAP (mouse IgG1, 1:1000, Sigma), doublecortin (DCX; goat polyclonal IgG, 1:500, Santa Cruz), BrdU (mouse IgG1, 1:200, BD), CldU (rat, 1:250, Accurate), Ki67 (rabbit, 1:500, Novocastra), dsRed (rabbit, 1:1000, Invitrogen), NeuN (mouse IgG1, 1:500, Chemicon) and Sox2 (polyclonal rabbit, 1:500, Chemicon), BMP4 (mouse IgG2b, 1:1000, Millipore), BMP4 (mouse IgG1, 1:50, Origene).
2.6. Thymidine Analog Administration
For each experiment, either bromodeoxyuridine (BrdU) or chlorodeoxyuridine? (CldU) was dissolved in saline and administered via intraperitoneal injection using an insulin syringe according to animal weight. CldU was administered in a single injection 24 hours and 12 hours prior to sacrifice at 42.5mg/kg. BrdU was administered for 3 days 4 times a day and every 2 hours at 50mg/kg. Due to low levels of proliferation in the aged mouse brain, 3 days of BrdU was used to ensure enough cells were labeled for accurate cell counts. Before primary antibody incubation, sections stained for BrdU or CldU were incubated in 10mM sodium citrate, 0.05% Tween 20, pH 6 for 20 minutes at 95°C and then were allowed to cool for 30 minutes at room temperature. Sections were washed in PBS and then incubated in 2N HCl for 30 minutes at room temperature. Sections were washed in 0.1M sodium tetraborate, pH 8.5 for 10 minutes, washed in PBS and continued normal immunohistochemistry protocol.
2.7. Human Tissue Preparation
Tissue samples were graciously provided by the Northwestern Cognitive Neurology & Alzheimer’s Disease Center (CNADC) Neuropathology Core. The samples all came from non-demented patients at the time of death. PFA-fixed wet human hippocampal tissue was sectioned on a microtome (HM 450, Microm) and stained as described previously. The samples were coded so that the subject was unknown during the analysis of the tissue.
2.8. Confocal Imaging and Quantification
All images were taken on a Leica SP5 Confocal Microscope using sequential scanning to prevent false-positive co-localization. Four or more images of the dentate gyrus were taken at 40x magnification per 40μm brain section, and 4 brain sections spanning 200 μm surrounding the coordinates used for viral injection (see description blow) were imaged and used for quantification. In viral injection experiments, only sections with visible viral infection were considered for quantification. Images were given random identifier by another lab member to ensure counts were blinded to conditions. ImageJ software was then used to track cells across multiple optical sections of a z-series and to discriminate individual cell staining. Granule cell layer volume was calculated by manual selection of DAPI dense dentate regions in all four imaged sections using ImageJ and used as the denominator for per volume quantification. Counts for Ki67, BrdU, CldU and NPC/NSC were only in the SGZ region while all other counts represent positive cells within the granule cell layer.
2.9. Viral Plasmids and Injections
Plasmid design and construction is described previously (Bond, et al., 2014). Briefly, pBOB-IRES2-mCherry (LV-Control), pBOB-secBMP4-IRES2-mCherry (LV-BMP4) and pBOB-secNoggin-IRES2-mCherry (LV-Noggin) were created to manipulate BMP signaling. Viruses were stereotaxically injected into the DG of 2-, 9- or 12-month-old male C57Bl/6 mice. Mice were anesthetized using isofluorane gas and secured in a Kopf stereotaxic frame (Model 900). After the skull was exposed, burr holes were drilled over the dentate gyrus and 2µl of virus was injected bilaterally using a MicroSyringe Pump Controller (Micro4, World Precision Instruments) fit with a pulled glass micropipette. Dentate gyrus coordinates from bregma: −2mmAP, ±1.5mmML, −2.5mmDV for 9- and 12-month-old mice and −2mmAP, ±1.5mmML, −1.9mmDV for 2-month-old mice. The lentivirus preferentially infects the SGZ and post-analysis was used to determine level of infection and remove uninfected samples.
2.10. Infusion
Infusion experiments were previously described (Gobeske, et al., 2009). C57BL/6 mice aged 2 months or 20 months were anesthetized with isoflurane gas and secured in a Kopf stereotaxic frame. Burr holes were drilled at −0.5mm and 1.0mm lateral to bregma, and an Alzet brain infusion cannula was then placed within the lateral ventricle. The Alzet micro-osmotic pumps (#1002) were pre-loaded with vehicle or noggin and attached to the cannula with a 1.5cm plastic catheter. Pumps were implanted into a subcutaneous pocket extending caudally from the initial incision. Each pump delivered noggin (R&D) at 50 ng/µl or vehicle for 14 days at 0.25µl/hr. After 3, 6, 13 and 15 full days of infusion, cognitive testing was performed as described below.
2.11. Novel Object Recognition/Novel Location Recognition
NOR testing in mice was slightly modified from Hashimoto, et al (Hashimoto, et al., 2005). The NOR apparatus consisted of an open box made of Plexiglas (52 cm L; 52 cm W; 31 cm H) with white walls and a solid floor. The box was positioned approximately 30 cm above the floor centered on a table such that the overhead lights could not provide a spatial cue. The animals were habituated to the empty NOR arena, as a group, for one hour, on each of the three days prior to the acquisition trial. During the acquisition trial, the mice were allowed to explore two identical objects (for e.g. A1 and A2) for 10 min, followed by a 6-hour inter-trial interval. During the retention trial, the mice were allowed to explore the familiar object (A) from the acquisition trial and a novel object (for e.g. B). Objects were made of hard plastic and cylindrical (4in × 2in × 2in) and were tested previously to maximize exploration time. The position of the novel object in the retention trial was randomly assigned for each mouse tested using a pseudorandom schedule to reduce the effects of object and place preference. Behavior was recorded on video for blind scoring of object exploration. Object exploration was defined as an animal licking, sniffing, or touching the object with the forepaws while sniffing. The exploration time (s) of each object in each trial was recorded manually by the use of two stopwatches, and if the mice failed to explore for <1(s) in both acquisition and retention trials, they were excluded from the analysis. The discrimination index (DI) [(time spent exploring the novel object - time spent exploring the familiar object)/total exploration time] was then calculated for retention trials.. For the novel location recognition test, one of the familiar objects (A) was moved to a new location within the arena, and mice were re-introduced to the field for the retention trial after 2.5 hours. During the retention trial, mice were allowed to explore the arena until amassing a cumulative 30 seconds of object-exploration time. Mice have a predilection for new items in their environment, so the percentage of time at the novel object or location indicates the animal's degree of memory for the familiar object or location.
2.12. Spontaneous alternation Y-maze
The Y-maze consists of three radial arms measuring 60cm in length, 18cm deep, 4cm wide at the bottom and 14cm wide at the top, providing no proximal visual landmarks inside the maze but allowing a view surrounding distal navigational cues. Mice were placed into the end of one arm, and were allowed to explore the maze freely for 5-6 minutes, amassing at least 15 arm entries. Arm entries were counted when the mouse's hind paws completely left the central hub and the mouse had taken two steps down the chosen arm. The efficiency of exploration was measured from the ratio of entry triads (3 consecutive arm entries) that contain entries into all three arms, divided by the total number of possible triads (total number of arm entries, minus 2).
2.13. Statistical Analyses
Group analyses were evaluated with one-way ANOVA, followed by Tukey post-hoc test using the PRISM software (GraphPad). Where appropriate, unpaired two-tailed Student’s t-tests were performed. P-values are noted throughout the text and data are presented as means ± SEM.
3. Results
3.1. Neurogenesis and hippocampus-dependent cognition decline with age
We first examined baseline markers of neurogenesis and hippocampus-dependent cognitive function at various ages (2-18 months) of C57BL/6 mice. Consistent with previous reports, we found significant age-related decreases both in neurogenesis (Fig. S1A,B) and in performance on tests of hippocampus-dependent cognitive function including the Y-Maze and Novel Object Recognition (NOR) tests (Fig. S1C,D). We observed that the decline in cognition did not occur until neurogenesis was greatly compromised, similar to what has been observed previously in other studies (Deng et al, 2010). This may reflect the ability to maintain behavioral performance until a certain threshold of cellular impairment is reached as well as the type of behavioral testing that was performed (Deng et al, 2010).
3.2. BMP signaling increases with age in mouse and human DG
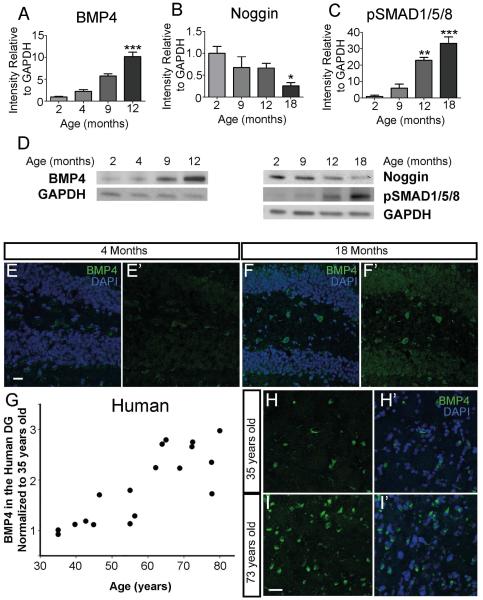
Decreased neurogenesis and impaired performance on hippocampus-dependent cognitive tests are observed with aging (Lazarov, et al., 2010). Since BMP signaling inhibits neurogenesis in the hippocampus (Bond, et al., 2014), we hypothesized that age-related decreases in neurogenesis might reflect changes in the BMP signaling pathway. We therefore examined BMP4 expression by western analysis of microdissected DG tissue at various ages and found a 5-fold increase in expression in 9 month old compared to 2 month old mice and a 10-fold increase by 12 months (Fig. 1A,D). In contrast, western analysis of noggin revealed a 40% decline in levels of expression at 9 and 12 months compared to 2 month old mice and a decline of more than 75% in the 18 month old DG (Fig. 1B,D). We observed no change in BMP4 and noggin mRNA levels, suggesting that changes at the protein level may reflect altered protein synthesis or turnover with age (Fig. S2D,E). Levels of phosphorylated-SMAD1/5/8 (pSMAD1/5/8), a measure of downstream BMP signaling, increased 5-fold in the DG by 9 months, more than 20-fold by 12 months, and more than 30-fold by 18 months of age (Fig. 1C,D). This increase is also present when pSMAD1/5/8 is compared to levels of SMAD1/5/8, which remains consistent throughout aging (Fig. S2). The increase of BMP signaling correlated closely with the age-related decline in behavioral performance (Fig. S1C,D). Immunohistochemical analysis of BMP4 showed that expression in the 18 month old mouse DG is markedly increased compared to a 4 month old mouse (Fig. 1E-F’). Thus there was a profound age-related increase in BMP signaling in the mouse DG.
Figure 1.
BMP signaling increases with age in both mouse and human DG. (A) Protein analyses of mouse DG at various ages show a 10-fold increase in BMP4 by 12-months of age (n=4; 2 months=0.97±0.12; 4 months=2.26±0.39; 9 months=5.77±0.47; 12 months=10.18±1.0). (B) Western blot analyses of mouse DG show noggin protein expression is reduced by more than 60% in 18 month old compared to 2 month old mice (n=4; 2 months=1.0±0.15; 9 months=0.68±0.24; 12 months=0.66±0.1; 18 months=0.25±0.079). (C) Protein analyses of the same samples analyzed show that levels of pSMAD1/5/8, increase more than 30-fold by 18 months compared to 2 month old mice (n=4; 2 months=1.0±0.81; 9 months=6.0±2.55; 12 months=22.99±1.8; 18 months=33.27±3.9). (D) Representative western blots at various ages of BMP4, pSMAD1/5/8 and noggin protein compared to GAPDH loading control. (E,F) IHC analysis of BMP4 shows an increase in immunoreactivity in the DG of a 18 month old mouse (F,F’) compared to a 4 month old mouse (E,E’). (G) IHC was performed on hippocampus from cognitively normal humans ranging in age from 35 to 80 years old. BMP4 expression exhibits an age-related increase, similar to what was observed in the mouse. Samples were normalized to 35-years-old (n=18). (H,I), Co-localization of BMP4 (green) and DAPI (blue) in the cognitively normal human DG is increased in a 73-year-old sample (I,I’) compared to a 35-year-old sample (H,H’). Scale bar=10µm. All data analyzed by one-way ANOVA and differs from control by Tukey post hoc test unless noted. All data are presented as means±SEM. Protein graphs show expression levels normalized to Gapdh and relative to protein expression in 2 month old mice. *p<0.05,**p<0.01,***p<0.001.
Given the BMP4 increase in the aged mouse DG, we next examined BMP4 expression by immunohistochemistry (IHC) in DG samples from non-demented humans of different ages. A 2-3 fold increase in the number of BMP4 immunoreactive cells was present between the ages of 35 and 65 (Fig. 1G). Though the number of human tissue samples that were obtained and analyzed is small (n=18), there is an apparent jump of BMP4 immunoreactivity occurring between 55-65 years old. A clear increase in BMP4 staining is observed in the 73-year-old DG compared to the 35-year-old DG (Fig. 1H,I).
3.3. Increased BMP signaling impairs hippocampus-dependent memory
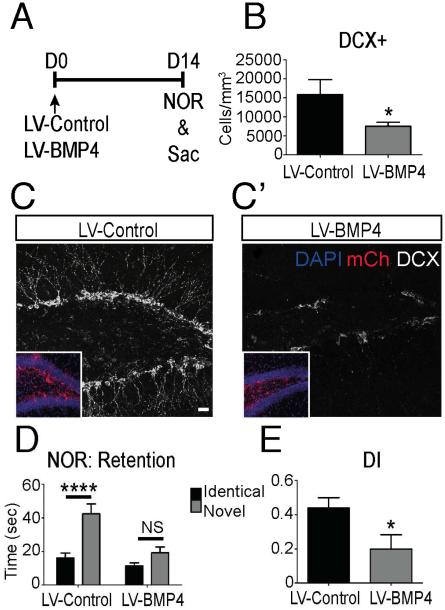
Overexpression of BMP4 in the DG of adult mice results in a sustained decrease in neurogenesis (Bond, et al., 2014). To determine whether a BMP4-mediated decrease in neurogenesis is associated with cognitive impairments similar to those observed aging, a mCherry fluorescent protein tagged BMP4-expressing lentivirus (LV-BMP4) or control virus (LV-Control) was injected bilaterally into the DG of 2 month old mice (Fig. 2A). Virus infection efficiency and specificity were confirmed by mCherry expression in the SGZ (Fig. 2C,C’ inset), and quantification of mCherry-expressing cell types demonstrated that hippocampal NSC/NPCs account for majority of the infected cells (Bond, et al., 2014)data not shown). Examination of DG at 14 days post viral injection revealed that overexpression of BMP4 impaired proliferation as observed in a more than 50% reduction of newly generated neurons (DCX+ cells; Fig. 2B-C’). Before sacrifice, cognition was analyzed with the NOR test. A significant preference for the novel object was observed in LV-Control mice in the retention trial, whereas LV-BMP4 mice had minimal preferential interest in the novel object (Fig. 2D). The discrimination index (DI), which is a measure of the ratio of time spent exploring each object, was markedly decreased in LV-BMP4 mice compared to LV-Control mice (Fig. 2E). Thus increased BMP4 signaling in the DG impairs not only new granule neuron generation but also hippocampal dependent cognition, suggesting that the age-related increase in BMP signaling could mediate the age-related declines in both neurogenesis and cognition.
Figure 2.
Modulation of BMP signaling in the DG impairs neurogenesis and cognitive function. (A) Experimental paradigm. LV-BMP4 or LV-Control was stereotaxically injected into the DG of 2-month-old mice at D0. Behavior analysis was performed on D14 and mice were then sacrificed for IHC analysis. (B) Quantification of neuroblasts (DCX+) in the SGZ of LV-BMP4 mice is significantly reduced at D14 compared to LV-Control. (n=7-8 mice; LV-Control=15884±3927; LV-BMP4=7502±1091; n=7-8 mice). (C,C’) A decline in DCX+ immunoreactivity (Walker, et al.) is observed in LV-BMP4 infected brains compared to LV-Control brains. Virus infection is labeled by mCherry fluorescence (red, inset). (D) Time spent exploring the novel object was significantly higher in the LV-Control group during the NOR retention trial, while the LV-BMP4 group showed little preference for the novel object (LV-Control: Identical=16, Novel=42, p=0.0007; LV-BMP4: Identical=11, Novel=19, p=0.054; two-tailed t-test; n=10-11 mice). (E) The Discrimination index (DI) was significantly higher in the LV-Control group compared to the LV-BMP4 group, indicating a greater preference for the novel object. (n=10-11 mice; LV-Control=0.4383±0.06; LV-BMP4=0.1980±0.08, p=0.031; two-tailed t-test; n=10-11). Scale bar = 50µm. All data analyzed by two-tailed t-test and is presented as mean±SEM. *p < 0.05, ****p<0.0001, ns=not significant.
3.4. Inhibiting BMP signaling in the aged brain increases proliferation and improves hippocampus-dependent cognitive function
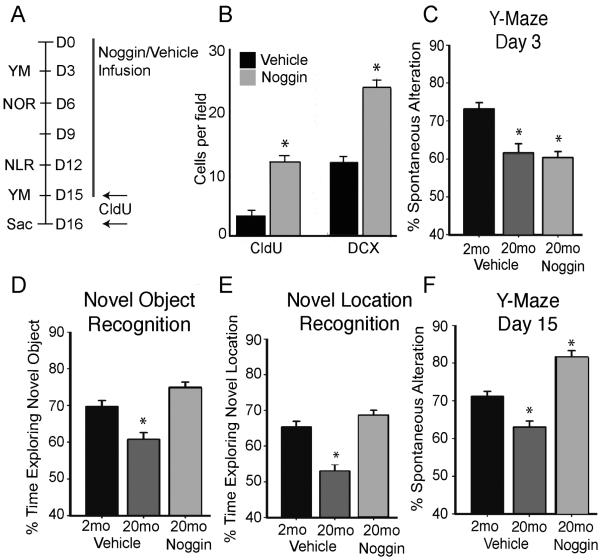
To directly test whether the increase in BMP signaling underlies age-related impairments in neurogenesis and cognition, we used intraventricular infusion of noggin (Gobeske et al, 2009; Mira et al, 2010) to block BMP signaling in the aging brain. Alzet minipumps were implanted into 2 and 20 month old mice for 15 days of either noggin or vehicle infusion (Fig. 3A). Infusion of noggin into 20 month old mice resulted in a 3-fold increase in both proliferation as measured by thymidine analog incorporation (CldU+) and generation of new neurons (DCX+ cells) compared to vehicle infused aged mice (Fig. 3B). Evaluation of behavior using the Y-maze at Day 3 (D3) exhibited the expected impairment in old compared to young mice (Fig. 3C). Notably there was no difference between the aged noggin and control groups at this baseline examination. However by D15, the noggin-infused aged mice had markedly improved performance on the Y-Maze, exceeding performance not only of vehicle-infused aged mice but also the 2 month old controls (Fig. 3F). Cognitive performance examined on D6 using the NOR test and on D12 using novel location recognition (NLR) test also revealed improved performance in noggin-infused aged group compared to vehicle-infused aged group (Fig. 3D-E). Thus inhibition of BMP signaling reversed age-related decreases in both neurogenesis and cognition.
Figure 3.
Inhibition of BMP signaling in the aged brain rescues age-related behavioral deficits. (A) Experimental design. Noggin or vehicle was infused into the lateral ventricles of young (2 month) or old (20 month) mice for 15 days. Mice received a series of hippocampus-dependent cognitive tests at various times during the experiment and were sacrificed for IHC at D16. (B) IHC analysis of DG showed a significant 3-fold increase in proliferating cells (CldU+) in aged mice that received infusions of noggin compared to old mice treated with vehicle. CldU was administered ip 24 hours before sacrifice and the number of CldU+ cells per field was counted. The number of neuroblasts (DCX+) per field in the SGZ increased significantly in old mice infused with noggin compared to mice infused with vehicle (analyzed by two-tailed t-test, *p<0.05, n=5-7). (C) Initial behavior analysis 3 days after initiation of noggin infusion was performed using the Y-Maze task. A behavior deficit is exhibited in both vehicle and noggin treated old mice compared to vehicle treated young mice. Percent of spontaneous alteration measures the likelihood of each mouse to enter a different arm of the maze over total possible different arm entries. (D) Infusion of noggin for 6 days into old mice improves behavior performance on the NOR task to levels comparable to a vehicle-treated young mouse. Measured by percent of time exploring the novel object during the retention trial. (E) After 12 days of noggin infusion behavior was analyzed using the NLR task. Percent of total time exploring the novel location shows a rescue of behavioral performance in the noggin-treated aged mice to a level equivalent to vehicle-treated young mice. (F) After D15 of noggin infusion in 20 month old mice, performance on the Y-Maze task, measured by % spontaneous alteration, improves significantly over vehicle-treated aged mice. Strikingly, behavioral performance of aged mice that received noggin infusions surpassed performance of vehicle-treated young mice. Figure 2C-F analyzed by one-way ANOVA and differs from control by Tukey post hoc test, n=5-7 mice, *p<0.05.
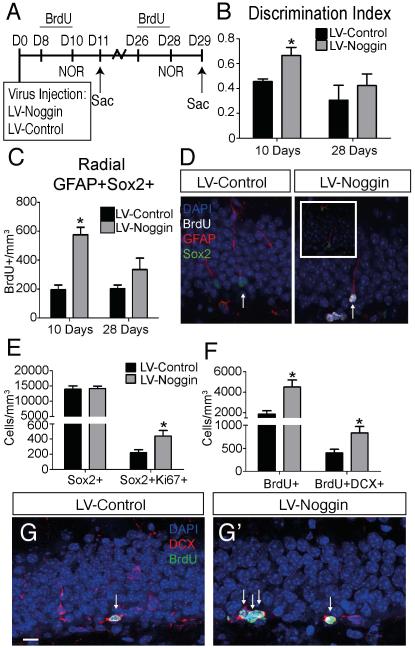
Because intraventricular infusion of noggin also could have effects outside the hippocampus, we examined effects of stereotaxic injection of lentivirus that overexpresses a secreted form of noggin (LV-Noggin) into the DG of aged mice. Cognitive function and neurogenesis was examined at D11 or D29 (Fig. 4A). BrdU was administered intraperitonealy for three consecutive days (D7-10 in the first group and D26-28 in the second group) before sacrifice. Again, virus infection was confirmed by mCherry expression and we found noggin overexpression significantly increased the DI score on the NOR test at D10 (Fig. 4B) and increased BrdU incorporation by ~40% on D11 (LV-Control=3139±455.7; LV-Noggin=4711±527.0; p=0.0469, two-tailed t-test). Though the total number of NSCs (radial GFAP+Sox2+) remained the same between groups (Fig. S3), inhibition of BMP signaling was able to enhance the number of activated NSCs (BrdU+) at D11 (Fig.4C,D). In addition, rate of reentry into quiescence as measured by BrdU+Ki67-Sox2+ divided by the total number of BrdU+ cells revealed a significant decrease in the LV-Noggin condition (12%) relative to the control (24%) (Fig. S3C). Thus noggin overexpression in the DG increased both cognitive function and neurogenesis, consistent with findings after intraventricular infusion of noggin. To determine whether the increase in NSC/NPC proliferation after noggin overexpression was preserved, we extended the experiment to D29. At this time BrdU incorporation more than doubled with noggin overexpression indicating a sustained effect on NCS/NPC proliferation (Fig. 4F). Further, BrdU incorporation into proliferating neuroblasts (DCX+ cells) was more than doubled by noggin overexpression (Fig. 4F-G’). Though noggin overexpression did not alter the total number of SOX2 immunoreactive NPCs at D29, it increased the number of SOX2+ cells in cell cycle as evidenced by co-expression of Ki67 (Fig. 4E).
Figure 4.
Inhibiting BMP signaling in the aged SGZ expands proliferating populations of cells and improves hippocampus-dependent cognitive function. (A) Experimental design. LV-Noggin or LV-Control was stereotaxically injected into DG of 12 month old mice at D0. Behavior performance was evaluated at D10 and animals were sacrificed on D11 and D29 for IHC analysis. (B) Cognition was analyzed using the NOR task at D10 and D28. Inhibition of BMP signaling with LV-Noggin significantly improved behavior at D10 (LV-Control=0.45±0.02; LV-Noggin=0.66±0.06; p=0.0342, n=7-9), but these benefits were not sustained to D28 (LV-Control=0.306±0.106; LV-Noggin=0.423±0.076; p=0.48; n=7-9). (C) A significant increase in activated NSCs (radial GFAP+Sox2+BrdU+ cells) is observed after 10 days in the LV-Noggin group (LV-Control=196.05±31.95, LV-Noggin=576.07±52.02, p=0.024). This increase is reduced at 28 days, though a slight increase in activated NSCs is still present in the LV-Noggin condition (LV-Control=199.78±28.26; LV-Noggin=334.35±79.92; p=0.25). (D) Immunohistochemistry at 10 days shows a proliferating BrdU+ (Walker, et al.) NSC (radial GFAP+Sox2+, arrow) in LV-Noggin condition. (E) At D28, the number of NSCs/NPCs (Sox2+) remains unchanged between groups (LV-Control=12368±980.9; LV-Noggin=14147±824.4; p=0.87; n=7-9 mice). NPCs in cell cycle (Sox2+Ki67+) are expanded in LV-Noggin mice D28 (LV-Control=220.3±46.3; LV-Noggin=438.2±73.2; p=0.0302; n=7-9 mice). (F) Proliferating cells (BrdU+) remain increased D28 in the LV-Noggin condition compared to LV-Control (LV-Control=1867±329.4; LV-Noggin=4501±507.9 p=0.0263; n=4-5 mice). The population of proliferating neuroblasts (DCX+BrdU+) expands 2-fold in LV-Noggin compared to LV-Control (LV-Control=400.9±77.7; LV-Noggin=832.7±119.5; p=0.0423; n=4-5 mice). (G) Co-expression of BrdU (green) and DCX (red) identifies proliferating neuroblasts (arrows) in the LV-Control (G) and LV-Noggin (G’) mice. Scale bar=10µm. All data analyzed by two-tailed t-test and is presented as mean±SEM. **p < 0.01, ****p<0.0001.
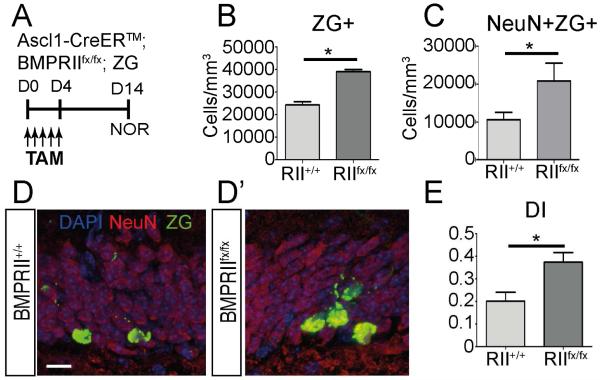
To exclude the possibility that LV-noggin altered hippocampal neurogenesis and behavior through non-cell autonomous effects by non-NSC/NPC cell types, we next examined transgenic mice carrying conditional ablation of BMP receptor type 2 (BMPRII) in Ascl1-positive NSC/NPCs (Battiste, et al., 2007,Ganat, et al., 2006). 10-12 month old Ascl1-CreERTM;BMPRIIflx; ROSAzsGreen/+ (Ascl1-RII cKO) mice were given Tamoxifen for 5 days to induce recombination at BMPRII and ROSA loci, and both neurogenesis and cognition were analyzed at 14 days (Fig. 5A). Expression of zsGreen (ZG) fluorescent protein marked recombined progeny of Ascl1 expressing NSC/NPCs, and was used to visualize cellular changes that resulted from BMP receptor ablation. The total number of recombined cells in the SGZ (ZG+) nearly doubled following ablation of BMP signaling (BMPRIIfx/fx; Fig. 5B). More importantly, we observed an expansion of recombined cells that had matured into newly generated neurons (ZG+NeuN+) in the BMPRIIfx/fx condition compared to the wild-type controls (BMPRII+/+; Fig. 5C-D’). While both BMPRIIfx/fx and BMPRII+/+ mice were able to learn on the NOR task, ablation of BMP signaling in Ascl1 NSC/NPCs enhanced their performance as seen on the DI score (Fig. 5E). These findings suggest that inhibition of BMP signaling specifically in NSC/NPCs results in an increase in neurogenesis as well as an improvement in hippocampal dependent cognitive behavior in aged mice.
Figure 5.
Ablation of BMP signaling in NPCs improves neurogenesis and hippocampus-dependent behavior in young mice. (A) Experimental paradigm. 10-12 month old Ascl1-CreERTM;BMPRIIfx/fx; ROSAzsGreen/+ (Ascl1-RII cKO) mice were administered Tamoxifen for 5 days and proliferation and hippocampus-dependent behavior were analyzed after 14 days.(B) Quantification of recombined zsGreen+ (ZG+) cells indicates an increase in proliferating cells with loss of BMP signaling (BMPRIIfx/fx) compared to wild type (BMPRII+/+; BMPRII+/+ =13593.75±2024.7; BMPRIIfx/fx=24529.89±3225.22; p=0.047; n=5-7). (C) Quantification of the recombined cells that developed into mature neurons (NeuN+ZG+) shows a 2-fold increase in those cells with loss of BMP signaling compared to wild type (BMPRII+/+=9720.5±1732.6; BMPRIIfx/fx = 20785.82±3795.1; p=0.045; n=5-7). (D) IHC analysis shows an increase in mature neurons (NeuN; red) that are ZG+ (green) in the BMPRIIfx/fx condition (D’) compared to wild type (D). (E) Hippocampus-dependent behavior was analyzed using the NOR task. The discrimination index (DI) is increased in the BMPRIIfx/fx condition indicating enhanced learning with ablation of BMP signaling (BMPRII+/+=0.201±0.0398; BMPRIIfx/fx=0.375±0.041; p=0.023; n=5-9). Scale bar=10µm. All data were analyzed by two-tailed t-test and are presented as means±SEM. n=4-5, **p < 0.01, ****p<0.0001.
3.5. Environmental enrichment combined with inhibition of BMP signaling increases neurogenesis and reverses behavior impairments in the aged mouse
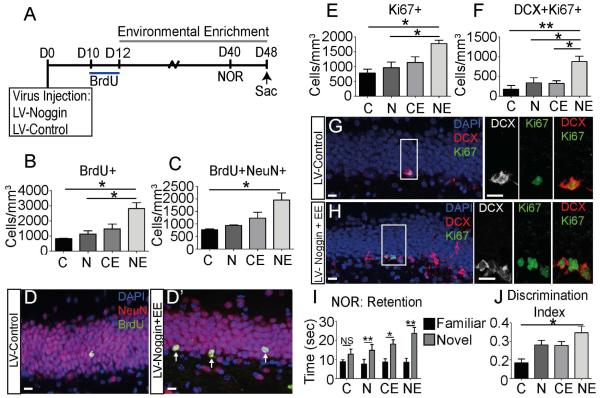
While LV-Noggin administration in aged mice resulted in an improvement in neurogenesis and cognitive ability at D10, the number of dividing NSCs was not significant increased (Fig. 4C) and minimal improvement was observed on the NOR task at D28 (Fig. 4B). Similarly, BMP inhibition in young mice increased neurogenesis at D7 and D14, but by D28 no differences were observed (Bond et al., 2014). These findings led us to hypothesize that blocking BMP signaling increases the proliferating and cycling NSCs/NPCs, but there is an impediment in the integration of these newly generated cells into existing circuitry. Exposure to EE has been shown increase integration and synaptogenesis of newly generated neurons through regulation of neurotrophic factors, (Birch, et al., 2013,Clemenson, et al., 2015,Kempermann, et al., 1997,Olson, et al., 2006) and together the stimulatory effects of exercise and EE on neurogenesis and cognition are additive (Fabel, et al., 2009). We therefore examined whether effects of EE and inhibition of BMP signaling are additive in aged mice (Fig. 6). Mice injected with LV-Noggin or LV-Control were housed in standard or enriched conditions and were evaluated with the NOR test on D40. A pulse of BrdU was given at D10 and persistence of labeled cells was examined at D48. In accordance with published findings, we did not observe significant changes in label retaining cells in the EE alone condition (Fig. 6B,C). However, Noggin combined with EE increased the number of surviving BrdU+ cells (Fig. 6B). Similarly overexpression of noggin with EE increased the number of BrdU labeled neurons (NeuN+BrdU+ cells), indicating enhanced neurogenesis or survival (Fig. 6C-D). The overall number of cells in cell cycle (Ki67+) also was increased in mice exposed to both LV-noggin and EE compared to all other groups, suggesting enhanced cell division (Fig. 6E). Further, the number of neuroblasts in cell cycle (DCX+Ki67+) was increased in mice with noggin expression and EE compared all other groups (Fig. 6F-H). Thus inhibition of BMP signaling combined with EE stimulated neurogenesis more than either one alone.
Figure 6.
Inhibition of BMP signaling combined with EE provides additive benefits in NSC/NPC proliferation and cognition. (A) Experimental design. LV-Noggin or LV-Control was stereotaxically injected into DG of 9 month old mice at D0. BrdU injections were given at D10 to mark dividing cells, a time point where expansion of the proliferating population was seen previously. At D12, LV-Noggin and LV-Control mice were divided into EE (LV-Control+EE or LV-Noggin+EE) or standard housing (LV-Control or LV-Noggin) groups. Behavior performance was evaluated using the NOR task at D40 and mice were sacrificed for IHC at D48. (B) The number of surviving BrdU+ cells that had incorporated BrdU at the time of labeling on D10 is increased in the LV-Noggin, LV-Control+EE and LV-Noggin+EE compared to the LV-Control group at D48. (LV-Control=825±27.39; LV-Noggin=1133±209.3; LV-Control+EE=1467±324.6; LV-Noggin+EE=2819±394.1). (C) The number of BrdU+ neurons (NeuN+BrdU+) is significantly increased at D48 in the LV-Noggin+EE group compared to LV-Control. (LV-Control=765.4±32.15; LV-Noggin=941.8±18.14; LV-Control+EE=1234±231.4; LV-Noggin+EE=1953±288.6). (D) Co-localization of NeuN (red) and BrdU (green) is increased in LV-Noggin+EE (D’) compared to LV-Control (D;arrows). (E) The total population of cells in cell cycle (Ki67+) at the point of sacrifice is expanded in LV-Noggin+EE compared to LV-Control and LV-Noggin at D48 (LV-Control=778±133.6; LV-Noggin=960±192.2; LV-Control+EE=1138±191.2; LV-Noggin+EE=1775±112.9). (F) The pool of neuroblasts in cell cycle (Ki67+DCX+) is significantly increased in LV-Noggin+EE compared to all other groups at D48. (LV-Control=178±93.7; LV-Noggin=337±132; LV-Control+EE=323±68.03; LV-Noggin+EE=878±134.6). (G,H) Neuroblasts in cell cycle co-localize Ki67 (green) with DCX (red, white) in LV-Noggin+EE (H) and LV-Control (G). High power images show co-localization in the SGZ. (I) Hippocampus-dependent cognitive function was evaluated using the NOR task at D40. Time spent exploring the novel object is higher in all groups during the retention trail (two-tailed t-test). (J) Performance on the NOR task is assessed using the discrimination index (DI). The DI is significantly increased in LV-Noggin+EE compared to LV-Control. (LV-Control=0.17±0.04; LV-Noggin=0.36±0.05; LV-Control+EE=0.36±0.04; LV-Noggin+EE=0.49±0.07). Scale bar=10µm. All data analyzed by one-way ANOVA and differs from control by Tukey post hoc test unless noted. All data are presented as means±SEM, n=4-5, *p<0.05,**p<.01, ns=not significant. Abbreviations: LV-Control (C), LV-Noggin (N), LV-Control+EE (CE), LV-Noggin+EE (NE).
Similar additive effects were observed on cognition, as analyzed with the NOR test. During the retention trial, mice preferred the novel object in the LV-Noggin (p<0.01), LV-Control+EE (p<0.05) and LV-Noggin+EE (p<0.001) groups but not in the LV-Control group (Fig. 6I). The combination of BMP inhibition and EE significantly increased the time the animals explored the novel object compared to all other groups. The DI increased in all groups compared to the LV-Control group and was significantly increased in the group that combined both BMP inhibition and EE (Fig. 6J). Thus the combined effects of BMP inhibition and EE on both neurogenesis and cognition were additive, analogous to the combined effects of exercise and EE. These data suggest that targeting the BMP pathway specifically along with exposure to an enriched environment can rescue aging deficits in both neurogenesis and cognition.
4. Discussion
Development of techniques for preventing or reversing age-related impairments in neurogenesis and cognition would greatly improve quality of life as the population ages. BMP signaling has been identified as a negative regulator of NSC/NPC proliferation (Bonaguidi, et al., 2008,Bond, et al., 2014,Gobeske, et al., 2009,Mira, et al., 2010). Here we observe changes in the BMP signaling pathway with age that establish a mechanism for age-related impairments in both neurogenesis and cognition. A robust increase in BMP4 expression, observed in both the human and mouse DG, coupled with a simultaneous decrease in noggin expression resulted in a profound increase in total BMP4 signaling. This was illustrated by a 30-fold increase in pSMAD1/5/8 expression in the mouse DG. Other BMP family members are known to be expressed in the developing and adult hippocampus and some have been implicated in aging related neural degenerative diseases (Li, et al., 2008,Tang, et al., 2009). The BMP4 antibody we used has been validated not to cross react with BMP2, a BMP family member that shares 90 percent amino acid identity (Masuhara, et al., 1995). While BMP5, 6 and 7 are also expressed in the rodent hippocampus, they share less than 60% sequence identity with the immunogen used for the BMP4 antibody, therefore not detected in our western blot or immunohistochemistry. Further examination of other BMP expression during aging is needed to determine whether similar increase is observed. Overexpression of BMP4 in the DG of young mice was sufficient to diminish both neurogenesis and hippocampus-dependent cognition. Conversely, blocking BMP signaling in the aged mouse, both by overexpression of noggin or by transgenic manipulation, increased neurogenesis in the SGZ and was associated with restored hippocampus-dependent cognitive function. Though the regulatory mechanism for the increase in BMP signaling is yet to be determined, it is interesting that an increase in BMP signaling has similar effects on aging hair follicles (Genander, et al., 2014). In toto our findings indicate that increases in BMP signaling contribute to the impairments in neurogenesis and cognition that are associated with aging.
The extent to which reduced hippocampal neurogenesis contributes to aging associated cognitive decline is not fully understood. Studies have shown that aged rodents display significantly different cognitive abilities with similar levels of decline in hippocampal neurogenesis, suggesting that hippocampal neurogenesis is not the sole contributing factor for age associated cognitive decline (Bizon, et al., 2004). We observed a decline in both neurogenesis and cognitive performance, though the timing does not directly correlate. A possible explanation could be that there are mechanisms in place that maintain behavioral performance until a certain threshold of neurogenic decline is reached (Deng et al, 2010). However, Increasing evidence indicates a causal relationship between neurogenesis and spatial learning in young adult mice (Deng, et al., 2010,Marin-Burgin and Schinder, 2012). A number of studies have used experimental manipulation of neurogenesis in the SGZ to effect learning and memory (Clelland, et al., 2009,Deng, et al., 2009,Jessberger, et al., 2009,Saxe, et al., 2006,Shors, et al., 2001). Further, previous work in our laboratory showed that infusion of the anti-mitotic drug cytosine arabinoside (AraC) into the ventricles of adult mice was able to block the benefits of BMP signaling inhibition, indicating that proliferating NSCs/NPCs are required for the enhancement of neurogenesis and cognition through manipulation of BMP signaling (Gobeske, et al., 2009). In this study, the causal relationship between BMP regulated hippocampal neurogenesis and hippocampal dependent cognition is further supported by the ablation of BMPRII in Ascl1+ NSCs/NPCs, which increase DG neurogenesis in a cell autonomous fashion and led to cognitive behavioral improvements in aged mice. The functional relationship between neurogenesis and behavior remains controversial, however together with previous studies, our data indicate that enhanced neurogenesis with inhibition of BMP signaling is associated with an improvement in hippocampus-dependent cognition.
Though the number of detectable NSC clearly declines precipitously with age, it has been unclear whether this reflects a permanent depletion of NSCs (Bonaguidi, et al., 2011,Encinas, et al., 2011,Furutachi, et al., 2013) or increased quiescence of a pool that nevertheless can be reactivated. This is a critical question since depletion of the stem cell pool would indicate that the neurogenic loss with age is irreversible. However, our findings suggest that an increase in BMP signaling with age, which promotes quiescence in multiple NPC subtypes in the SGZ (Bond, et al., 2014), silences proliferation, and that inhibiting this signaling is sufficient to activate NSCs/NPCs. Inhibition of BMP signaling was accomplished experimentally by three different techniques, ventricular infusion of noggin, viral mediated expression of noggin in the DG, and Ascl1+ NSC specific ablation of the BMP receptor II subunit. Though the total number of NSCs/NPC (Sox2+) in the SGZ remained the same, expression of noggin in the aged DG expanded the actively cycling population of NSCs/NPCs (Sox2+Ki67+). However, the lack of quiescent NSC markers hindered our ability to determine whether this increase reflects reactivation of quiescent NSCs. Ablation of BMPRII in Ascl1+ NSCs/NPCs suggests that actively dividing stem and progenitors remain responsive to changes in BMP signaling in aged mice, and the absence of BMP signaling in these cells and their progeny is sufficient to improve hippocampal dependent cognition.
Exercise leads to an increase in neurogenesis in the SGZ associated with improvements in cognition (Deng, et al., 2009,van Praag, et al., 2005). At least some of the neurological benefits of exercise are due to decreased BMP signaling in the hippocampus (Gobeske, et al., 2009). Interestingly, our data together with previously published work showed an increase in the number of multiple NPC populations with inhibition of BMP signaling from 7 to 14 days, along with improved hippocampal cognition at 14 days. Since inhibition of BMP signaling accelerates division of late DG progenitors including DCX+ neuroblasts in young mice (Bond, et al., 2014), it is possible that a wave of rapidly generated granule neurons could contribute to the short term improvements in cognitive function in aged mice. This idea is also supported by our behavioral analysis of aged Ascl1-RII cKO mice, which also demonstrated rapid cognitive behavior improvements at 14 days post initial Tamoxifen injection. While development from a radial NSC to a fully matured granule neuron in the adult SGZ takes approximately 28 days (Toni and Sultan, 2011), the 14 day time point correlates with the initial establishment of GABAergic synaptic connections of newly born neurons (Esposito, et al., 2005,Ge, et al., 2006). The process may be further accelerated by BMP inhibition as it affects cell division of both early and late NPCs.
We did not observe changes in the number of newly generated neurons and cognitive behavior with BMP inhibition at 28 days (Bond, et al., 2014; Figure 4). This observation led us to hypothesize that though inhibition of the BMP pathway amplifies proliferating NSC/NPCs, additional signaling is required for survival of newly generated neurons. Though most of the benefits to neurogenesis with EE can be attributed to exercise (Kobilo, et al., 2011,Mustroph, et al., 2012), EE without running enhances incorporation of newly generated neurons into existing circuitry through increased neurotrophic factor release and synaptogenesis (Birch, et al., 2013,Clemenson, et al., 2015,Fabel, et al., 2009). Enhanced performance on a number of behavior tasks is also observed following EE without running (Birch, et al., 2013,Clemenson, et al., 2015). To this end, we found that the combination of BMP signaling inhibition with EE led to a significant increase in the number of newly generated neurons, suggesting an effect on progenitor expansion and/or neuronal survival. Similarly, we found that modulating BMP signaling, which mimics the benefits of exercise, and exposing aging mice to EE had additive effects on cognition. Together, these data indicate that, regardless of the specific role of EE on neurogenesis, inhibition of the BMP pathway combined with exposure to EE may offer a viable therapeutic approach to age-related declines in hippocampus-dependent cognitive functions.
Increases in BMP4 expression were observed in the cognitively normal aged human as well as mouse DG. Multiple BMP family members have been studied in a number of age-related neurodegenerative diseases (Chen, et al., 2003,Crews, et al., 2010,Li, et al., 2008). Notably, hippocampus levels of BMP6 rise and levels of noggin fall in models of Alzheimer’s disease (Tang, et al., 2009) suggesting that some of the early changes in cognitive function could reflect perturbation of this critical signaling system. We have shown an increase in pSMAD1/5/8 expression with age that occurs downstream of BMP4 signaling. It is also possible that non-canonical signaling through the p38 MAPK pathway may have aging-related effects.
Together, our findings identify BMP signaling as a possible druggable target for reversing normal age-related declines in neurogenesis and cognitive function and possibly for ameliorating symptoms of disorders that lead to declines in cognition. In conclusion, BMP signaling increases in the aged mouse brain and mediates aging-related impairments observed in neurogenesis and cognition. Inhibition of BMP signaling in the aged hippocampus reverses impairments in both neurogenesis and cognition and these effects are further enhanced with exposure to an enriched environment. These findings together with an observed increase in BMP signaling in the human brain identify BMP signaling is a promising druggable target for impairments occurring with aging.
Supplementary Material
Highlights.
BMP signaling increases with age in the mouse and human dentate gyrus
Blocking BMP signaling expands proliferating NSC/NPC populations in the aged SGZ
Inhibition of BMP signaling in the aged hippocampus rescues cognitive deficits
Blocking BMP signaling with EE are additive to improve neurogenesis and cognition
6. Acknowledgements
This work was supported by the National Institutes of Health grant NS 20778 (to JAK), grant P30 AG013854 and in part by an Alzheimer’s Disease Core Center grant (P30 AG013854) from the National Institute on Aging to Northwestern University, Chicago Illinois. We gratefully acknowledge the assistance of the Neuropathology Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Disclosure Statement
The authors report no actual or potential conflict of interest.
7. Author Contributions
E.A.M: conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing; K.T.G: collection and assembly of data and data analysis and interpretation; A.M.B. & C-Y.P: collection and assembly of data, data analysis and interpretation, and manuscript writing; J.C.J: data collection; J.A.K: Conception and design, manuscript writing and final approval of manuscript.
8. References
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134(2):285–93. doi: 10.1242/dev.02727. doi:10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Beppu H, Lei H, Bloch KD, Li E. Generation of a floxed allele of the mouse BMP type II receptor gene. Genesis. 2005;41(3):133–7. doi: 10.1002/gene.20099. doi:10.1002/gene.20099. [DOI] [PubMed] [Google Scholar]
- Birch AM, McGarry NB, Kelly AM. Short-term environmental enrichment, in the absence of exercise, improves memory, and increases NGF concentration, early neuronal survival, and synaptogenesis in the dentate gyrus in a time-dependent manner. Hippocampus. 2013;23(6):437–50. doi: 10.1002/hipo.22103. doi:10.1002/hipo.22103. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–35. doi: 10.1038/nature08983. doi:10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging cell. 2004;3(4):227–34. doi: 10.1111/j.1474-9728.2004.00099.x. doi:10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA. Noggin expands neural stem cells in the adult hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(37):9194–204. doi: 10.1523/JNEUROSCI.3314-07.2008. doi:10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145(7):1142–55. doi: 10.1016/j.cell.2011.05.024. doi:10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Developmental neurobiology. 2012;72(7):1068–84. doi: 10.1002/dneu.22022. doi:10.1002/dneu.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AM, Peng CY, Meyers EA, McGuire T, Ewaleifoh O, Kessler JA. BMP signaling regulates the tempo of adult hippocampal progenitor maturation at multiple stages of the lineage. Stem cells. 2014 doi: 10.1002/stem.1688. doi:10.1002/stem.1688. [DOI] [PubMed] [Google Scholar]
- Chen HL, Lein PJ, Wang JY, Gash D, Hoffer BJ, Chiang YH. Expression of bone morphogenetic proteins in the brain during normal aging and in 6-hydroxydopamine-lesioned animals. Brain research. 2003;994(1):81–90. doi: 10.1016/j.brainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–3. doi: 10.1126/science.1173215. doi:10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemenson GD, Lee SW, Deng W, Barrera VR, Iwamoto KS, Fanselow MS, Gage FH. Enrichment rescues contextual discrimination deficit associated with immediate shock. Hippocampus. 2015;25(3):385–92. doi: 10.1002/hipo.22380. doi:10.1002/hipo.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, Hansen L, Masliah E. Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(37):12252–62. doi: 10.1523/JNEUROSCI.1305-10.2010. doi:10.1523/JNEUROSCI.1305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature reviews Neuroscience. 2010;11(5):339–50. doi: 10.1038/nrn2822. doi:10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(43):13532–42. doi: 10.1523/JNEUROSCI.3362-09.2009. doi:10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell stem cell. 2011;8(5):566–79. doi: 10.1016/j.stem.2011.03.010. doi:10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(44):10074–86. doi: 10.1523/JNEUROSCI.3114-05.2005. doi:10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Frontiers in neuroscience. 2009;3:50. doi: 10.3389/neuro.22.002.2009. doi:10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Harris MA, Ghosh-Choudhury N, Feng M, Mundy GR, Harris SE. Structure and sequence of mouse bone morphogenetic protein-2 gene (BMP-2): comparison of the structures and promoter regions of BMP-2 and BMP-4 genes. Biochim Biophys Acta. 1994;1218(2):221–4. doi: 10.1016/0167-4781(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Furutachi S, Matsumoto A, Nakayama KI, Gotoh Y. p57 controls adult neural stem cell quiescence and modulates the pace of lifelong neurogenesis. The EMBO journal. 2013;32(7):970–81. doi: 10.1038/emboj.2013.50. doi:10.1038/emboj.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(33):8609–21. doi: 10.1523/JNEUROSCI.2532-06.2006. doi:10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–93. doi: 10.1038/nature04404. doi:10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M, Cook PJ, Ramskold D, Keyes BE, Mertz AF, Sandberg R, Fuchs E. BMP Signaling and Its pSMAD1/5 Target Genes Differentially Regulate Hair Follicle Stem Cell Lineages. Cell stem cell. 2014 doi: 10.1016/j.stem.2014.09.009. doi:10.1016/j.stem.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, Kessler JA. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PloS one. 2009;4(10):e7506. doi: 10.1371/journal.pone.0007506. doi:10.1371/journal.pone.0007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Shimizu E, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur J Pharmacol. 2005;519(1-2):114–7. doi: 10.1016/j.ejphar.2005.07.002. doi:10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr., Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learning & memory. 2009;16(2):147–54. doi: 10.1101/lm.1172609. doi:10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Annals of neurology. 2002;52(2):135–43. doi: 10.1002/ana.10262. doi:10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–5. doi: 10.1038/386493a0. doi:10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE. Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PloS one. 2011;6(3):e18472. doi: 10.1371/journal.pone.0018472. doi:10.1371/journal.pone.0018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learning & memory. 2011;18(9):605–9. doi: 10.1101/lm.2283011. doi:10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiology of aging. 2006;27(10):1505–13. doi: 10.1016/j.neurobiolaging.2005.09.016. doi:10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(6):2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends in neurosciences. 2010;33(12):569–79. doi: 10.1016/j.tins.2010.09.003. doi:10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Tang J, Xu H, Fan X, Bai Y, Yang L. Decreased hippocampal cell proliferation correlates with increased expression of BMP4 in the APPswe/PS1DeltaE9 mouse model of Alzheimer's disease. Hippocampus. 2008;18(7):692–8. doi: 10.1002/hipo.20428. doi:10.1002/hipo.20428. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28(3):713–26. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Marin-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behavioural brain research. 2012;227(2):391–9. doi: 10.1016/j.bbr.2011.07.001. doi:10.1016/j.bbr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Masuhara K, Nakase T, Suzuki S, Takaoka K, Matsui M, Anderson HC. Use of monoclonal antibody to detect bone morphogenetic protein-4 (BMP-4) Bone. 1995;16(1):91–6. doi: 10.1016/s8756-3282(94)00014-x. [DOI] [PubMed] [Google Scholar]
- Mikawa S, Sato K. Noggin expression in the adult rat brain. Neuroscience. 2011;184:38–53. doi: 10.1016/j.neuroscience.2011.03.036. doi:10.1016/j.neuroscience.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Mikawa S, Wang C, Sato K. Bone morphogenetic protein-4 expression in the adult rat brain. The Journal of comparative neurology. 2006;499(4):613–25. doi: 10.1002/cne.21125. doi:10.1002/cne.21125. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. doi:10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, Colak D, Gotz M, Farinas I, Gage FH. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell stem cell. 2010;7(1):78–89. doi: 10.1016/j.stem.2010.04.016. doi:10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. doi:10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–60. doi: 10.1002/hipo.20157. doi:10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17501–6. doi: 10.1073/pnas.0607207103. doi:10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–6. doi: 10.1038/35066584. doi:10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Tang J, Song M, Wang Y, Fan X, Xu H, Bai Y. Noggin and BMP4 co-modulate adult hippocampal neurogenesis in the APP(swe)/PS1(DeltaE9) transgenic mouse model of Alzheimer's disease. Biochemical and biophysical research communications. 2009;385(3):341–5. doi: 10.1016/j.bbrc.2009.05.067. doi:10.1016/j.bbrc.2009.05.067. [DOI] [PubMed] [Google Scholar]
- Toni N, Sultan S. Synapse formation on adult-born hippocampal neurons. The European journal of neuroscience. 2011;33(6):1062–8. doi: 10.1111/j.1460-9568.2011.07604.x. doi:10.1111/j.1460-9568.2011.07604.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(38):8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. doi:10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TL, White A, Black DM, Wallace RH, Sah P, Bartlett PF. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(20):5240–7. doi: 10.1523/JNEUROSCI.0344-08.2008. doi:10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in neurosciences. 2011;34(10):515–25. doi: 10.1016/j.tins.2011.06.006. doi:10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451(7181):1004–7. doi: 10.1038/nature06562. doi:10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Jou J, Wolff LJ, Sun H, Gage FH. Spine morphogenesis in newborn granule cells is differentially regulated in the outer and middle molecular layers. The Journal of comparative neurology. 2014;522(12):2756–66. doi: 10.1002/cne.23581. doi:10.1002/cne.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.