Abstract
Objectives
IL-13 is a cytokine classically produced by anti-inflammatory T-helper-2 lymphocytes; it is decreased in the circulation of type 2 diabetic patients and impacts positively on liver and skeletal muscle. Although IL-13 can exert positive effects on beta-cell lines, its impact and mode of action on primary beta-cell function and survival remain largely unexplored.
Methods
Beta-cells were cultured for 48 h in the presence of IL-13 alone or in combination with IL-1β or cytokine cocktail (IL-1β, IFNγ, TNFα).
Results
IL-13 protected human and rat beta-cells against cytokine induced death. However, IL-13 was unable to protect from IL-1β impaired glucose stimulated insulin secretion and did not influence NFκB nuclear relocalization induced by IL-1β. IL-13 induced phosphorylation of Akt, increased IRS2 protein expression and counteracted the IL-1β induced regulation of several beta-cell stress response genes.
Conclusions
The prosurvival effects of IL-13 thus appear to be mediated through IRS2/Akt signaling with NFκB independent regulation of gene expression. In addition to previously documented beneficial effects on insulin target tissues, these data suggest that IL-13 may be useful for treatment of type 2 diabetes by preserving beta-cell mass or slowing its rate of decline.
Keywords: Beta-cells, Apoptosis, Cytokines, Gene expression, Akt
Highlights
-
•
IL-13 decreases human beta-cells apoptosis.
-
•
IL-13 protects primary beta-cells form cytokine induced apoptosis.
-
•
The IRS2/akt pathway mediates IL-13 protective effects.
-
•
IL-13 modulates the expression of genes involved in stress response.
1. Introduction
A decrease in functional beta-cell mass is a key feature of type 2 diabetes [1], [2]. Increasing evidence indicates that alterations in the balance between anti- and pro-inflammatory cytokines may play a role in the pathogenesis not only of type 1 but also type 2 diabetes in humans and may possibly underlie decreased beta-cell function/mass in the latter condition. Type 2 diabetes is thus associated with chronic low-grade inflammation and immune cell infiltration in islets, though this occurs at a lesser degree than in islets from type 1 diabetic patients [3]. Additionally, it is known that islet endocrine cells are able to produce certain cytokines, both pro- and anti-inflammatory [4], [5]. The detrimental effects of pro-inflammatory pathways triggered by T-helper-1 (Th1) cytokines (e.g. IL-1β, TNFα, IFNγ) in islets have been widely studied and are implicated in beta-cell failure [6]. By contrast, the effects of Th2 cytokines (e.g. IL-4 and IL-13) on islets and more specifically on beta-cells have been investigated only in few studies [7] and mainly on transformed rodent beta-cell lines.
IL-13 is secreted by activated Th2 cells and classified as an anti-inflammatory cytokine due to its ability to suppress the secretion of several macrophage and monocyte-derived inflammatory cytokines. IL-13 mediates its effects by interacting with a complex receptor system comprised of IL-4Rα and IL-13Rα1 with downstream activation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling cascade and the PI3kinase pathway through recruitment of insulin receptor substrate members [8], [9].
IL-13 plays a key role in the regulation of hepatic glucose production in mice [10]. Moreover, IL-13 plasma levels are reduced in type 2 diabetic patients compared to healthy individuals and IL-13 exerts an autocrine effect on glucose metabolism in skeletal muscle [11]. Most recently, it has been found that IL-13 is induced in adipose tissue of obese humans and high-fat fed mice, and that the source of such IL-13 is primarily adipocytes [6].
IL-13 is able to impact positively on transformed rodent beta-cell lines and on intact rat islets [12], [13], [14]. Indeed, IL-13 increases Rinm5F cell viability and improves that of INS1E cells following serum starvation or when challenged with palmitate. IL-13 has also been found to counteract the suppression of rat islet glucose oxidation induced by IL-1β [14].
Against this background, we wanted to explore the impact of IL-13 on primary human or rat beta-cell function and survival and the mechanism underlying these putative effects. Our study shows for the first time that IL-13 protects primary beta-cells against IL-1β and mixed cytokine induced cell death without affecting proliferation or insulin secretion. These effects are mediated through the IRS2/Akt signaling cascade, and IL-13 further regulates the expression of a small number of specific genes involved in the beta-cell response to stress, albeit independently of NFκB.
2. Materials and methods
2.1. Human islets, sorted beta-cells
Human islets were kindly provided by the Cell Isolation and Transplant Centre of the University of Geneva (JDRF award 31-2008-413, ECIT Islet for Basic Research Program). Human islets were dispersed by Acutase (PAA Laboratories, Austria) and beta-cells were sorted by FACS after labeling with Newport Green using a FACSVantage (Becton Dickinson, Franklin Lakes, NJ, USA) as previously described [15].
For functional analysis and immunostaining, human islets, non sorted single cells or sorted beta-cells were cultured in CMRL-1066 medium containing 5 mM glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamax, 250 μg/ml gentamycin, 10 mM Hepes and 10% fetal calf serum (FCS) (Invitrogen, Switzerland) on plastic dishes coated with extracellular matrix secreted by 804G rat bladder cancer cells (804G ECM), as described elsewhere [15] and were left in islet medium for 24 h to adhere and spread before initiation of the experiments.
2.2. Rat islets, sorted beta-cells
Islets were isolated by collagenase digestion of pancreas from adult male Wistar rats. Beta-cells were separated from non beta-cells by autofluorescence using a FACSVantage as described previously [15]. Islet dispersed cells, sorted beta-cells and sorted non beta-cells were cultured in DMEM medium containing 11 mM glucose, 0.05 mg/ml gentamicin, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% FCS (Invitrogen, Switzerland). Cells were cultured on 804G ECM-coated plates as described elsewhere [15] and were left for 24 h to adhere and spread before initiation of the experiments.
2.3. Western blotting
Beta-cell protein lysate was separated by SDS-PAGE, transferred to Immobilon-P membranes (Merck Millipore, Germany) and probed with anti-NFkB and anti-phospho-NFkB (p65) (Santa Cruz Biotechnology, USA), anti-IRS2, Akt1, Akt2, anti-phospho Akt (Serine 473) (Cell Signaling Technology, USA) and anti-actin (Merck Millipore, Germany).
2.4. RNA extraction, library preparation, sequencing, mapping and expression quantification
Total RNA was prepared according to the manufacturer's instructions (ReliaPrep RNA Cell Miniprep System, Promega, USA). PolyA + RNA library construction and sequencing were performed as described elsewhere [16]. The 49 bp paired-ends reads were mapped with gemtools v1.7.1 [17], [18] onto the Rnor_5.0 genome and onto the Rnor_5.0.73 gene annotation. A maximum of 5 mismatches was allowed for the alignment and reads having a mapping quality score below 150 were filtered out.
The differential expression analysis was performed with the DESeq2 software [19] by taking into account the batch and the GC content of each sample.
2.5. Insulin secretion
For acute insulin release in response to glucose, islets and beta-cells were preincubated for 2 h (2.8 mM glucose) and incubated in Krebs–Ringer bicarbonate Hepes buffer, 0.5% BSA (KRB) containing 2.8 mM glucose for 1 h followed by 1 h incubation in KRB containing 16.7 mM glucose. Total insulin was extracted with 0.18 M HCl in 70% ethanol for determination of insulin content. Insulin was measured by radioimmunoassay.
2.6. Immunofluorescence, cell proliferation and cell death
Dispersed islet cells or sorted beta-cells were treated for 48 h with IL-13 (Preprotech, USA) in the continued presence of BrdU. The incorporated BrdU was detected with the BrdU-detection kit (Roche, Switzerland) and beta-cells were co-stained with anti-insulin antibody (Dako, Denmark). The free 3-OH strand breaks were detected by the terminal deoxynucleotidyl-transferase-mediated deoxyuridine 5-triphosphate nick-end labeling (TUNEL) technique according to the manufacturer's instructions (In situ cell death detection kit, Roche, Switzerland). The cells were co-stained with either an anti-insulin or an anti-glucagon antibody (Dako, Denmark). NFκB was localized by an anti-NFκB p65 antibody (Santa Cruz Biotechnology, USA), and beta-cells appeared co-stained with an anti-insulin antibody. The numbers of TUNEL-positive or BrdU-positive cells, or cells with nuclear localization of NFκB were determined by blinded counting with a fluorescent microscope. For each condition in each individual experiment, over 1000 cells were analyzed.
2.7. Immunofluorescence and confocal microscopy
Primary antibodies were as follows: rabbit anti-phospho (Y397) FAK pAb and mouse anti-paxillin monoclonal (BD Transduction Laboratories (San Jose, CA, USA)); secondary antibodies were as follows: donkey anti-rabbit Alexa Fluor 488 and donkey anti-mouse Alexa Fluor 555 (Invitrogen, Switzerland). To visualize F-actin, Alexa Fluor 647-phalloidin was used (Invitrogen, Switzerland). Immunofluorescence and confocal microscopy on sorted beta-cells were performed as previously described [20]. The basal membranes of cells were observed under a Zeiss LSM510 Meta confocal microscope using a 63x oil immersion lens. Images were acquired and processed using the LSM510 software (Carl Zeiss AG, Germany).
2.8. Statistical analyses
Data are expressed as means ± SEM, with the number of individual experiments presented in the figure legends. All data were tested for normality and analyzed with PRISM (GraphPad, USA). Differences were evaluated using Student's t test and ANOVA with Bonferroni post hoc test for multiple comparison analysis. Significance was set as p < 0.05.
3. Results
3.1. IL-13 decreases beta-cell apoptosis without affecting insulin secretion and proliferation
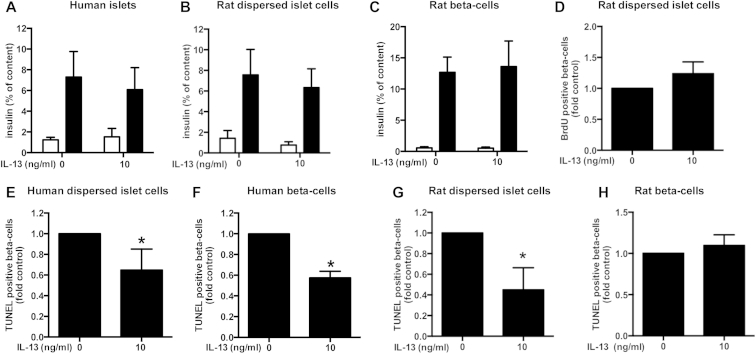
We first analyzed the impact of IL-13 on the function (insulin content and secretion) of human islets, rat dispersed islet cells and sorted rat beta-cells. Neither insulin secretion (Figure 1A,B and C) nor cellular insulin content (data not shown) was significantly influenced by IL-13 in any cell preparation. We also investigated the impact of IL-13 on rat beta-cell proliferation (note that this was not possible using adult human beta-cells since they do not replicate to any meaningful extent in vitro [21]). Replication was assessed by BrdU incorporation over 48 h. As for insulin secretion, IL-13 did not affect rat beta-cell proliferation (Figure 1D).
Figure 1.
IL-13 decreases human and rat beta-cell death. Human islets, human or rat dispersed islets or sorted human or rat beta-cells were cultured for 48 h with IL-13 (10 ng/ml). A–C: Insulin secretion. A: Human islet insulin secretion: 2.8 mM glucose (open bars), 16.7 mM glucose (closed bars); n = 5. Rat dispersed islet cells (B; n = 5) or sorted beta-cells (C; n = 7) insulin secretion: 2.8 mM glucose (open bars), 16.7 mM glucose (closed bars). D: Rat beta-cell proliferation. BrdU-positive beta-cells in dispersed rat islet cells (normalized to control = 9.98 ± 1.93% BrdU-positive beta-cells); n = 5. E-H Beta-cell death. E: TUNEL-positive beta-cells among dispersed human islet cells (normalized to control = 0.47 ± 0.1% TUNEL-positive beta-cells); n = 5. F: TUNEL-positive sorted human beta-cells (normalized to control = 1.06 ± 0.32% TUNEL-positive beta-cells); n = 4. G: TUNEL-positive beta-cells in dispersed rat islet cells (normalized to control = 0.12 ± 0.03% TUNEL-positive beta-cells); n = 3. H: TUNEL-positive sorted rat beta-cells (normalized to control = 0.06 ± 0.02% TUNEL-positive beta-cells); n = 5. *p < 0.05 vs. control as tested by Student's t-test.
By contrast, IL-13 significantly decreased death of human beta-cells under standard culture conditions (“basal” cell death) independently of the presence of other islet cell types (human dispersed islet cells (Figure 1E) versus sorted beta-cells (Figure 1F). Using rat primary islet cells, IL-13 was able to decrease basal cell death of beta-cells within a population of dispersed (non-sorted) rat islets (Figure 1G). It was not possible to observe any significant effect of IL-13 on death of sorted rat beta-cells under basal culture conditions due to the very low level of dying cells in the control situation (0.06 ± 0.02% TUNEL-positive cells, Figure 1H).
3.2. IL-13 induces Akt phosphorylation and upregulates IRS2
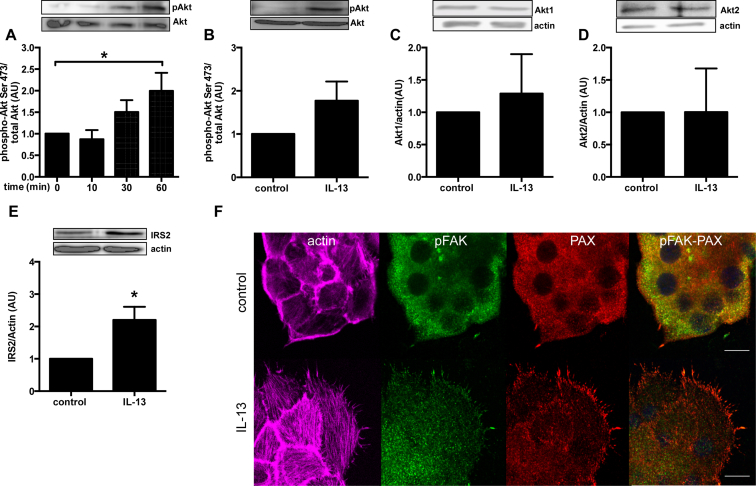
To investigate the putative pathway(s) involved in IL-13 decreased beta-cell apoptosis, we measured Akt phosphorylation, a central player in beta-cell survival [22], in sorted primary rat beta-cells since human material is not readily available and to avoid noise from non beta-cells. Already after 60 min treatment, IL-13 was able to increase Akt serine 473 phosphorylation (Figure 2A). This effect tended to be maintained after 48 h IL-13 treatment (Figure 2B), albeit without achieving statistical significance, and there was no change in protein expression of either Akt1 or Akt2 isoforms (Figure 2C, D). Interestingly, IRS2 total protein expression was increased after 48 h treatment with IL-13 (Figure 2E).
Figure 2.
IL-13 induces Akt phosphorylation, increases IRS2 protein expression and induces actin and focal adhesion remodeling in primary sorted rat beta-cells. Rat sorted beta-cells were cultured with IL-13 (10 ng/ml) for the indicated times. Phosphorylation and total protein levels were quantified by western blot. A: Akt serine-473 phosphorylation after 10–60 min exposure to IL-13; B: Akt serine-473 phosphorylation after 48 h exposure to IL-13; C: Akt1 total protein after 48 h exposure to IL-13; D: Akt2 total protein after 48 h exposure to IL-13. E. IRS2 total protein after 48 h exposure to IL-13. n = 4. F: Primary β-cell cells were cultured in complete medium for 48 h ± IL-13 (10 ng/ml). Cells were subsequently fixed and stained for actin (with phalloidin, purple), pY397FAK (green) and PAX (red). All images are fully representative of three independent experiments. *p < 0.05 vs. control as tested by ANOVA followed by Bonferroni post hoc test (A) or Student's t-test (E).
It has been previously demonstrated that actin and focal adhesion (FA) remodeling are essential for insulin secretion. Indeed, glucose stimulation led to bundling of actin filaments, with FAK and PAX activation by phosphorylation and their localization in filopodial extensions called FAs in beta-cells [23]. However, in embryonic fibroblasts, FAK has been also demonstrated to be involved in cell survival via NF-κB and ERK signaling pathways [24]. We have treated rat primary beta-cells with IL-13 for 48 h and observed the actin and FA remodeling by confocal microscopy (Figure 2F). IL-13 induced an important remodeling of actin in thick filament and increase the number of protrusions at basal cell membrane containing FAK phosphorylated form and paxillin. Moreover, western blot analyses of paxillin protein expression revealed an increase level of this protein upon IL-13 treatment (1.24 ± 0.07 (AU) fold over control). IL-13-induced adhesion could be involved in beta-cell survival observed in Figure 1.
3.3. IL-13 increases the expression of 4 beta-cell genes
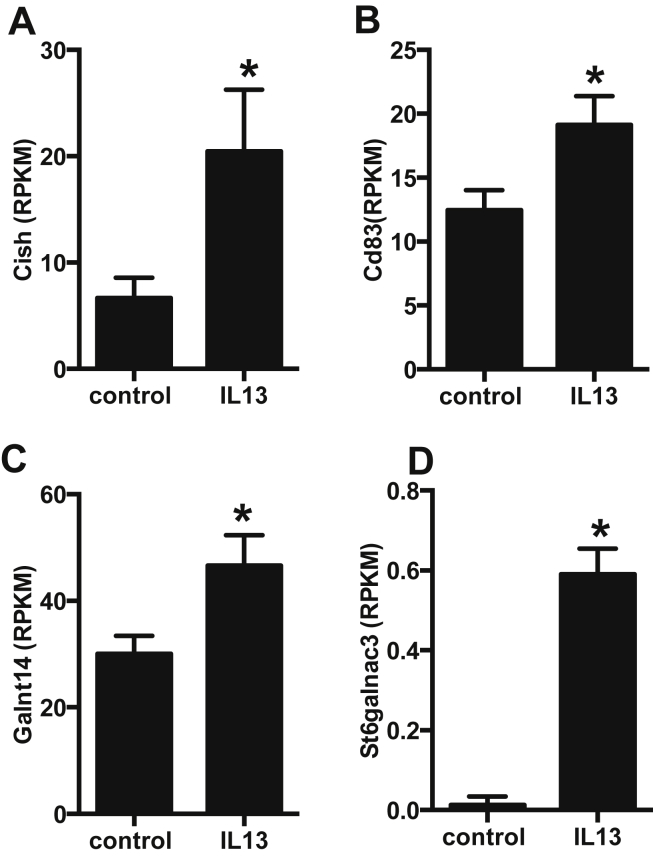
The transcriptome analysis of rat beta-cells treated for 48 h with IL-13 revealed only 5 genes that were differentially expressed compared to control cells. Among these 5 genes, one was a pseudogene. Interestingly, IL-13 upregulated Cish and Cd83 gene expression (Figure 3A,B), two genes involved in cytokine response and antigen presentation. Cish is part of a classical negative feedback system that regulates cytokine signal transduction [25]. Cd83 is involved in antigen presentation and single-nucleotide polymorphisms (SNPs) in this gene have been shown to be associated with type 1 diabetes [25]. The two other genes upregulated by IL-13, Galnt14 and St6galnac3 (Figure 3C,D), are transferases that have so far never been investigated in beta-cells or in the context of diabetes.
Figure 3.
IL-13 increases expression of 4 beta-cell genes. Sorted rat primary beta-cells were cultured for 48 h ± IL-13 (10 ng/ml) and their transcriptome profiled by RNAseq. A: Cish; B: Cd83; C: Galnt14; D: St6galnac3. Data for respective levels of mRNA are presented as reads per kilobase per million mapped reads (RPKM), n = 3. *p < 0.05 vs. control, dseq2 adjusted p value.
3.4. IL-13 protects beta-cells from IL-1β induced cell death but not from the associated impairment of glucose-stimulated insulin secretion
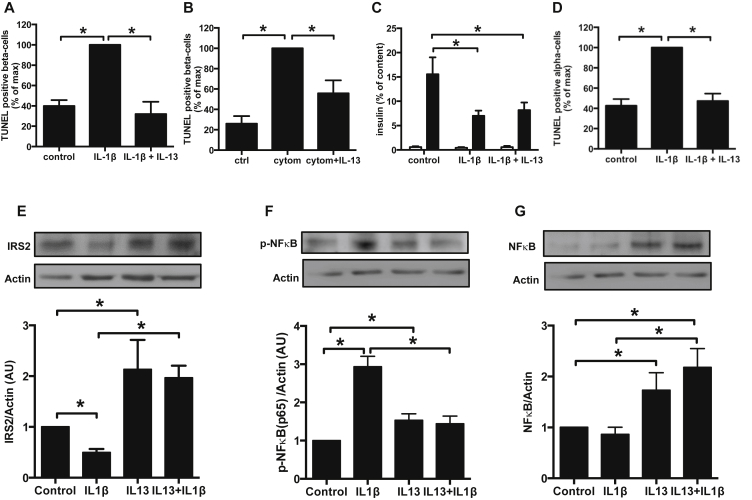
As mentioned earlier, in our experimental approach (cells coated on ECM) basal level of apoptosis in sorted rat beta-cells is almost non-detectable (<0.1% TUNEL-positive cells). Therefore, sorted rat beta-cells were exposed to IL-1β alone or in combination with IFNγ and TNFα, known to be cytotoxic towards beta-cells and believed to be implicated in decreased beta-cell function and mass in diabetic states. IL-13 was able to protect beta-cells from IL-1β induced death alone (Figure 4A) or in combination with IFNγ and TNFα (Figure 4B). However, IL-13 was unable to preserve beta-cell glucose stimulated insulin secretion impaired by 48 h treatment with IL-1β (Figure 4C).
Figure 4.
Protection by IL-13 against cytokine-induced islet cell death. A–G: Sorted rat primary beta-cells or alpha-cells were treated for 48 h with IL-13 (10 ng/ml) ± IL-1β (20 ng/ml) or cytomix (IL-1β + TNFα + INFγ, each 20 ng/ml). A: TUNEL-positive rat sorted beta-cells; n = 3. B: TUNEL-positive rat sorted beta-cells; n = 3. C: Rat beta-cell insulin secretion: open bars 2.8 mM glucose; closed bars 16.7 mM glucose; n = 4. D: TUNEL-positive alpha-cells in a population of sorted rat islet non-beta cells; n = 3. E–G: Phosphorylation and total protein expression were quantified by western blot. E: IRS2 total protein expression (n = 4). F: NFkB p65 phosphorylation (n = 4). G: NFkB total protein expression (n = 4). *p < 0.05 as tested by ANOVA followed by Bonferroni post hoc test.
We show here also that IL-1β induced alpha-cell death in primary rat non beta-cells (containing over 75% alpha-cells) after FACS sorting (Figure 4D). Interestingly, alpha-cells were also protected by IL-13 from IL-1β induced death.
IL-13 treatment prevents the impact of IL-1β on IRS2 protein expression (Figure 4E) and decreases NFκB p65 phosphorylation induced by IL-1β stimulation (Figure 4F). Moreover IL-13 exposure induces an increase of NFκB (p65) protein expression similar to what was observed for IRS2 (Figure 4G).
3.5. IL-13 counter-regulates IL-1β gene expression without affecting the nuclear translocation of NFκB
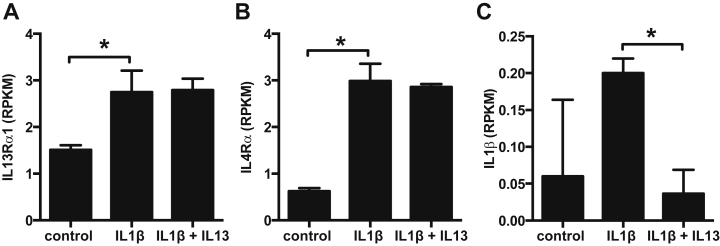
We next investigated whether the protective effects of IL-13 were perhaps mediated by NFκB. Rat primary beta-cells treated for 48 h with IL-13 in the presence or not of IL-1β were stained for NFκB (p65). The number of nuclei positive for NFκB was then carefully counted in order to quantify NFκB nuclear localization (Figure 5). IL-13 alone did not influence NFκB localization. Moreover, IL-13 did not counteract the nuclear re-localization of NFκB induced by IL-1β treatment (Figure 5), suggesting that IL-13 acts independently of this key transcription factor. This notwithstanding, and in an attempt to uncover genes possibly involved in the protective effects of IL-13 against IL-1β induced beta-cell death, we performed a transcriptome analysis of rat primary beta-cells treated for 48 h with IL-1β alone or in the presence of IL-13. Primary sorted rat beta-cells express the 2 receptor subunits IL-13Rα1 (Figure 6A) and IL-4Rα (Figure 6B) indispensible for a cellular IL-13 response. This confirms a recently published transcriptome analysis comparing human islets, a beta-cell fraction and a non beta-cell fraction showing that these 2 receptors are also expressed in human islets and beta-cells [16]. In rat beta-cells, the expression of the 2 receptors was upregulated upon IL-1β treatment, suggesting that in the presence of a pro-inflammatory cytokine beta-cells are sensitized towards the anti-inflammatory actions of IL-13. As documented previously in beta-cells [26], there was a tendency for IL-1β to upregulate its own expression (Figure 6C) even if this failed to achieve statistical significance in the present small series of experiments due to the low levels of expression of IL-1β in beta-cells and high degree of variability in the control situation. More importantly, IL-13 was able to downregulate such self-induction of IL-1β (Figure 6C).
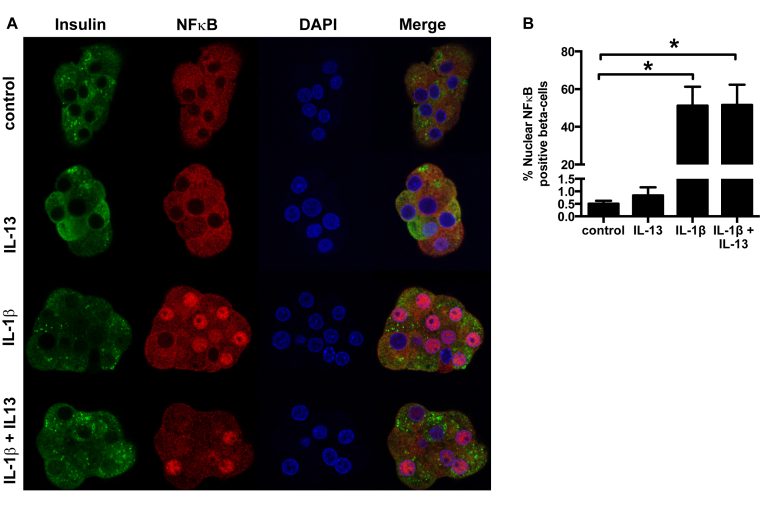
Figure 5.
IL-13 does not influence IL-1β induced NFκB nuclear relocalization. Primary rat sorted beta-cells were cultured for 48 h with IL-1β (20 ng/ml) ± IL-13 (10 ng/ml). A: Representative images showing NFκB (red) localization. B: Beta-cells with nuclear localization of NFκB (n = 6). *p < 0.05 as tested by ANOVA followed by Bonferroni post hoc test.
Figure 6.
IL-13 receptor subunit and IL-1β expression in beta-cells. Sorted rat primary beta-cells were cultured for 48 h with IL-1β (20 ng/ml) ± IL-13 (10 ng/ml) and transcriptomes profiled by RNAseq. A: IL-13Rα1; B: IL-4Rα; C: IL-1β. Data are presented as reads per kilobase per million mapped reads (RPKM), n = 3. *p < 0.05 vs. control, dseq2 adjusted p value.
Among the over 6000 genes affected by treatment of rat beta-cells with IL-1β, only 110 were significantly counter regulated by IL-13 (Table 1). The IL-13 counter regulation was entire (IL-1β + IL-13 condition back to control value) only for 19 among these 110 genes. Of these 110 genes, 63 were upregulated by IL-1β with total or partial abrogation of this increase by IL-13 and 47 genes were downregulated by IL-1β with total or partial abrogation of this decrease by IL-13 (Table 1). Of the 63 genes with increased expression upon IL-1β treatment and partial or full protection by IL-13, 9 were singled out as being of particular interest in the context of beta-cell apoptosis and dysfunction (grey lines in Table 1). These genes are implicated in inflammation (Csf3, Ccl20, IL-23), stress responses (Ern1, CYBB, Cat, IGFbp1), transcription (Myc) or insulin secretion (Slc1a2). Similarly, of the 47 genes downregulated by IL-1β with partial or full protection by IL-13, 6 were of particular interest in the context of our study (grey lines in Table 1). These genes are involved in insulin processing and secretion (Pcsk1, Slc30a8, Vsnl1) or encode secreted proteins (IGFbp3, Itnl1). Interestingly, Galnt14 is highly increased in cells treated with IL-13 and IL-1β compared to IL-1β alone.
Table 1.
IL-13 counterregulates the expression of genes regulated by IL-1β. Sorted rat primary beta-cells were cultured for 48 h with IL-1β (20 ng/ml) ± IL-13 (10 ng/ml) and transcriptomes profiled by RNAseq. Data are presented as fold-changes between control and IL-1β and between IL-1β and IL-1β + IL-13 conditions, n = 3.
| Gene name | Gene ID | Control versus IL1β |
IL1β versus IL1β + IL13 |
||
|---|---|---|---|---|---|
| Fold change | p value | Fold change | p value | ||
| Slc1a2 | ENSRNOG00000005479 | 6.06 | 7.61E-17 | 0.86 | 4.51E-02 |
| Myc | ENSRNOG00000004500 | 24.42 | 1.79E-46 | 0.82 | 4.49E-02 |
| SRXN1 | ENSRNOG00000031167 | 3.27 | 2.48E-29 | 0.78 | 4.49E-02 |
| Aldoa | ENSRNOG00000023647 | 2.07 | 6.01E-59 | 0.90 | 4.32E-02 |
| Fhl2 | ENSRNOG00000016866 | 23.43 | 1.26E-81 | 0.77 | 4.30E-02 |
| Lcn2 | ENSRNOG00000013973 | 372.22 | 0.00E+00 | 0.90 | 4.25E-02 |
| Dvl1 | ENSRNOG00000019423 | 1.33 | 1.47E-05 | 0.83 | 3.61E-02 |
| Kcnn3 | ENSRNOG00000020706 | 3.94 | 1.59E-10 | 0.82 | 3.40E-02 |
| Saal1 | ENSRNOG00000011895 | 3.92 | 1.35E-20 | 0.72 | 3.00E-02 |
| Hnrnpa0 | ENSRNOG00000039876 | 1.24 | 1.91E-02 | 0.80 | 2.93E-02 |
| Adm | ENSRNOG00000027030 | 15.35 | 1.01E-133 | 0.79 | 2.82E-02 |
| Il23a | ENSRNOG00000003254 | 10.41 | 1.35E-62 | 0.72 | 2.46E-02 |
| Ern1 | ENSRNOG00000012864 | 2.07 | 6.36E-31 | 0.87 | 2.39E-02 |
| Pfkfb3 | ENSRNOG00000018911 | 18.00 | 1.19E-132 | 0.79 | 2.31E-02 |
| Pfkl | ENSRNOG00000001214 | 1.84 | 1.93E-19 | 0.85 | 2.16E-02 |
| Fblim1 | ENSRNOG00000011774 | 2.16 | 4.98E-12 | 0.73 | 2.11E-02 |
| Ddit4 | ENSRNOG00000000577 | 1.93 | 6.60E-12 | 0.78 | 1.84E-02 |
| Ccl20 | ENSRNOG00000015992 | 67.65 | 2.44E-27 | 0.59 | 1.64E-02 |
| Sesn2 | ENSRNOG00000042785 | 1.23 | 1.15E-04 | 0.87 | 1.58E-02 |
| Raph1 | ENSRNOG00000014722 | 2.04 | 3.18E-15 | 0.80 | 1.51E-02 |
| Cybb | ENSRNOG00000003622 | 86.22 | 3.70E-98 | 0.77 | 1.22E-02 |
| Sqstm1 | ENSRNOG00000003147 | 2.75 | 5.13E-100 | 0.88 | 1.08E-02 |
| Stc2 | ENSRNOG00000020729 | 21.11 | 3.27E-09 | 0.77 | 9.37E-03 |
| Ugdh | ENSRNOG00000002643 | 4.89 | 2.13E-170 | 0.86 | 9.13E-03 |
| Ugt1a8 | ENSRNOG00000018740 | 3.81 | 8.36E-125 | 0.87 | 9.13E-03 |
| Fhdc1 | ENSRNOG00000024594 | 1.58 | 5.21E-14 | 0.81 | 8.71E-03 |
| Rusc2 | ENSRNOG00000045843 | 2.03 | 1.15E-13 | 0.77 | 8.71E-03 |
| Tle3 | ENSRNOG00000013013 | 1.37 | 2.63E-07 | 0.86 | 8.25E-03 |
| Gtpbp2 | ENSRNOG00000019332 | 3.12 | 8.63E-79 | 0.83 | 5.43E-03 |
| NA | ENSRNOG00000007930 | 2.23 | 4.75E-20 | 0.77 | 5.40E-03 |
| Nqo1 | ENSRNOG00000012772 | 3.34 | 2.86E-117 | 0.86 | 4.29E-03 |
| Slc16a1 | ENSRNOG00000019996 | 22.94 | 7.13E-87 | 0.73 | 4.19E-03 |
| RGD1310819 | ENSRNOG00000018366 | 4.20 | 2.53E-65 | 0.80 | 3.79E-03 |
| Ppp1r15a | ENSRNOG00000020938 | 5.74 | 6.26E-223 | 0.83 | 3.67E-03 |
| Ets2 | ENSRNOG00000001647 | 1.78 | 6.49E-24 | 0.86 | 3.47E-03 |
| Fndc3b | ENSRNOG00000024089 | 1.35 | 4.00E-07 | 0.84 | 3.40E-03 |
| Csf3 | ENSRNOG00000008525 | 182.28 | 1.84E-20 | 0.48 | 3.09E-03 |
| Abcc3 | ENSRNOG00000002948 | 1.49 | 7.99E-10 | 0.83 | 2.91E-03 |
| Ldlr | ENSRNOG00000009946 | 1.36 | 7.46E-07 | 0.84 | 2.85E-03 |
| Dgat2 | ENSRNOG00000016573 | 16.34 | 2.89E-117 | 0.75 | 1.95E-03 |
| Jund | ENSRNOG00000019568 | 2.45 | 7.44E-20 | 0.78 | 1.89E-03 |
| Arnt2 | ENSRNOG00000013017 | 1.89 | 1.49E-31 | 0.84 | 1.73E-03 |
| Nceh1 | ENSRNOG00000013313 | 3.39 | 1.07E-76 | 0.83 | 7.64E-04 |
| Cat | ENSRNOG00000008364 | 2.35 | 3.29E-38 | 0.85 | 5.95E-04 |
| Ak4 | ENSRNOG00000045738 | 28.25 | 1.84E-95 | 0.68 | 1.16E-04 |
| Gys1 | ENSRNOG00000020812 | 3.81 | 1.68E-76 | 0.78 | 1.08E-04 |
| Slc2a1 | ENSRNOG00000007284 | 11.24 | 0.00E+00 | 0.80 | 9.99E-05 |
| Gstp1 | ENSRNOG00000018237 | 9.99 | 2.00E-174 | 0.81 | 8.15E-05 |
| Bhlhe40 | ENSRNOG00000007152 | 4.35 | 5.08E-65 | 0.76 | 8.01E-05 |
| Elmsan1 | ENSRNOG00000010653 | 5.03 | 1.36E-109 | 0.80 | 6.17E-05 |
| Pnpla7 | ENSRNOG00000008190 | 3.20 | 4.35E-36 | 0.71 | 5.79E-05 |
| Pcsk6 | ENSRNOG00000011526 | 34.78 | 4.38E-30 | 0.84 | 5.21E-05 |
| Ptgs1 | ENSRNOG00000007415 | 9.58 | 4.15E-40 | 0.60 | 2.16E-05 |
| NA | ENSRNOG00000021752 | 2.58 | 1.49E-14 | 0.59 | 2.16E-05 |
| Lrp11 | ENSRNOG00000014303 | 1.96 | 1.55E-38 | 0.85 | 8.61E-06 |
| Igfbp1 | ENSRNOG00000008371 | 4.50 | 2.88E-14 | 0.44 | 5.05E-06 |
| Vegfa | ENSRNOG00000019598 | 1.95 | 1.09E-53 | 0.82 | 8.88E-07 |
| Gdf15 | ENSRNOG00000019661 | 13.83 | 3.20E-77 | 0.73 | 5.66E-08 |
| Mmp13 | ENSRNOG00000008478 | 45.57 | 3.40E-86 | 0.54 | 3.26E-10 |
| Orm1 | ENSRNOG00000007886 | 218.28 | 1.06E-34 | 0.55 | 2.90E-12 |
| Fabp5 | ENSRNOG00000049075 | 39.12 | 9.32E-84 | 0.71 | 2.69E-12 |
| Akr1b8 | ENSRNOG00000009734 | 89.26 | 9.74E-47 | 0.63 | 9.94E-17 |
| Slc7a11 | ENSRNOG00000010210 | 17.51 | 1.37E-71 | 0.71 | 9.70E-18 |
| Slc30a8 | ENSRNOG00000004747 | 0.13 | 9.42E-247 | 1.21 | 9.52E-04 |
| Slc38a5 | ENSRNOG00000027767 | 0.15 | 5.68E-206 | 1.24 | 1.50E-05 |
| Gnas | ENSRNOG00000047374 | 0.32 | 3.87E-155 | 1.18 | 3.22E-05 |
| Myh10 | ENSRNOG00000002886 | 0.31 | 1.36E-142 | 1.26 | 1.86E-06 |
| Pcsk1 | ENSRNOG00000011107 | 0.29 | 8.41E-126 | 1.34 | 7.44E-09 |
| Gm20721 | ENSRNOG00000048879 | 0.33 | 3.22E-125 | 1.15 | 1.58E-02 |
| Galnt14 | ENSRNOG00000007951 | 0.14 | 2.59E-114 | 4.03 | 7.05E-61 |
| Lbh | ENSRNOG00000039902 | 0.22 | 1.38E-102 | 1.25 | 1.58E-02 |
| Pam | ENSRNOG00000033280 | 0.35 | 1.57E-81 | 1.14 | 1.81E-03 |
| Maob | ENSRNOG00000029778 | 0.35 | 3.17E-80 | 1.47 | 1.82E-14 |
| Pgm5 | ENSRNOG00000015406 | 0.37 | 2.42E-70 | 1.33 | 3.23E-06 |
| Vsnl1 | ENSRNOG00000005345 | 0.20 | 5.26E-66 | 1.46 | 4.25E-03 |
| Sphkap | ENSRNOG00000016388 | 0.53 | 1.72E-33 | 1.30 | 9.26E-11 |
| Cd83 | ENSRNOG00000018092 | 0.27 | 7.78E-31 | 1.79 | 2.36E-04 |
| Matn2 | ENSRNOG00000006060 | 0.29 | 7.38E-30 | 1.87 | 1.98E-08 |
| AW551984 | ENSRNOG00000005960 | 0.52 | 1.62E-28 | 1.61 | 1.59E-21 |
| Aass | ENSRNOG00000039494 | 0.42 | 2.85E-26 | 1.52 | 6.93E-12 |
| Scg2 | ENSRNOG00000015055 | 0.64 | 3.44E-26 | 1.12 | 1.84E-02 |
| Ggh | ENSRNOG00000007351 | 0.52 | 1.00E-23 | 1.28 | 3.71E-04 |
| Tspan7 | ENSRNOG00000003229 | 0.65 | 6.49E-21 | 1.15 | 9.83E-03 |
| Scfd1 | ENSRNOG00000031203 | 0.59 | 1.17E-20 | 1.16 | 4.60E-02 |
| Crp | ENSRNOG00000000053 | 0.44 | 2.20E-18 | 2.14 | 2.15E-22 |
| Atp6v1a | ENSRNOG00000001992 | 0.66 | 3.37E-16 | 1.16 | 1.21E-02 |
| Dnah9 | ENSRNOG00000004171 | 0.22 | 6.24E-16 | 3.12 | 5.64E-08 |
| Rtn4rl1 | ENSRNOG00000003121 | 0.54 | 1.10E-14 | 1.69 | 4.58E-13 |
| Lrrtm4 | ENSRNOG00000021938 | 0.26 | 3.70E-13 | 2.11 | 3.03E-03 |
| NA | ENSRNOG00000021687 | 0.09 | 6.44E-12 | 14.32 | 1.49E-19 |
| Gc | ENSRNOG00000003119 | 0.72 | 6.60E-11 | 1.16 | 2.55E-03 |
| Gad1 | ENSRNOG00000000007 | 0.65 | 9.84E-11 | 1.24 | 8.71E-07 |
| Camk2n1 | ENSRNOG00000016322 | 0.63 | 3.82E-10 | 1.18 | 3.50E-02 |
| Trh | ENSRNOG00000011824 | 0.70 | 1.21E-09 | 1.15 | 4.35E-02 |
| Galr1 | ENSRNOG00000016654 | 0.25 | 1.94E-09 | 2.75 | 4.05E-04 |
| Cox7b | ENSRNOG00000028451 | 0.64 | 1.01E-08 | 1.25 | 1.84E-02 |
| Sema5a | ENSRNOG00000011977 | 0.66 | 1.24E-07 | 1.35 | 1.09E-04 |
| Fam92a1 | ENSRNOG00000016338 | 0.68 | 5.32E-07 | 1.28 | 1.84E-02 |
| Hgfac | ENSRNOG00000009572 | 0.58 | 7.49E-07 | 1.42 | 2.76E-02 |
| Hrsp12 | ENSRNOG00000005437 | 0.68 | 4.03E-06 | 1.37 | 1.90E-04 |
| Marcks | ENSRNOG00000000579 | 0.65 | 4.70E-06 | 1.38 | 8.20E-04 |
| Col6a3 | ENSRNOG00000019648 | 0.23 | 6.77E-05 | 3.01 | 3.68E-02 |
| Atp1b1 | ENSRNOG00000002934 | 0.84 | 5.49E-04 | 1.14 | 3.09E-03 |
| Bche | ENSRNOG00000009826 | 0.59 | 1.07E-03 | 1.96 | 9.84E-06 |
| Igfbp3 | ENSRNOG00000008645 | 0.48 | 1.85E-03 | 2.06 | 1.14E-02 |
| Accsl | ENSRNOG00000042533 | 0.23 | 1.83E-02 | 4.79 | 6.49E-03 |
| Tsc22d3 | ENSRNOG00000013786 | 0.81 | 1.84E-02 | 1.52 | 1.50E-05 |
| Il1r1 | ENSRNOG00000014504 | 0.89 | 2.10E-02 | 1.57 | 1.17E-42 |
| Itln1 | ENSRNOG00000004678 | 0.78 | 2.39E-02 | 1.34 | 2.17E-02 |
| Tmprss2 | ENSRNOG00000001976 | 0.79 | 3.14E-02 | 1.51 | 2.75E-08 |
4. Discussion
It has recently been shown that IL-13 plays an important role in glucose homeostasis and that its level is decreased in the circulation of type 2 diabetic patients but increased in the adipose tissue of obese individuals [6], [10], [11]. IL-13 regulates glucose homeostasis in the liver and plays a unique protective role by limiting adipose tissue inflammation [6], [10]. The impact that IL-13 could exert on beta-cells has so far been studied mainly on beta-cell lines [7]. Our study shows for the first time, that IL-13 directly impacts primary rat and, more importantly, human beta-cells, acting to improve survival both under standard culture conditions and in the face of cytotoxic cytokines. We thus show that IL-13 decreases human beta-cell death, either in a pure population or in the presence of other islet cell types. These results, together with those obtained by transcriptome analysis of human purified beta-cells or islets [16] where IL-13Rα1 and IL4Rα, the 2 receptor subunits indispensable for IL-13 signaling, were shown to be expressed at the mRNA level in beta-cells and in islets, may suggest that IL-13 directly interacts with beta-cells. Only one study has so far shown that IL-13 might impact human islet cell signaling pathways with the induction of STAT3 and STAT6 phosphorylation [12].
We demonstrate that the decrease in basal death of rat beta-cells was associated with increased IRS2 protein expression and Akt phosphorylation. The IL-13 receptor subunit IL-4Rα has been shown previously to be able to interact with IRS2 [7], and, in turn, this insulin receptor substrate is known to be essential in maintaining proper functional beta-cell mass [27]. It was also associated with an increase in the spreading of beta-cells and in the protein expression associated with this process. These data are in line with a previous study on embryonic fibroblast demonstrating that focal adhesion remodeling is involved in cell survival [24]. Moreover, the transcriptome analysis of rat beta-cells in the presence of IL-13 revealed that it is able to increase the expression of Cish. This protein forms part of a classical negative feedback system that regulates cytokine signal transduction and is involved in the negative regulation of cytokines that signal through the JAK-STAT5 pathway [25]. Together our results thus show that IL-13 positively impacts beta-cell survival via the IRS2/Akt pathway and also by inhibiting cytokine signaling. Moreover, IL-13 through adhesion formation could protect cells from apoptosis by regulating the activation of NF-κB as demonstrated in fibroblasts [24].
IL-13 is also able to protect beta-cells against a cocktail of cytokines (IL-1β, TNFα and IFNγ) thought to contribute to decreased beta-cell mass in both major types of diabetes. Moreover, as recently shown, IL-13 is decreased in patients with type 2 diabetes [11]. The imbalance between pro- and anti-inflammatory cytokines has been suggested to be central in the beta-cell damage that occurs during progression towards clinically manifest type 2 diabetes. Our results showing the protective effect of IL-13 against cell death induced by pro-inflammatory cytokine are in perfect keeping with this hypothesis.
IL-13-induced IRS2 protein expression is not influenced by IL-1β treatment, suggesting that IRS2 is implicated in the mediation of the protective role played by IL-13 against IL-1β−induced apoptosis. IL-13 protection of beta-cells against IL-1β-induced death is independent of the translocation (and thus activation) of NFκB, but it prevents the decrease in NFκB phosphorylation and total protein expression induced by IL-1β treatment. However, this does not mean that the IL-13 effect is transcription independent since we could identify genes regulated by IL-1β and counter-regulated by IL-13. IL-13 impacts NFκB turnover by modifying its protein expression, without impacting its localization or mRNA expression. IL-1β impacts drastically the transcriptome of beta-cells; whereas IL-13 does not massively counter-regulate the perturbed expression of all these genes it may perhaps correct only the expression of those few genes that are important for beta-cell survival.
While it is hard to interpret the data without considering the combined effect of all the changes exerted by IL-13 in the face of IL-1β, some of the impacted genes do stand out as logical candidates that may contribute towards the protective effects of IL-13. Among them, some genes appear of particular interest including: Cybb which forms reactive oxygen species (ROS) and has been shown to be involved in beta-cell dysfunction in response to glycotoxic or pro-inflammatory cytokine response [28], [29]; Ern1 which is an important player of the endoplasmic reticulum (ER) stress and unfolded protein responses [30]; Myc which has been shown to induce beta-cell dysfunction and apoptosis [31]; Csf3 and Ccl20 that code for cytokines implicated in inflammation. By dampening the increased expression of the above-mentioned genes in response to IL-1β, IL-13 may improve beta-cell survival in the presence of cytotoxic cytokines.
Among the genes downregulated by IL-1β but not in the presence of IL-13, some are of particular interest in the context of beta-cell resistance to inflammatory stress. Cd83 is involved in antigen presentation and SNPs in this gene have been shown to be associated with type 1 diabetes [25]. Thus, its upregulation by IL-13, also in the presence of IL-1β, might be part of a beta-cell survival process. Ltn1, also known as omentin-1, is a recently discovered adipokine that is reduced in type 2 diabetic patients [32]. The impact of this adipokine on beta-cells remains to be studied; however, omentin-1 suppresses myocyte apoptosis in an ischemia model through Akt and AMPK activation [33]. This suggests that this adipokine might play a role in the IL-13 protective effect against IL-1β, since IL-13 is able to restore its transcription level in IL-1β treated beta-cells.
In other tissues, Galnt14 has been shown to impact on IGFBP3 to decrease apoptosis [34]. As well, an increase of a 2,6 sialyted carbohydrate produced by ST6galnac3 has been shown to render cells resistant to apoptosis [35]. Therefore, the increase of Galnt14 and St6galnac3 expression upon IL-13 treatment could partly explain the decrease of baseline beta-cell apoptosis observed after IL-13 treatment.
Interestingly, IL-13 exerts its protective effect against IL-1β not only on beta-cells but also on alpha-cells. Although glucagon is the major secretory product of alpha-cells, acting to increase hepatic glucose production, alpha-cells can also produce GLP-1 under certain particular situations, allowing for a possible paracrine effect on beta-cells to enhance their glucose-stimulated insulin secretion and improve their survival [36]. We thus speculate that under conditions of islet inflammation, when alpha-cell GLP-1 production is believed to be increased, preserving alpha-cells may actually be beneficial for preserving beta-cell functional mass, contrary to received thinking. Furthermore, it has been shown that under conditions of extreme beta-cell ablation in rodents, alpha-cells can be trans-differentiated into beta-cells [37], raising the interesting hypothesis that in the clinical setting of type 2 diabetes, protecting alpha-cells against IL-1β may indirectly help preserve beta-cell mass.
5. Conclusions
Our study is in line with previous studies showing a positive impact of IL-13 on transformed beta-cell lines (for review see [7]). However, we show for the first time that IL-13 can directly affect human beta-cell survival. We show that IL-13 protects beta-cells from IL-1β induced apoptosis, a cytokine known to play an important role in type 2 diabetes. These positive effects are mediated via the IRS2/Akt pathway and the regulation of genes implicated in the cellular stress response. While the complete pathway underlying the IL-13 protective effects on beta-cell survival remains to be fully elucidated, our study indicates that IL-13 may be an important new player in helping beta-cells survive cytokine attack in both major forms of diabetes and may be a useful adjunct to existing or novel anti-proinflammatory cytokine therapy, acting not only on the liver and adipocytes as previously suggested by others [6], [10] but also on islets.
Acknowledgments
Human islets were kindly provided by the Islet for Basic Research program through the European Consortium for Islet Transplantation, supported by the Juvenile Diabetes Research Foundation (JDRF award 31-2008-413). We thank Katharina Rickenbach, Luciana Romano and Deborah Bielser for expert technical assistance. This study was supported by the Juvenile Diabetes Research Foundation (JDRF grant 17-2012-27).
Conflict of interest
The authors declare that they have no conflict of interest to disclose in relation to this work.
References
- 1.Prentki M., Nolan C.J. Islet beta cell failure in type 2 diabetes. Journal of Clinical Investigation. 2006;116(7):1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donath M.Y., Halban P.A. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47(3):581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 3.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 4.Donath M.Y., Boni-Schnetzler M., Ellingsgaard H., Halban P.A., Ehses J.A. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends in Endocrinology & Metabolism. 2010;21(5):261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Rutti S., Arous C., Schvartz D., Timper K., Sanchez J.C., Dermitzakis E. Fractalkine (CX3CL1), a new factor protecting beta-cells against TNFalpha. Molecular Metabolism. 2014;3(7):731–741. doi: 10.1016/j.molmet.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon H., Laurent S., Tang Y., Zong H., Vemulapalli P., Pessin J.E. Adipocyte-specific IKKbeta signaling suppresses adipose tissue inflammation through an IL-13-dependent paracrine feedback pathway. Cell Reports. 2014 doi: 10.1016/j.celrep.2014.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell M.A., Morgan N.G. The impact of anti-inflammatory cytokines on the pancreatic beta-cell. Islets. 2014;6(3):e950547. doi: 10.4161/19382014.2014.950547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly-Welch A.E., Hanson E.M., Boothby M.R., Keegan A.D. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300(5625):1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 9.Wills-Karp M., Finkelman F.D. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Science Signaling. 2008;1(51) doi: 10.1126/scisignal.1.51.pe55. pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanya K.J., Jacobi D., Liu S., Bhargava P., Dai L., Gangl M.R. Direct control of hepatic glucose production by interleukin-13 in mice. Journal of Clinical Investigation. 2013;123(1):261–271. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang L.Q., Franck N., Egan B., Sjogren R.J., Katayama M., Duque-Guimaraes D. Autocrine role of interleukin-13 on skeletal muscle glucose metabolism in type 2 diabetic patients involves microRNA let-7. American Journal of Physiology Endocrinology & Metabolism. 2013;305(11):E1359–E1366. doi: 10.1152/ajpendo.00236.2013. [DOI] [PubMed] [Google Scholar]
- 12.Russell M.A., Cooper A.C., Dhayal S., Morgan N.G. Differential effects of interleukin-13 and interleukin-6 on Jak/STAT signaling and cell viability in pancreatic beta-cells. Islets. 2013;5(2):95–105. doi: 10.4161/isl.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souza K.L., Gurgul-Convey E., Elsner M., Lenzen S. Interaction between pro-inflammatory and anti-inflammatory cytokines in insulin-producing cells. Journal of Endocrinology. 2008;197(1):139–150. doi: 10.1677/JOE-07-0638. [DOI] [PubMed] [Google Scholar]
- 14.Sternesjo J., Sandler S. Interleukin-13 counteracts suppression induced by interleukin-1beta of glucose metabolism but not of insulin secretion in rat pancreatic islets. Autoimmunity. 1997;26(3):153–159. doi: 10.3109/08916939708994737. [DOI] [PubMed] [Google Scholar]
- 15.Parnaud G., Bosco D., Berney T., Pattou F., Kerr-Conte J., Donath M.Y. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51(1):91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- 16.Nica A.C., Ongen H., Irminger J.C., Bosco D., Berney T., Antonarakis S.E. Cell-type, allelic, and genetic signatures in the human pancreatic beta cell transcriptome. Genome Research. 2013;23(9):1554–1562. doi: 10.1101/gr.150706.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marco-Sola S., Sammeth M., Guigo R., Ribeca P. The GEM mapper: fast, accurate and versatile alignment by filtration. Nature Methods. 2012;9(12):1185–1188. doi: 10.1038/nmeth.2221. [DOI] [PubMed] [Google Scholar]
- 18.Center de Regulation Genomica, B. 2008.
- 19.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomas A., Yermen B., Min L., Pessin J.E., Halban P.A. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. Journal of Cell Science. 2006;119(Pt 10):2156–2167. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 21.Rutti S., Sauter N.S., Bouzakri K., Prazak R., Halban P.A., Donath M.Y. In vitro proliferation of adult human beta-cells. PLoS One. 2012;7(4):e35801. doi: 10.1371/journal.pone.0035801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Xu H., Xiao T., Cong L., Love M.I., Zhang F. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biology. 2014;15(12):554. doi: 10.1186/s13059-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rondas D., Tomas A., Halban P.A. Focal adhesion remodeling is crucial for glucose-stimulated insulin secretion and involves activation of focal adhesion kinase and paxillin. Diabetes. 2011;60(4):1146–1157. doi: 10.2337/db10-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang D., Khoe M., Befekadu M., Chung S., Takata Y., Ilic D. Focal adhesion kinase mediates cell survival via NF-kappaB and ERK signaling pathways. American Journal of Physiology Cell Physiology. 2007;292(4):C1339–C1352. doi: 10.1152/ajpcell.00144.2006. [DOI] [PubMed] [Google Scholar]
- 25.Trengove M.C., Ward A.C. SOCS proteins in development and disease. American Journal of Clinical and Experimental Immunology. 2013;2(1):1–29. [PMC free article] [PubMed] [Google Scholar]
- 26.Boni-Schnetzler M., Thorne J., Parnaud G., Marselli L., Ehses J.A., Kerr-Conte J. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. The Journal of Clinical Endocrinology & Metabolism. 2008;93(10):4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terauchi Y., Takamoto I., Kubota N., Matsui J., Suzuki R., Komeda Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. Journal of Clinical Investigation. 2007;117(1):246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowluru A., Kowluru R.A. Phagocyte-like NADPH oxidase [Nox2] in cellular dysfunction in models of glucolipotoxicity and diabetes. Biochemical Pharmacology. 2014;88(3):275–283. doi: 10.1016/j.bcp.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N., Li B., Brun T., Deffert-Delbouille C., Mahiout Z., Daali Y. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes. 2012;61(11):2842–2850. doi: 10.2337/db12-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eizirik D.L., Miani M., Cardozo A.K. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56(2):234–241. doi: 10.1007/s00125-012-2762-3. [DOI] [PubMed] [Google Scholar]
- 31.Cheung L., Zervou S., Mattsson G., Abouna S., Zhou L., Ifandi V. c-Myc directly induces both impaired insulin secretion and loss of beta-cell mass, independently of hyperglycemia in vivo. Islets. 2010;2(1):37–45. doi: 10.4161/isl.2.1.10196. [DOI] [PubMed] [Google Scholar]
- 32.Tan B.K., Adya R., Randeva H.S. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends in Cardiovascular Medicine. 2010;20(5):143–148. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Kataoka Y., Shibata R., Ohashi K., Kambara T., Enomoto T., Uemura Y. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. Journal of the American College of Cardiology. 2014;63(24):2722–2733. doi: 10.1016/j.jacc.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Wu C., Shan Y., Liu X., Song W., Wang J., Zou M. GalNAc-T14 may be involved in regulating the apoptotic action of IGFBP-3. Journal of Biosciences. 2009;34(3):389–395. doi: 10.1007/s12038-009-0045-z. [DOI] [PubMed] [Google Scholar]
- 35.Lu J., Gu J. Significance of beta-galactoside alpha2,6 sialyltranferase 1 in cancers. Molecules. 2015;20(5):7509–7527. doi: 10.3390/molecules20057509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donath M.Y., Burcelin R. GLP-1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care. 2013;36(Suppl. 2):S145–S148. doi: 10.2337/dcS13-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorel F., Nepote V., Avril I., Kohno K., Desgraz R., Chera S. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]