Abstract
Objective
The ventromedial nucleus of the hypothalamus (VMH) controls energy and glucose homeostasis through direct connections to a distributed network of nuclei in the hypothalamus, midbrain, and hindbrain. Structural changes in VMH circuit morphology have the potential to alter VMH function throughout life, however, molecular signals responsible for specifying its neural connections are not fully defined. The VMH contains a high density of neurons that express brain-derived neurotrophic factor (BDNF), a potent neurodevelopmental effector known to regulate neuronal survival, growth, differentiation, and connectivity in a number of neural systems. In the current study, we examined whether BDNF impacts the afferent and efferent connections of the VMH, as well as energy homeostatic function.
Methods
To determine if BDNF is required for VMH circuit formation, a transgenic mouse model was used to conditionally delete Bdnf from steroidogenic factor 1 (SF1) expressing neurons of the VMH prior to the onset of establishing neural connections with other regions. Projections of SF1 expressing neurons were visualized with a genetically targeted fluorescent label and immunofluorescence was used to measure the density of afferents to SF1 neurons in the absence of BDNF. Physiological changes in body weight and circulating blood glucose were also evaluated in the mutant mice.
Results
Our findings suggest that BDNF is required to establish normal densities of GABAergic afferents onto SF1 neurons located in the ventrolateral part of the VMH. Furthermore, loss of BDNF from VMH SF1 neurons results in impaired physiological responses to insulin-induced hypoglycemia.
Conclusion
The results of this study indicate that BDNF is required for formation and/or maintenance of inhibitory inputs to SF1 neurons, with enduring effects on glycemic control.
Keywords: Ventromedial nucleus of the hypothalamus, Brain-derived neurotrophic factor, Steroidogenic factor 1, Hypoglycemia, GABA, Connectivity
Highlights
-
•
BDNF is highly expressed in the VMH during neural development.
-
•
Loss of BDNF results in increased inhibitory synapse number onto SF1 neurons.
-
•
BDNF is not required for axonal outgrowth from SF1 neurons.
-
•
Animals lacking BDNF in SF1 neurons have impaired responses to hypoglycemia.
1. Introduction
The hypothalamus coordinates a variety of extrinsic and intrinsic signals that regulate energy metabolism [1]. Similar to other brain regions, hypothalamic function is dependent on the organization of neural pathways that connect discrete populations of hypothalamic neurons. The development of these circuits is clearly influenced by multiple factors, but how mature patterns of hypothalamic connectivity are established is poorly understood [2], [3].
The ventromedial nucleus of the hypothalamus (VMH) has long been a focal point for examination of neural circuits underlying regulation of food intake and body weight [4], [5]. Early lesion studies identified the VMH as a central regulator of hyperphagia [6] and more recent findings indicate that it also impacts autonomic function and regulation of circulating blood glucose [7], [8], [9], [10], [11]. The VMH contains several distinct and partially overlapping subpopulations of neurons that have been implicated in the regulation of body weight and glucose homeostasis [12], [13], [14], [15], [16], including cells that express the steroidogenic transcription factor (SF1; approved mouse gene name, Nr5a1). In the brain, SF1 is uniquely expressed in the VMH beginning at approximately E9.5 [17], and is required for terminal differentiation of VMH neurons [18]. Disruption of SF1 expression leads to metabolic dysfunction including increased body weight and impaired regulation of blood glucose [9], [19], [20]. Deletion of SF1 also disrupts formation of VMH neuronal projections and reduces expression of the brain derived neurotrophic factor (BDNF) [9], [18]. In many brain regions BDNF is required for normal circuit formation [21], [22], but it remains unknown if BDNF is required to establish connections between the VMH and other parts of the forebrain.
In addition to its neurodevelopmental actions, BDNF has also been implicated in the regulation of energy balance and its expression is altered by nutritional status as both prolonged fasting and high-fat diet reduce expression of BDNF specifically in the VMH, but not in other regions, such as the hippocampus [9], [23], [24], [25]. Mice with impaired expression of BDNF or its receptor, the tyrosine receptor kinase TrkB, exhibit obesity and are diabetic [23], [26], [27], [28], and rescue of BDNF signaling in the VMH improves this metabolic phenotype [29].
To examine a potential role for BDNF in development of VMH neural connections and maturation of metabolic phenotype, we evaluated BDNF expression in the VMH of perinatal mice and used Cre-loxP technology to conditionally delete BDNF in SF1 neurons prior to VMH circuit formation. Simultaneous genetic targeting of the fluorescent reporter tdTomato to SF1 neurons was used to evaluate their projections in the absence of BDNF, as well as to visualize neuronal inputs to SF1 neurons. Corresponding changes in body weight and glucose regulatory physiology were also evaluated in the mutant mice. The results suggest that expression of BDNF by SF1 neurons is required to establish normal patterns of GABA innervation and maintain hypothalamic responses to hypoglycemia.
2. Materials and methods
2.1. Animals
Mice expressing Cre recombinase under the control of the steroidogenic factor 1 promoter (Sf1-Cre; stock #012462), and mice expressing the cre-dependent reporter, tdTomato (Ai14, stock #007914), were obtained from The Jackson Laboratory. Similarly, mice containing alleles with LoxP sites that flank the coding exon of the Bdnf gene (Bdnfflox/flox; stock# 004339) were also obtained from The Jackson Laboratory. The Sf1-Cre mouse line was on a FVB/NJ background, the tdTomato mouse line was on a C57/BL6J background, and the Bdnfflox/flox mouse line was on a 129S4/SvJae background. Therefore, progeny produced by intercross of these lines to produce Sf1-Cre; tdTomato;Bdnfflox/flox mice, and littermates, were maintained on a mixed background. The terms Sf1-Cre; tdTomato;Bdnf+/+ or control mice refer to mice with normal expression of the Bdnf gene, while the term Sf1-Cre; tdTomato;Bdnfflox/flox mice refers to mice with targeted deletion of the Bdnf gene in SF1 neurons. All animals were housed at 22 °C on a 13/11-hour light/dark cycle (lights on at 0600 h/lights off at 1900 h) with ad libitum access to a standard chow diet (PicoLab Rodent Diet 20, #5053). All animal care and experimental procedures were performed in accordance with the Institutional Animal Care and Usage Committee of the Saban Research Institute, Children's Hospital Los Angeles.
2.2. In situ hybridization
On postnatal day (P) 0, 4, 10, and at 7 weeks of age, male mice were anesthetized and perfused transcardially with normal saline followed by fixative (4% paraformaldehyde in borate buffer, pH 9.5). On embryonic day (E) 17.5, mouse embryos were not perfused, but acutely decapitated and neural tissue was dissected. Following post-fixation in a solution of 20% sucrose in fixative, tissue was embedded in OCT and frozen in powdered dry ice for later processing. 20 μm thick sections were collected and fixed in 4% paraformaldehyde for in situ hybridization. Briefly, sections were digested with proteinase K, acetylated in 0.0025% triethanolamine, and dehydrated in ascending concentrations of ethanol. Sections were incubated overnight at 58 °C with digoxigenin-labeled anti-sense BDNF probe (probe template provided by Dr. Baoji Xu, The Scripps Research Institute, Jupiter, FL), in hybridization buffer (65% formamide, 13% Dextran Sulfate, 0.26M NaCl, 2.6% Denhardt's Solution, 0.013M Tris, 0.0013M EDTA). Slides were rinsed in sodium citrate solution, digested with RNase-A, and desalted in descending concentrations of sodium citrate buffer. Anti-digoxigenin conjugated alkaline phosphatase antibody (Roche) was used to detect labeled probe and visualized through chromogenic staining with NBT and BCIP.
2.3. PCR
On P12, male mice were anesthetized with tribromoethanol and decapitated. Brains were removed and cut in 200 μm thick sections using a tissue slicer (OTS – 5000, Electron Microscopy Sciences). Sections containing the VMH were identified under a dissecting microscope equipped with contrast optics and the VMH was microdissected. VMH tissue was pooled into samples containing the VMH from 5 to 6 animals of the same genotype. mRNA was isolated using the PureLink RNA mini Kit (Life Technologies) with on column DNase digestion. The mRNA concentration was measured, and 1 μg mRNA used to generate cDNA using the High Capacity RNA-to-cDNA Kit (Life Technologies). Using TaqMan Gene Expression assays (Bdnf assay ID Mm04230607_s1, GAPDH 4352339E), and TaqMan Gene Expression Master Mix (Life Technologies), Bdnf and Gapdh mRNA expression was measured using the Applied Biosystems ABI 7900HT Fast Reat-Time PCR System. Expression was calculated using in the 2−ΔΔCT method, following normalization relative to Gapdh expression [30].
2.4. Immunohistochemistry
On P12 or 60, male mice were anesthetized and perfused transcardially with saline followed by fixative (4% paraformaldehyde in borate buffer, pH 9.5). Brains were postfixed in a solution of 20% sucrose in fixative, cryoprotected overnight in 20% sucrose in 0.2M KPBS, and frozen in powdered dry ice for later processing. 20 μm thick sections were collected and used for immunohistochemical staining, as described previously [31]. Briefly, tissue sections were incubated overnight in blocking buffer (0.2M KPBS, 2% normal goat serum, 0.3% Triton X-100) at 4 °C followed by incubation for 72 h at 4 °C in blocking buffer containing combinations of antibodies directed against VGLUT2 (Synaptic Systems), VGAT (Synaptic Systems), or HuC/D (Life Technologies). After several rinses in KPBS, sections were incubated in blocking buffer containing a cocktail of species-specific Alexa Fluor conjugated secondary antibodies (Life Technologies). Sections were rinsed in KPBS and mounted on gel-subbed slides, and coverslipped using Fluoromount G mounting medium (Southern Biotech).
2.5. Image acquisition and analysis
To measure the density of labeled axons and terminals in defined regions of interest, images were acquired by using a Zeiss LSM 710 confocal microscope equipped with 20× (N.A. 0.8) and 63× (N.A.1.4) oil-immersion objective lenses. For analysis of VMH projections to various targets, cytoarchitectonic features were visualized with the cytoplasmic neuronal marker HuC/D and 10 μm-thick image stacks were collected through anatomically defined regions of interest at a frequency of 0.4 μm. Images were stored on a 35TB storage area network and ported to Volocity software (Perkin Elmer). Labeled fibers were binarized according to a defined intensity value threshold and then skeletonized to a thickness of one-pixel, allowing measurement of fiber length. The fiber lengths throughout the reference volume were then summed to obtain an estimate of the density of labeled fibers contained in each region of interest.
For analysis of synaptic inputs onto SF1 neurons, three-channel image stacks were collected in matched regions of interest within the dorsomedial and ventrolateral VMH through a z-axis distance of 10 μm. Three-dimensional (3D) reconstructions of the image volumes were then rendered for each multichannel set of images using Volocity visualization software. The number of VGLUT2-or VGAT-immunoreactive punctae determined to be in close apposition to SF1 neurons (voxel to voxel apposition) were counted without user interaction, by applying the same Volocity analysis criteria to all image volumes. Briefly, VGLUT2 and VGAT immunolabeled punctae were identified by using the Find Spots function in Volocity software. This analysis routine identifies local intensity maxima for labeled objects within a user defined three-dimensional radius. This radius was determined empirically through measurement of the minimum distance between immunoreactive punctae, and served to accurately and reliably identify individual puncta. Because puncta volume is larger than a single voxel, identified spots were digitally dilated by using the Dilate command in order to more closely approximate the volume of labeled puncta. The extent of dilation was empirically determined based on average puncta volume measured in a subset of images. Voxels encompasing labeled puncta that did not make contact with voxels marking tdTomato labeled cell bodies were excluded from the analysis. Total numbers of VGLUT2 or VGAT labeled punctae touching tdTomato labeled cell bodies were then summed and divided by the total surface area of labeled SF-1 cell bodies captured in each image stack to obtain an estimate of the density of glutamatergic or GABAergic inputs onto SF1 neurons in each region of interest. All modules of Volocity software were run on a Mac Pro computer (Apple Inc.) supported by a dedicated high performance cluster computing system (Dell Computer Corp.) and Imaging Computing Server utility software (Improvision).
2.6. Glucose and insulin tolerance tests and blood collection
At 6–7 weeks of age, male mice were fasted for 12 h, beginning at 1900 h for glucose tolerance testing (GTT). At 7–8 weeks of age, male mice were fasted for 6 h, beginning at 0800 h for insulin tolerance testing (ITT). During the GTT or ITT, a bolus of either 1.5 mg/kg d-glucose or 0.75 mU/kg insulin (Humalin) was delivered intraperitoneally, and blood glucose was measured using a FreeStyle Lite glucometer and test strips (Abbott Diabetes Care) at time 0, 15, 30, 45, 60, 90, and 120 min following injection.
In addition, blood samples were collected for measurement of plasma glucagon concentration. Following a 6-hour fast, blood samples were taken from uninjected control animals, and from animals 60 min after injection of 0.75 mU/kg insulin (Humalin). Plasma samples were treated with aprotinin to inactivate proteases, and glucagon was measured by enzyme-linked immunosorbent assay (Millipore).
During the ITT or blood collection following insulin injection, animals that failed to reach glucose values below 80 mg/dl were considered to have received misplaced insulin injections and were excluded from analysis. This failed insulin response occurred equally between genotypes, and is therefore unlikely to be the result of manipulation of BDNF expression in SF1 cells.
2.7. Statistical analyses
All results were analyzed using GraphPad Prism software and are expressed as mean values ± SEM. For two group comparisons, a two-tailed unpaired t test was used. For multiple comparisons with one independent variable, a one-way ANOVA with a bonferroni post hoc test was used. For multiple comparisons with two independent variables (e.g. GTT/ITT), a two-way ANOVA followed by Bonferroni's post hoc test was used. p values < 0.05 were considered significant.
3. Results
3.1. BDNF is highly expressed in the VMH during neural development
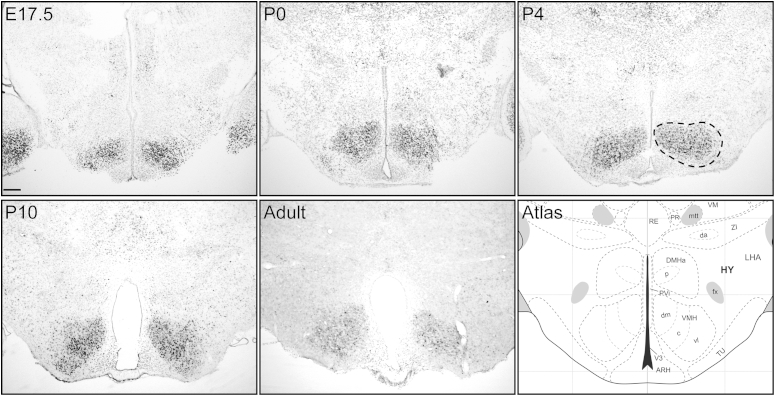
BDNF is expressed in the VMH of adult mice [27]. To confirm that BDNF expression is also enriched in the VMH during perinatal development, in situ hybridization was used to visualize Bdnf mRNA in embryonic and postnatal mice (Figure 1). In contrast to the surrounding hypothalamus, Bdnf mRNA was abundant in the developing VMH at all of the developmental time points assayed. Within the VMH, BDNF was expressed throughout the entire nucleus, including the dorsomedial, central, and ventrolateral components. In agreement with previous studies [32], in situ hybridization for TrkB mRNA revealed broad expression of the TrkB receptor within the hypothalamus during the same developmental period (data not shown), demonstrating that expression of Bdnf and its cognate receptor, TrkB, temporally coincides with the establishment of VMH connectivity [33], [34].
Figure 1.
Perinatal expression of Bdnf in the VMH. Representative images of in situ hybridization labeling for Bdnf mRNA at embryonic day (E) 17.5, postnatal day (P) 0, 4, 10, and in adult C57/BL6J mice, illustrating the pattern of Bdnf expression in the developing and adult VMH. Outline in the top right panel indicates the morphological boundaries of the VMH that corresponds to a reference atlas level (bottom right panel), adapted from [66]. Scale bar, 120 μm.
3.2. Conditional reduction of Bdnf expression in the VMH
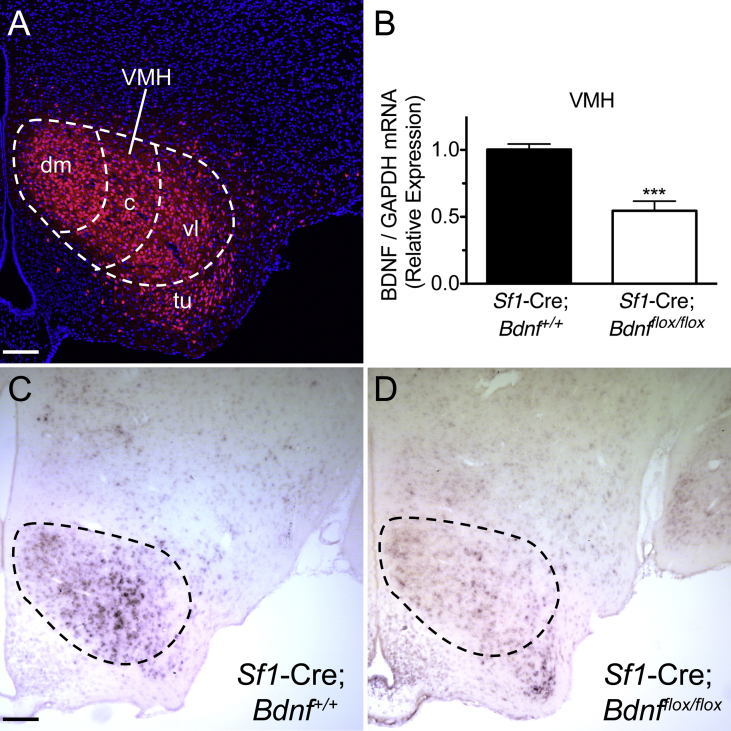
To determine whether BDNF is required for the formation of VMH circuits controlling body weight and glucose homeostasis, a loss-of-function model was generated that takes advantage of localized expression of SF1 in the VMH [12], [19], [35]. BDNF was deleted in VMH neurons by crossing Sf1-Cre mice with Bdnfflox/flox mice. These mice also contained an allele for the tdTomato reporter protein allowing visualization of axons derived from neurons that express Sf1-Cre. In the Sf1-Cre; tdTomato mice approximately 76% of the neurons within the VMH expressed the tdTomato reporter and matched the distribution of SF1 mRNA (Figure 2A) [9], [17], [18]. It should be noted that while Sf1 is expressed in both the dorsomedial and ventrolateral subcompartments of the VMH during early embryonic development, in the mature VMH Sf1 expression is restricted nearly entirely to the dorsomedial compartment [34]. Therefore, in Sf1-Cre; tdTomato;Bdnf+/+ mice, following cre recombination in early embryonic development, both the Bdnf gene and the transcription stop sequence preceding the tdTomato exon are permanently excised resulting in lasting deletion of the Bdnf gene as well as lasting expression of the reporter in all subcompartments of the VMH, irrespective of whether SF1 continues to be expressed. Here, we will use the term, SF1 neuron, to collectively refer to the entire population of VMH neurons that has, at any time, expressed SF1 and therefore undergone Cre recombinase-mediated expression of the tdTomato reporter and/or deletion of the Bdnf gene. In Sf1-Cre; tdTomato mice, the full pattern of VMH axonal projections was apparent, as verified by comparison with earlier reports of VMH [36], [37], demonstrating that neither Sf1-Cre nor tdTomato alleles altered the pattern of VMH projections. In addition, a small subpopulation of neurons in the cortex was found to exhibit Cre recombination, as described previously [12].
Figure 2.
Selective deletion of Bdnf from SF1 neurons. A, Visualization of Sf1 positive neurons in a Sf1-Cre; tdTomato;Bdnf+/+ mouse at the level of the VMH. tdTomato-labeled cells, shown in red, are found in all subregions of the VMH, demonstrating that Cre-mediated recombination has occurred throughout the entire extent of the nucleus. DAPI stained nuclei are shown in blue. Scale bar, 100 μm. B, Quantification of Bdnf mRNA by qPCR on postnatal day 12 in Sf1-Cre;Bdnfflox/flox animals (n = 5 pooled VMH samples) and control littermates (n = 4 pooled VMH samples). Data are expressed as mean ± SEM of Bdnf mRNA relative to Gapdh mRNA. Significance between genotypes was determined by unpaired t-test. ***p < 0.001 between control littermates and Sf1-Cre;Bdnfflox/flox animals. C, D, Representative images of in situ hybridization for Bdnf mRNA in Sf1-Cre;Bdnfflox/flox mice (C) and control littermates on P12. Outlines denote the morphological boundaries of the VMH. Scale bar, 140 μm dm, dorsomedial subregion; c, central subregion; vl, ventrolateral subregion, of the VMH; tu, tuberal nucleus.
Both semi-quantitative RT-PCR and in situ hybridization were used to validate that Bdnf mRNA expression is reduced in Sf1-Cre; tdTomato;Bdnfflox/flox mice. In Sf1-Cre; tdTomato;Bdnfflox/flox mice there was a significant reduction in Bdnf expression in the VMH on P12, compared to levels detected in Sf1-Cre; tdTomato;Bdnf+/+ mice as determined by semi-quantitative RT-PCR (Figure 2B). While total Bdnf mRNA in the VMH is significantly reduced in the VMH of Sf1-Cre; tdTomato;Bdnfflox/flox mice, a subpopulation of non-SF1 positive neurons continue to expresses Bdnf. In situ hybridization confirmed the reduction of Bdnf mRNA within the developing VMH of Sf1-Cre; tdTomato;Bdnfflox/flox mice (Figure 2C–D). Bdnf expression appeared unaltered in regions outside the VMH. For example, the same overall pattern of Bdnf expression was observed in regions such as in the dorsomedial nucleus of the hypothalamus and paraventricular nucleus of the hypothalamus, demonstrating the specificity of the conditional Bdnf deletion.
3.3. Loss of BDNF in SF1 neurons results in increased inhibitory synapse formation onto ventrolateral VMH neurons
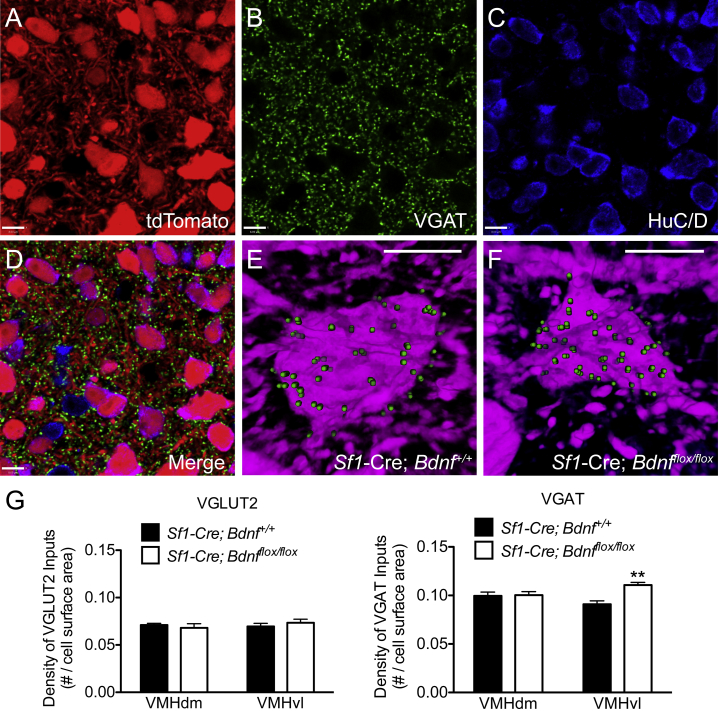
To test the effect of VMH-derived BDNF on excitatory and inhibitory inputs to the VMH, immunohistochemistry was used to visualize glutamatergic and GABAergic imputs by using the presynaptic markers, VGLUT2, and VGAT, respectively. The density of presynaptic glutamatergic and GABAergic terminals in direct contact with tdTomato labeled neurons of the VMH was measured in Sf1-Cre; tdTomato;Bdnfflox/flox mice and in control mice. Analysis of inputs to the VMH was subdivided into the dorsomedial and ventrolateral compartments of the VMH based on known anatomical differences in afferents to the two subregions [38], [39], [40]. A significant increase (21.7%) in the number of VGAT immmunoreactive puncta in contact with SF1 neurons in the ventrolateral compartment of the VMH was observed in Sf1-Cre; tdTomato;Bdnfflox/flox mice (Figure 3G), suggesting that the loss of BDNF in SF1 neurons promotes a sustained increase in inhibitory synapse formation onto VMH neurons. In contrast, no significant difference in the number of VGLUT2 puncta in contact with SF1 neurons was observed between genotypes in either the dorsomedial or ventrolateral parts of the VMH (Figure 3G). The number of tdTomato labeled neurons in the VMH was not significantly different between Sf1-Cre; tdTomato;Bdnf+/+ mice and control littermates, suggesting that the difference in GABAergic inputs cannot be attributed to a relative loss in VMH target neurons.
Figure 3.
Deletion of Bdnf expression from SF1 neurons results in increased GABAergic innervation in the ventrolateral VMH. Within the VMH, regions of interest were imaged for A, tdTomato fluorescence, B, VGLUT2 or VGAT immunoreactivity, and C, HuC/D immunoreactivity. D, illustrates the merged image of all channels. Scale bar, 8 μm. The number of VGLUT2 or VGAT immunoreactive puncta in direct contact with SF1 cell bodies expressing tdTomato was quantified. E,F, Representative images of VGAT puncta in contact with an SF1 neuron in the VMHvl of a control (E) and Sf1-Cre; tdTomato;Bdnfflox/flox animal (F). No difference in the size of SF1 cell bodies was observed between genotypes. Scale bar, 8.47 μm. G, Quantification of VGLUT2 and VGAT inputs onto SF1 neurons in Sf1-Cre; tdTomato;Bdnfflox/flox mice and control littermates. Data are expressed as mean ± SEM of the density of VGLUT2 or VGAT immunoreactive puncta in contact with SF1 neuronal cell bodies, normalized to cell body surface area (Sf1-Cre; tdTomato;Bdnf+/+ n = 3–4, Sf1-Cre; tdTomato;Bdnfflox/flox n = 5–6). Significance between genotypes was determined using unpaired t-test; **p < 0.01 between genotypes within each subregion.
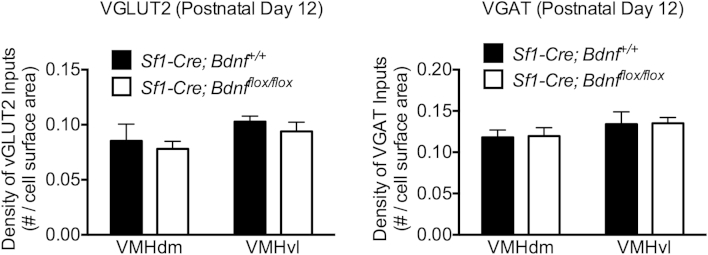
The increase in inhibitory innervation of the ventrolateral VMH observed in Sf1-Cre; tdTomato;Bdnfflox/flox mice could arise either in early postnatal life as the result of altered synapse formation onto VMH neurons, or later on as a result of refinement or plasticity of an already established circuit. To test these possibilities, antibody labeling of VGLUT2 and VGAT was used to compare the densities of presynaptic inputs onto SF1 neurons in Sf1-Cre; tdTomato;Bdnfflox/flox mice and control littermates on P12. Our data suggested that by P12 the total density of VGAT in the VMH has already reached a density that is similar to that of the mature VMH. Therefore, any difference in synaptic density observed by P12 likely reflects a difference in synapse formation, whereas differences in synaptic density that can only be observed later more likely reflect differences in synaptic refinement, or activity dependent plasticity. No difference in the density of either VGLUT2 or VGAT in contact with SF1 neurons was found between genotypes on P12 (Figure 4). Innervation densities were similar in both the dorsomedial and ventrolateral compartments of the VMH in all samples analyzed at this age, suggesting that the effects of BDNF deletion on innervation of SF1 neurons observed in adults are not apparent at P12.
Figure 4.
Early postnatal innervation of SF1 neurons is not dependent upon their expression of BDNF. Quantification of VGLUT2 and VGAT inputs onto SF1 neurons in Sf1-Cre; tdTomato;Bdnfflox/flox mice and control littermates on postnatal day 12. Data are expressed as mean ± SEM of the density of VGLUT2 or VGAT immunoreactive puncta in contact with SF1 neuronal cell bodies, normalized to cell body surface area (Sf1-Cre; tdTomato;Bdnf+/+ n = 4, Sf1-Cre; tdTomato;Bdnfflox/flox n = 4). Significance between genotypes was determined using unpaired t-test; **p < 0.01 between genotypes within each subregion.
3.4. BDNF is not required for axonal outgrowth from SF1 neurons
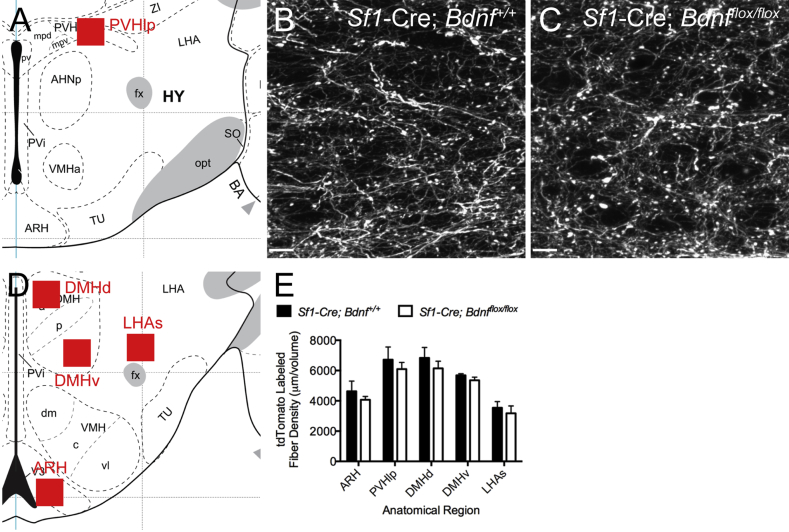
In addition to the ability to alter the dynamics of synaptogenesis, BDNF also induces axonal initiation and outgrowth [21], [22]. To test whether BDNF deletion from SF1 neurons of the VMH alters patterns of efferent SF1 innervation, we measured the density of tdTomato labeled fibers targeting regions involved in regulation of energy balance, including the arcuate nucleus of the hypothalamus (ARH), lateral part of the paraventricular nucleus of the hypothalamus (PVHlp), dorsal (d) and ventral (v) subregions of the dorsomedial nucleus of the hypothalamus (DMH), and the suprafornical region of the lateral hypothalamic area (LHAs). No differences in the densities of tdTomato labeled axonal projections to any of the terminal fields analyzed were found between genotypes (Figure 5), suggesting that axonal growth and guidance of VMH axons to these target regions is independent of BDNF expression in SF1 neurons.
Figure 5.
BDNF is not required for normal development of SF1 neuronal projections. The density of SF1 neuronal projections to several key hypothalamic target regions that regulate energy balance and glucose homeostasis was quantified in Sf1-Cre; tdTomato;Bdnfflox/flox animals and control littermates. Regions of interest (ROIs) that were measured for the ARH, PVHlp, DMHd, DMHv, and LHAs are indicated in reference atlas diagrams by red boxes (A and D). Atlas drawings were adapted from [66]. Representative z-projected composite image stacks of tdTomato-labeled axonal projections in the PVHlp of Sf1-Cre; tdTomato;Bdnf+/+ (B) and Sf1-Cre; tdTomato;Bdnfflox/flox (C) mice. E, Quantification of tdTomato-labeled axonal fiber density in Sf1-Cre; tdTomato;Bdnfflox/flox mice and control littermates on postnatal day 60. Data are expressed as mean ± SEM of the density of tdTomato labeled axons within each target region in a defined volume located within each ROI (Sf1-Cre; tdTomato;Bdnf+/+ n = 3–4, Sf1-Cre; tdTomato;Bdnfflox/flox n = 3–5).
3.5. Animals lacking BDNF in SF1 neurons have impaired responses to hypoglycemia
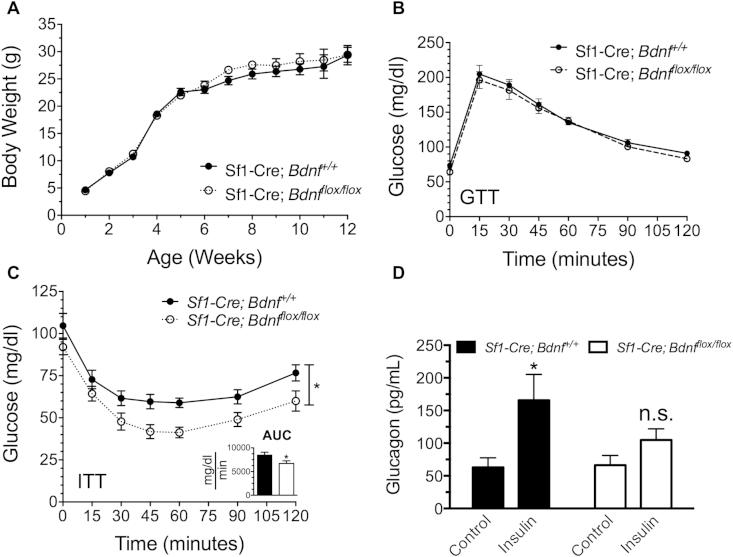
The activity of SF1 neuronal populations is required for normal regulation of body weight and maintenance of circulating concentrations of blood glucose [8], [9], [10], [19], [20]. In the current study Sf1-Cre; tdTomato;Bdnfflox/flox mice were found to have increased inhibitory innervation of SF1 neurons, suggesting that the activity of SF1 neurons may be suppressed in the absence of BDNF. However, body weights were similar throughout the first 12 weeks of age, as previously reported [12], suggesting that expression of BDNF by SF1 neurons does not affect body weight (Figure 6).
Figure 6.
Sf1-Cre;Bdnfflox/flox mice have impaired responses to insulin-induced hypoglycemia. Physiological measurements of A, body weight, B, glucose tolerance (n = 8 for each genotype) C, insulin tolerance and area under the curve (AUC, inset) (n = 11–12 for each genotype) and D, plasma glucagon in Sf1-Cre;Bdnfflox/flox animals and control littermates (n = 7–8 for each genotype). Data are expressed as mean ± SEM. For GTT and ITT, significance between groups was determined by two-way ANOVA; *p < 0.05 between genotypes. For glucagon measurement, significance between groups was determined by one-way ANOVA followed by Bonferroni's multiple comparison post-hoc test; *p < 0.05 between control animals treated with vehicle and control animals treated with insulin.
The consequences of BDNF deletion from SF1 neurons on glucose homeostasis were assessed using glucose (GTT) and insulin tolerance tests (ITT). In both control and Sf1-Cre;Bdnfflox/flox mice, glucose injection increased blood glucose to peak values within 15 min (Figure 6B). Between genotypes, no difference in blood glucose was observed at any time point during the GTT.
In contrast, insulin injection reduced blood glucose, which reached a nadir after 45 min. At this time point blood glucose values tended to be lower in Sf1-Cre;Bdnfflox/flox mice (41.75 ± 4.13) than in control littermates (59.64 ± 4.29). Overall, animals lacking BDNF in SF1 neurons of the VMH exhibited significantly reduced blood glucose over time in the ITT (Figure 6C), which was also evidenced by a 20% decrease in the area under the curve in Sf1-Cre;Bdnfflox/flox mice (see Figure 6C, inset).
Following detection of hypoglycemia by various central and peripheral sites, the VMH coordinates secretion of glucagon from the pancreas, and stimulates glycogenolysis and gluconeogenesis to raise blood glucose levels [10], [11], [41], [42]. To determine whether the decreased blood glucose values observed in Sf1-Cre;Bdnfflox/flox mice during the ITT are the result of reductions in glucagon secretion, blood samples were collected 1 h after insulin injection for measurement of glucagon. In Sf1-Cre;Bdnf+/+ mice acute insulin injection significantly increased plasma glucagon levels (Figure 6D). However, insulin injection failed to significantly increase plasma glucagon in Sf1-Cre;Bdnfflox/flox mice (Figure 6D), indicating that impaired glucagon secretion underlies the reduced glucose levels measured in these animals during the ITT. Together, these data suggest that BDNF expressed by SF1 neurons is required for normal GABAergic innervation of the VMH and robust counter-regulatory responses to insulin-induced hypoglycemia.
4. Discussion
Although there is widespread appreciation that the VMH plays an important role in the central control of feeding behavior and glucose homeostasis [5], [43], [44], until recently technical limitations have frustrated elucidation of underlying neurobiological mechanisms. Similar to other brain regions, the ability of the VMH to regulate these physiological functions is dependent upon the organization and maintenance of neural connections established during development. BDNF expression is enriched in the VMH during development and represents a dynamically regulated factor involved in neuronal plasticity with acute effects on energy balance [22], [23], [24]. In the present study, we demonstrate that BDNF acts as a critical signal required for normal patterns of GABAergic synapses onto SF1 neurons in the VMH, and provide evidence that these alterations in VMH connectivity are associated with lasting impairments in physiological response to hypoglycemia.
4.1. BDNF and connections to SF1 neurons
BDNF impacts synaptogenesis, axonal guidance and cell death in a number of developing neural systems [21], [22]. By using the Sf1-Cre;Bdnfflox/flox model, we were able to conditionally prevent BDNF expression in SF1 neurons during the period when these connections are established [33], [34], [45]. Because the onset of BDNF expression does not normally occur until after VMH neurons have already begun to extend axons to target regions [34], it is unlikely that BDNF is required for primary axon outgrowth from SF1 neurons. Our observation that deletion of BDNF in SF1 neurons did not affect the density of axons innervating VMH targets such as the DMH and LHA supports this interpretation.
Development of afferents to the VMH begins at approximately E17.5, when the VMH starts to express BDNF [9], [45], [46]. The early onset of BDNF expression and VMH synaptogenesis is consistent with our finding that loss of BDNF in SF1 neurons alters the density of GABAergic inputs to SF1 neurons. The increase in GABA innervation was detected specifically in the ventrolateral compartment of the VMH, which contains the highest density of SF1 neurons coexpressing BDNF [9]. In addition to its developmental roles, BDNF also functions to regulate synaptic plasticity in mature animals [22], [47], [48], [49]. That the density of GABA terminals on SF1 neurons at P12 appeared mature, but unaffected in Sf1-Cre;Bdnfflox/flox mice, suggests that this is a late onset circuitry phenotype. However, our results do not rule out a developmental action of BDNF on GABA circuit formation in the VMH, as electrophysiological properties of afferent GABAergic inputs, such as IPSC frequency and amplitude, were not assessed. Furthermore, BDNF action on other developmental events such as neuronal differentiation may be possible. Terminal differentiation of VMH neurons occurs around the onset of BDNF expression and relies upon expression of the orphan nuclear receptor, Steroidogenic Factor 1 (SF1) [18]. SF1 null animals retain expression of markers associated with immature neurons, such as Nkx2.1, and exhibit diminished BDNF expression even after birth, suggesting that they remain in an immature state [18]. Therefore, it is possible that BDNF mediates other developmental actions in the VMH.
The source of the enhanced GABAergic inputs to the VMH observed in Sf1-Cre;Bdnfflox/flox mice is unknown, but the bed nucleus of the stria terminalis, dorsal subregions of the medial amygdala, median preoptic nuclei, and AgRP-expressing neurons of the ARH are candidates. Each region provides direct inputs to the VMHvl, are comprised of predominantly GABAergic neurons, and express TrkB [29], [36], [50], [51], [52].
Interestingly, recent work has identified cholecystokinin-expressing neurons in the superior lateral parabrachial nucleus for their ability to drive downstream responses to hypoglycemia, apparently acting through SF1 neurons of the VMH. Optogenetic stimulation of these neurons was found to increase glucagon and epinephrine secretion, glucogenic enzyme activity in the liver, and circulating blood glucose [11]. However, parabrachial neurons are predominantly glutamatergic (http://www.brain-map.org) [53], [54] and are therefore unlikely to contribute to the observed increase in GABAergic innervation in Sf1-Cre;Bdnfflox/flox mice observed in our studies. VMH afferents also include axon terminals that are localized to the cell-poor shell region surrounding the VMH and innervate dendrites of VMH neurons that extend radially from the nucleus into the shell zone [55], [56]. Although our technical approach precludes visualization of inputs onto SF1 neuronal dendrites, an increase in GABAergic axo-dendritic terminals similar to that observed for axo-somatic GABAergic contacts may be present in the Sf1-Cre;Bdnfflox/flox mice, which would further enhance inhibitory input to SF1 neurons. Furthermore, while no difference in axo-somatic glutamatergic input number onto SF1 neurons was observed between genotypes, axo-dendritic alterations in synapse number could also potentially be present. Consistent with this idea, mice with neuronal depletion of BDNF exhibit reduced expression of the thrombospondin receptor α2δ-1 and a significant decrease in glutamatergic tone onto VMH neurons [57], suggesting that BDNF also regulates excitatory innervation of the VMH, which likely occurs in the dendritic compartment.
BDNF signals through the tyrosine kinase receptor, TrkB, which is expressed in many neurons that provide inputs to the VMH [51], [52]. However, the immature pro-BDNF isoform is also capable of signaling through the p75 neurotrophin receptor, p75NTR [21], [22]. While binding of BDNF to TrkB often drives axonal growth and synaptogenesis, binding of pro-BDNF to p75NTR often exerts opposite effects, suppressing axonal elaboration and synapse formation [22]. Therefore, loss of BDNF-p75NTR signaling can result in enhanced innervation. Indeed, mice with a global mutation of p75NTR exhibit an increased density of cholinergic projections to the hippocampus [58]. Thus, it is possible that GABAergic afferents to the VMH express p75NTR, and that the loss of BDNF-p75NTR signaling in Sf1-Cre;Bdnfflox/flox mice results in an increased density of GABAergic afferents. Interestingly, p75NTR−/− mice also display impairments in insulin tolerance similar to that we observed in Sf1-Cre;Bdnfflox/flox mice, however, this phenotype was attributed to increased insulin sensitivity by the liver and white adipose tissue [59]. It remains to be determined whether the loss of p75NTR in the central nervous system, or more specifically, in VMH afferents, also contributes to the glucose homeostatic phenotype observed in p75NTR−/− mice.
4.2. BDNF and obesity phenotypes
In contrast to the findings of Unger et al., which demonstrated that deletion of BDNF from the mature VMH results in hyperphagia and increased body weight, it is certainly surprising that Sf1-Cre;Bdnfflox/flox mice exhibit normal body weights [24]. The discrepancy between our study and that published by Unger et al., may be explained by the timing and extent of the deletion of the Bdnf gene. Using stereotaxic injections, Unger, et al., deleted Bdnf expression in adulthood from a region encompassing the VMH and DMH [24]. In the current study, we delete BDNF expression during embryonic development from a subset of VMH neurons that express SF1. Therefore, it is possible that a subset of neurons exists in the VMH that expresses BDNF, but not SF1, and that these neurons are responsible for regulation of body weight. Using qPCR we demonstrate that VMH expression of Bdnf is reduced by about 45% on postnatal day 12, suggesting that while these animals exhibit a significant reduction in Bdnf expression, a number of non-SF1 expressing neurons continue to express Bdnf. These data are consistent with previous studies that have demonstrated a colocalization of SF1 protein in 60% of BDNF expressing neurons [9]. This BDNF positive, SF1 negative subpopulation of VMH neurons may be critical for the regulation of feeding behavior and metabolic rate.
In addition, the timing of BDNF deletion may be a critical factor. Following early deletion of the Bdnf gene in the embryo, network level reorganization may compensate for the effect that loss of BDNF during adulthood normally has on body weight. Similar compensatory mechanisms in the circuits controlling body weight have been demonstrated in Agouti-Related Peptide (AgRP) expressing neurons of the ARH. While cell-type specific ablation of AgRP neurons in adulthood results in loss of appetite to the point of starvation, ablation during early postnatal life has almost no impact on body weight [60]. Similarly, it is possible that reduced BDNF expression during hypothalamic development results in network level reorganization of feeding circuits, which compensate for deficits in the ability of the VMH to control body weight.
4.3. BDNF and VMH responses to hypoglycemia
The vast majority of VMH neurons are glutamatergic, and this excitatory activity is thought to be responsible for autonomic stimulation of glucagon secretion from the pancreas, and epinephrine release from the adrenals. Deletion of the vesicular glutamate transporter gene from SF1 neurons reduces glutamate release from the VMH and results in impaired glucagon and epinephrine secretion during insulin-induced hypoglycemia [10]. Thus, following a hypoglycemic event, excitatory drive from the VMH is required to return blood glucose concentrations to within the normal range [10], [11], [61]. CCK inputs to SF1 neurons from the parabrachial nucleus appear to drive this counter-regulatory response (CRR) to hypoglycemia [11]. By contrast, direct injection of GABA agonist into the VMH impairs CRR, including glucagon and epinephrine secretion [62], suggesting that increased inhibitory transmission in the VMH impairs glycemic regulation. Our finding that mice lacking BDNF in SF1 neurons have increased afferent GABAergic innervation of the VMHvl, suggests that glutamatergic output from VMHvl neurons may be suppressed in Sf1-Cre;Bdnfflox/flox mice, which may be responsible for their impaired responses to hypoglycemia and insulin-induced glucagon secretion. Taken together, these data suggest that BDNF may modulate the CRR through changes in inhibitory GABAergic innervation of the VMHvl. Although it remains to be determined whether increases in GABAergic innervation specifically to the VMHdm have a similar impact on glycemic control, enhanced GABA input to SF1 neurons in the VMHvl may be sufficient to impact responses to insulin-induced hypoglycemia. However, SF1 neurons in the dorsomedial part of the VMH likely mediate the effects of leptin on body weight [12], [63]. Similarly, CCK containing inputs from the parabrachial nucleus regulate the activity of SF1 neurons in the VMHdm and counter-regulatory responses to hypoglycemia [11]. It is also worth noting that ERa-expressing neurons in the VMHvl regulate energy expenditure [14], reproduction and aggressive behavior [16], [64]. Although a degree of overlap between Era-expressing neurons with SF1 neurons is likely, it is unknown whether these subpopulations and the behaviors they drive are impacted by altered BDNF expression.
While much more common in patients with diabetes, hypoglycemia can occur in the absence of diabetes or metabolic disease, and can result from a number of factors, such as liver, kidney, or pancreatic disease, gastric bypass, and certain kinds of tumors and medications [65]. Germaine to the findings using the Sf1-Cre;Bdnfflox/flox mouse model system, hypoglycemia in the absence of a body weight or diabetic phenotype is sometimes observed clinically, secondary to endocrine deficits in glucagon, cortisol, growth hormone, or epinephrine secretion [65]. The data presented here support reduced BDNF expression in the VMH as a novel mechanism through which the connectivity of glucose homeostatic circuits can be altered, as well as contribute to pathophysiological neuroendocrine responses to hypoglycemia.
In summary, we have demonstrated that expression of BDNF by SF1 neurons is required for normal patterns of GABA innervation in the VMH. The loss of BDNF from SF1 neurons results in increased GABAergic innervation in the ventrolateral part of the VMH, as well as reduced glucagon secretion in response to insulin-induced hypoglycemia. Failure to mount effective physiological responses to hypoglycemia represents a significant clinical challenge for management of patients with defective glucose homeostasis. The data presented here may provide insight into the neuropathophysiology associated with impaired responses to hypoglycemia, and suggest that altered synapse formation and stability may underlie circuit level changes associated with metabolic disease. Furthermore, the results of this study implicate neurotrophic activity as a potential mechanism through which programming of glucose homeostatic circuits occurs, with lasting effects on normal and pathophysiological responses to hypoglycemia.
Acknowledgments
We thank Caroline Johnson for her assistance in quantifying axonal projection density. This work was supported by grants from the National Institutes of Health to BX and RBS (R01 DK089237) and by The Saban Research Institute, Children's Hospital Los Angeles to AKK.
Conflict of interest
None declared.
References
- 1.Williams K.W., Elmquist J.K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature Neuroscience. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouret S.G. Frontiers in neuroendocrinology. Frontiers in Neuroendocrinology. 2013;34:18–26. doi: 10.1016/j.yfrne.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elson A.E.T., Simerly R.B. Accepted manuscript. Frontiers in Neuroendocrinology. 2015:1–62. doi: 10.1016/j.yfrne.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King B.M. Amygdaloid lesion-induced obesity: relation to sexual behavior, olfaction, and the ventromedial hypothalamus. AJP: Regulatory, Integrative and Comparative Physiology. 2006;291:R1201–R1214. doi: 10.1152/ajpregu.00199.2006. [DOI] [PubMed] [Google Scholar]
- 5.Kim K.W., Sohn J.-W., Kohno D., Xu Y., Williams K., Elmquist J.K. SF-1 in the ventral medial hypothalamic nucleus: a key regulator of homeostasis. Molecular and Cellular Endocrinology. 2011;336:219–223. doi: 10.1016/j.mce.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hetherington A.W., Ranson S.W. Hypothalamic lesions and adiposity in the rat. Anatomical Record. 1940;78:149–172. [Google Scholar]
- 7.Smith F.J., Campfield L.A. Pancreatic adaptation in VMH obesity: in vivo compensatory response to altered neural input. American Journal of Physiology. 1986;251:R70–R76. doi: 10.1152/ajpregu.1986.251.1.R70. [DOI] [PubMed] [Google Scholar]
- 8.Borg W.P., During M.J., Sherwin R.S., Borg M.A., Brines M.L., Shulman G.I. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. Journal of Clinical Investigation. 1994;93:1677–1682. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran P.V., Akana S.F., Malkovska I., Dallman M.F., Parada L.F., Ingraham H.A. Diminished hypothalamic bdnfexpression and impaired VMH function are associated with reduced SF-1 gene dosage. Journal of Comparative Neurology. 2006;498:637–648. doi: 10.1002/cne.21070. [DOI] [PubMed] [Google Scholar]
- 10.Tong Q., Ye C., McCrimmon R.J., Dhillon H., Choi B., Kramer M.D. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metabolism. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garfield A.S., Shah B.P., Madara J.C., Burke L.K., Patterson C.M., Flak J. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metabolism. 2014;20:1030–1037. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhillon H., Zigman J.M., Ye C., Lee C.E., McGovern R.A., Tang V. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Kurrasch D.M., Cheung C.C., Lee F.Y., Tran P.V., Hata K., Ingraham H.A. The Neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. Journal of Neuroscience. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musatov S., Chen W., Pfaff D.W., Mobbs C.V., Yang X.-J., Clegg D.J. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klöckener T., Hess S., Belgardt B.F., Paeger L., Verhagen L.A.W., Husch A. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nature Neuroscience. 2011:1–10. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa S.M., Newstrom D.W., Warne J.P., Flandin P., Cheung C.C., Lin-Moore A.T. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Reports. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda Y., Luo X., Abbud R., Nilson J.H., Parker K.L. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Molecular Endocrinology. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 18.Tran P.V., Lee M.B., Marín O., Xu B., Jones K.R., Reichardt L.F. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Molecular and Cellular Neuroscience. 2003;22:441–453. doi: 10.1016/S1044-7431(03)00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majdic G. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 20.Kim K.W., Zhao L., Donato J., Kohno D., Xu Y., Elias C.F. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proceedings of the National Academy of Sciences. 2011;108:10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annual Review of Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu B., Pang P.T., Woo N.H. vol. 6. Nature Publishing Group; 2005. pp. 603–614. (The yin and yang of neurotrophin action). [DOI] [PubMed] [Google Scholar]
- 23.Xu B., Goulding E.H., Zang K., Cepoi D., Cone R.D., Jones K.R. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nature Neuroscience. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unger T.J., Calderon G.A., Bradley L.C., Sena-Esteves M., Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. Journal of Neuroscience. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y., Wang Q., Huang X.F. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience. 2009;160:295–306. doi: 10.1016/j.neuroscience.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 26.Lyons W.E., Mamounas L.A., Ricaurte G.A., Coppola V., Reid S.W., Bora S.H. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proceedings of the National Academy of Sciences U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kernie S.G., Liebl D.J., Parada L.F. BDNF regulates eating behavior and locomotor activity in mice. Embo Journal. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rios M., Fan G., Fekete C., Kelly J., Bates B., Kuehn R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Molecular Endocrinology. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 29.Liao G.-Y., An J.J., Gharami K., Waterhouse E.G., Vanevski F., Jones K.R. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nature Medicine. 2012;18:564–571. doi: 10.1038/nm.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Bouyer K., Simerly R.B. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. Journal of Neuroscience. 2013;33:840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coupé B., Dutriez-Casteloot I., Breton C., Lefèvre F., Mairesse J., Dickes-Coopman A. Perinatal undernutrition modifies cell proliferation and brain-derived neurotrophic factor levels during critical time-windows for hypothalamic and hippocampal development in the male rat. Journal of Neuroendocrinology. 2009;21:40–48. doi: 10.1111/j.1365-2826.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 33.McClellan K.M., Parker K.L., Tobet S. Development of the ventromedial nucleus of the hypothalamus. Frontiers in Neuroendocrinology. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Cheung C.C., Kurrasch D.M., Liang J.K., Ingraham H.A. Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. Journal of Comparative Neurology. 2013;521:1268–1288. doi: 10.1002/cne.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellovade T.L., Young M., Ross E.P., Henderson R., Caron K., Parker K. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. Journal of Comparative Neurology. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Canteras N.S., Simerly R.B., Swanson L.W. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. Journal of Comparative Neurology. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg D., Chen P., Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. Journal of Comparative Neurology. 2013;521:3167–3190. doi: 10.1002/cne.23338. [DOI] [PubMed] [Google Scholar]
- 38.Canteras N.S., Swanson L.W. The dorsal premammillary nucleus: an unusual component of the mammillary body. Proceedings of the National Academy of Sciences U S A. 1992;89:10089–10093. doi: 10.1073/pnas.89.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risold P.Y., Canteras N.S., Swanson L.W. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. Journal of Comparative Neurology. 1994;348:1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- 40.Canteras N.S., Simerly R.B., Swanson L.W. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. Journal of Comparative Neurology. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- 41.Bohland M., Matveyenko A.V., Saberi M., Khan A.M., Watts A.G., Donovan C.M. Activation of hindbrain neurons is mediated by portal-mesenteric vein glucosensors during slow-onset hypoglycemia. Diabetes. 2014;63:2866–2875. doi: 10.2337/db13-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jokiaho A.J., Donovan C.M., Watts A.G. The rate of fall of blood glucose determines the necessity of forebrain-projecting catecholaminergic neurons for male rat sympathoadrenal responses. Diabetes. 2014;63:2854–2865. doi: 10.2337/db13-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King B.M. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiology & Behavior. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Chan O., Sherwin R. Influence of VMH fuel sensing on hypoglycemic responses. Trends in Endocrinology & Metabolism. 2013;24:616–624. doi: 10.1016/j.tem.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto A., Arai Y. Development of sexual dimorphism in synaptic organization in the ventromedial nucleus of the hypothalamus in rats. Neuroscience Letters. 1986;68:165–168. doi: 10.1016/0304-3940(86)90135-7. [DOI] [PubMed] [Google Scholar]
- 46.Tobet S.A., Henderson R.G., Whiting P.J., Sieghart W. Special relationship of gamma-aminobutyric acid to the ventromedial nucleus of the hypothalamus during embryonic development. Journal of Comparative Neurology. 1999;405:88–98. doi: 10.1002/(sici)1096-9861(19990301)405:1<88::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 47.Poo M.M. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 48.Lu B. BDNF and activity-dependent synaptic modulation. Learning & Memory. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein R., Parada L.F., Coulier F., Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. Embo Journal. 1989;8:3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merlio J.P., Ernfors P., Jaber M., Persson H. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neuroscience. 1992;51:513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- 52.Masana Y., Wanaka A., Kato H., Asai T., Tohyama M. Localization of trkB mRNA in postnatal brain development. Journal of Neuroscience Research. 1993;35:468–479. doi: 10.1002/jnr.490350503. [DOI] [PubMed] [Google Scholar]
- 53.Niu J.-G., Yokota S., Tsumori T., Qin Y., Yasui Y. Glutamatergic lateral parabrachial neurons innervate orexin-containing hypothalamic neurons in the rat. Brain Research. 2010;1358:110–122. doi: 10.1016/j.brainres.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 54.Kaur S., Pedersen N.P., Yokota S., Hur E.E., Fuller P.M., Lazarus M. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. Journal of Neuroscience. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millhouse O.E. Certain ventromedial hypothalamic afferents. Brain Research. 1973;55:89–105. doi: 10.1016/0006-8993(73)90490-3. [DOI] [PubMed] [Google Scholar]
- 56.Flanagan-Cato L.M., Fluharty S.J., Weinreb E.B., LaBelle D.R. Food restriction alters neuronal morphology in the hypothalamic ventromedial nucleus of male rats. Endocrinology. 2007;149:93–99. doi: 10.1210/en.2007-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cordeira J.W., Felsted J.A., Teillon S., Daftary S., Panessiti M., Wirth J. Hypothalamic dysfunction of the thrombospondin receptor 2-1 underlies the overeating and obesity triggered by brain-derived neurotrophic factor deficiency. Journal of Neuroscience. 2014;34:554–565. doi: 10.1523/JNEUROSCI.1572-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeo T.T., Chua-Couzens J., Butcher L.L., Bredesen D.E., Cooper J.D., Valletta J.S. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. Journal of Neuroscience. 1997;17:7594–7605. doi: 10.1523/JNEUROSCI.17-20-07594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baeza-Raja B., Li P., Le Moan N., Sachs B.D., Schachtrup C., Davalos D. p75 neurotrophin receptor regulates glucose homeostasis and insulin sensitivity. Proceedings of the National Academy of Sciences. 2012;109:5838–5843. doi: 10.1073/pnas.1103638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luquet S. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 61.Shimazu T., Ishikawa K. Modulation by the hypothalamus of glucagon and insulin secretion in rabbits: studies with electrical and chemical stimulations. Endocrinology. 1981;108:605–611. doi: 10.1210/endo-108-2-605. [DOI] [PubMed] [Google Scholar]
- 62.Chan O., Zhu W., Ding Y., McCrimmon R.J., Sherwin R.S. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes. 2006;55:1080–1087. doi: 10.2337/diabetes.55.04.06.db05-0958. [DOI] [PubMed] [Google Scholar]
- 63.Bingham N.C., Anderson K.K., Reuter A.L., Stallings N.R., Parker K.L. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee H., Kim D.-W., Remedios R., Anthony T.E., Chang A., Madisen L. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cryer P.E., Axelrod L., Grossman A.B., Heller S.R., Montori V.M., Seaquist E.R. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2009;94:709–728. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 66.Dong H.-W. Wiley; 2008. The Allen Institute for brain science, the Allen reference atlas, (Book + CD-ROM) [Google Scholar]