Abstract
Sipuleucel-T treatment is associated with a significant and consistent survival benefit in patients with metastatic castrate-resistant prostate cancer. Most adverse events are infusion related, manageable, and of short duration. Early screening and diagnosis of metastatic disease is important, as the greatest survival benefit may occur in patients with a lower disease burden. The short duration of sipuleucel-T treatment facilitates the use of subsequent therapies. Sipuleucel-T is now being used in the clinic for patients with a lower disease burden. We present our own experience with the use of sipuleucel-T in the setting of a large urology practice.
Key words: Metastatic castrate-resistant prostate cancer, Immunotherapy, Sipuleucel-T, Clinical practice
Immunotherapies are designed to redirect the patient’s immune system to recognize and remove cancerous cells. Sipuleucel-T was the first autologous cellular immunotherapy to be approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of asymptomatic or minimally symptomatic, metastatic castrate-resistant prostate cancer (mCRPC).1,2 Other immunotherapies, such as vaccines with different actions (GVAX and PSATRICOM), anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4; ipilimumab), and anti-PD-L1 or -L2 monoclonal antibodies (pidilizumab), are in late-stage clinical development for prostate cancer and may provide clinicians and patients with additional future treatment options. This article provides clinicians with information about the best practices for sipuleucel-T treatment in the clinic, based on currently available data and our experience in a community practice.
Overview of Sipuleucel-T
Sipuleucel-T is manufactured from a patient’s own immune cells.1–4 These cells are removed by leukapheresis and sent to a processing facility, where they are enriched for peripheral blood mononuclear cells, which include antigen-presenting cells (APCs), T cells, B cells, and natural killer cells.5 The cells are then cultured with PA2024, a fusion protein combining granulocyte macrophage colony-stimulating factor with prostatic acid phosphatase (PAP). This leads to activation of APCs. The resulting product is sipuleucel-T, which is returned to the clinic where it is administered to the patient by intravenous infusion. In a full course of therapy, this process is repeated twice at approximately 2-week intervals.1,2
Given that sipuleucel-T is designed to target PAP, a protein almost exclusively expressed in prostate tissue,6 this treatment approach aims to specifically focus on the immune response to prostate cancer. Once infused, the APCs in the sipuleucel-T product are thought to present PAP peptides in vivo to activate CD4+ and CD8+ T cells.1–3 Sipuleucel-T treatment generates broad and durable systemic immune responses.7 These antitumor immune respons es were reported to be sustained for at least 26 weeks following initial sipuleucel-T treatment.7 In contrast, the activity of cytotoxic therapies is not sustained beyond treatment discontinuation.8 Additionally, evidence from the Sipuleucel-T as Neoadjuvant Treatment in Prostate Cancer (NeoACT) open-label, phase 2 study of patients with localized prostate cancer prior to radical prostatectomy shows that sipuleucel-T induces infiltration of activated T cells to the site of the tumor.9
The FDA and EMA approval of sipuleucel-T was based on efficacy and safety findings from three phase 3 clinical studies: D9901, D9902A, and Immunotherapy Prostate Adenocarcinoma Therapy (IMPACT).4,10,11 In the phase 3 IMPACT study, a significant treatment effect was observed with sipuleucel-T across more than 20 subgroups, including patients who had received prior docetaxel.4 In other studies, there were no deleterious effects of prior docetaxel on sipuleucel-T efficacy.12,13 In the phase 4, Registry of Sipuleucel-T Therapy in Men With Advanced Prostate Cancer (PROCEED) study, similar sipuleucel-T product parameters were reported in patients with or without previous docetaxel therapy.12 In addition, a survival benefit was reported with sipuleucel-T in patients with subsequent docetaxel use (hazard ratio [HR] 0.825; 95% confidence interval [CI], 0.619–1.101) and those without subsequent docetaxel use (HR 0.693; 95% CI, 0.545–0.880).13 Concurrent abiraterone acetate has also been shown to have no detrimental effects on sipuleucel-T.14 Furthermore, a positive correlation was reported between overall survival (OS) and APC activation, APC count, and total nucleated cell count.7 Sipuleucel-T was generally well tolerated across the phase 3 studies.4,10,11 The majority of reported adverse events (AEs) were infusion related, grade 1 or 2, and of short duration.4,10,11
Identifying Patients Most Suited to Sipuleucel-T Treatment
Early screening and diagnosis are important to identify patients who may benefit most from sipuleucel-T treatment. Data from the IMPACT study have shown that the greatest magnitude of benefit occurs in patients with a lower disease burden.15,16 In a retrospective subgroup analysis, median survival was improved by 13.0 months with sipuleucel-T vs control among the subgroup of patients within the lowest quartile of baseline prostate-specific antigen (PSA; PSA ≤ 22.1 ng/mL) compared with an improvement of 2.8 months in the subgroup with the highest quartile (PSA > 134.1 ng/mL; Table 1).16 Patients with a lower disease burden are likely to have a more robust immune system than those with more advanced disease, and as such will demonstrate a more effective immunologic response to sipuleucel-T.17,18 This improved response is likely to occur as a result of less immunosuppression, both systemically and within the tumor microenvironment.16 Additionally, this may also result from the delayed onset of action and sustained immune response of sipuleucel-T.16 Data from the phase 4, multicenter PROCEED registry of patients with advanced prostate cancer suggest that treatment practices with sipuleucel-T in the real-world setting are changing in accordance with these findings. Among patients in the registry treated from January 2011 through February 2013, baseline disease characteristics of patients receiving sipuleucel-T have improved over time, with decreasing baseline PSA and lower use of prior docetaxel.19
Table 1.
Clinical Outcomes Based on Quartiles of Baseline PSA: Overall Survival Outcome in Patients Participating in the IMPACT Trial
| Quartiles of Baseline PSA (ng/mL) |
||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| ≤22.1 | > 22.1–50.1 | > 50.1–134.1 | > 134.1 | |
| (n = 128) | (n = 128) | (n = 128) | (n = 128) | |
| Overall survival, mo (sipuleucel-T group) | 41.3 | 27.1 | 20.4 | 18.4 |
| Overall survival, mo (control group) | 28.3 | 20.1 | 15 | 15.6 |
| Difference in survival, mo (sipuleucel-T – control) | 13.0 | 7.1 | 5.4 | 2.8 |
| Hazard ratio (95% CI) | 0.51 (0.31, 0.85) | 0.74 (0.47, 1.17) | 0.81 (0.52, 1.24) | 0.84 (0.55, 1.29) |
Values are median (range) months.
CI, confidence interval; IMPACT, Immunotherapy for Prostate Adenocarcinoma Treatment; PSA, prostate-specific antigen; Q, quartile.
Data from Schellhammer PF et al.16
Our experience in a community-based urology practice demonstrates a different population from that of the IMPACT trial; our patients had a lower baseline PSA, indicating an earlier stage of mCRPC. The baseline quartile distribution in our practice is remarkably different from the IMPACT data and all four quartiles had much lower PSA cutoff than IMPACT (Table 2). Of the 21 deceased patients in our patient population, six (28.6%) died in the first 6 months, four (19%) in the first 3 months, seven (33%) in 6 to 12 months, three (14.3%) in 12 to 18 months, and five (23.8%) > 18 months after the last sipuleucel-T injection. In our patient population with an earlier stage of mCRPC (as shown by lower PSA level), it would seem reasonable to initiate immunotherapy with an attempt to improve OS by changing the natural history of the disease.
Table 2.
Clinical Outcomes Based on Quartiles of Baseline PSA: Demographic and Clinical Data Recorded in Patients With mCRPC Attending the Associated Medical Professionals Urology Practice, Syracuse, NY
| Quartiles of Baseline PSA (ng/mL) |
||||
|---|---|---|---|---|
| Variable | Q1 | Q2 | Q3 | Q4 |
| < 3.89 ng/mL | 3.9–14.60 ng/mL | 14.61–83.9 ng/mL | > 84 ng/mL | |
| (n = 18) | (n = 18) | (n = 18) | (n = 18) | |
| Age, y | 74 (58–90) | 73.5 (61–90) | 76 (53–87) | 73.5 (54–89) |
| BMI, kg/m2 | 29.4 (20.2–43.1) | 29.3 (21.9–35.4) | 29.5 (22–38.2) | 28.7 (29.7–39.9) |
| Time of follow-up, mo | 9 (1–29) | 15 (1–25) | 12 (2–28) | 9 (2–27) |
| Number of subjects died (%) | 2 (11) | 5 (28) | 7 (39) | 7 (39) |
| Number of subjects alive (%) | 16 (89) | 13 (72) | 11 (61) | 11 (61) |
Values are median (range).
BMI, body mass index; mCRPC, metastatic castrate-resistant prostate cancer; PSA, prostate-specific antigen; Q, quartile.
Data from Schellhammer PF et al.16
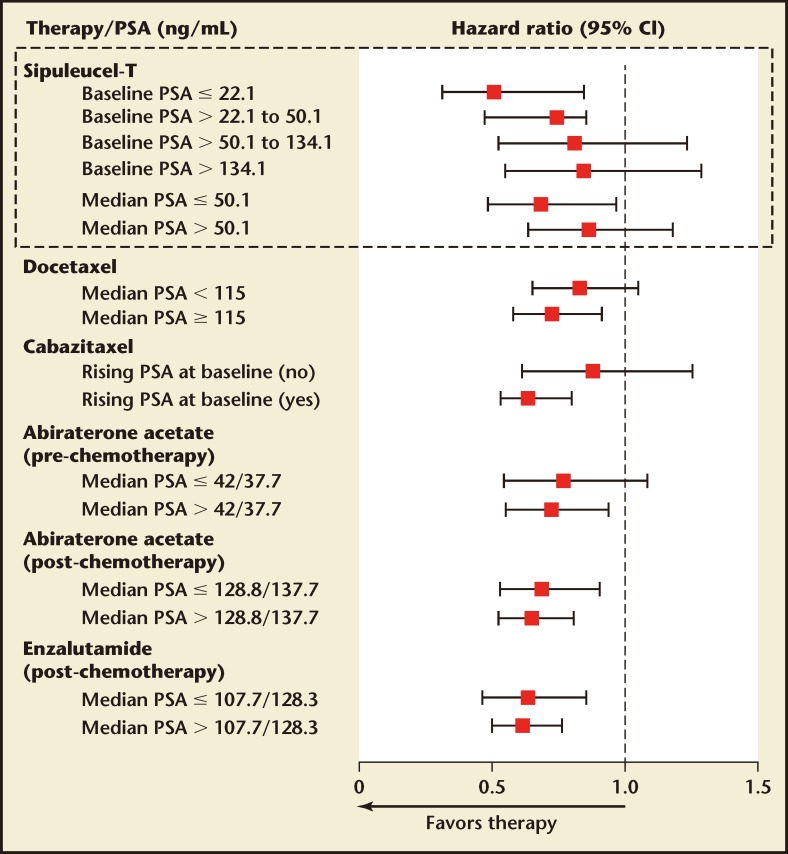
The greater benefit in earlier mCRPC that is seen with sipuleucel-T is in contrast to other mCRPC therapies, in which a larger treatment effect is observed with higher versus lower baseline PSA values (Figure 1). Therefore, patients with mCRPC should be identified as early as possible in their disease course (ie, upon detection of metastatic [M1] disease) in order for them to receive the maximum possible benefit from sipuleucel-T treatment. Early detection of M1 disease is therefore critical and baseline PSA level, as a marker of disease burden, may help to guide patient identification. However, within the minimally symptomatic or asymptomatic mCRPC patient population, no single factor carries enough weight to be used as a sole criterion for identifying patients for whom sipuleucel-T treatment would be suitable. Clinical judgment and imaging data are currently the basis for patient identification. For example, it may be appropriate to prioritize sipuleucel-T for those with a relatively slow PSA increase and those considered unlikely to require rapid reduction of tumor burden with cytoreductive treatment in the short term. In addition to clinical judgment, an integrated assessment of the Halabi factors may aid the identification of suitable patients.20 Finally, asymptomatic or minimally symptomatic disease may be defined in practical terms by an absence of opioid analgesic use,10 which is aligned with the IMPACT study eligibility criteria.
Figure 1.
Overall survival by baseline PSA levels across metastatic castrate-resistant prostate cancer clinical trials. CI, confidence interval; PSA, prostate-specific antigen. Reprinted with permission from Schellhammer PF et al.16
Integration of Sipuleucel-T Within the mCRPC Treatment Paradigm
A number of treatments have now been approved for mCRPC prior to chemotherapy. The National Comprehensive Cancer Network (NCCN) recommends use of sipuleucel-T in patients with minimally symptomatic disease, a good performance level (Eastern Cooperative Oncology Group performance status 0–1) and at least 6 months of life expectancy.21 Sequencing sipuleucel-T before other therapies is also supported by the NCCN.21 The rationale for sequencing sipuleucel-T early in the treatment paradigm for such patients is multifold. First, patients with a low disease burden appear to receive the greatest benefit from sipuleucel-T based on a retrospective analysis of the IMPACT trial16 and data compiled from our community-based urology practice.
Second, sipuleucel-T has a short treatment duration (∼4 wk), providing an opportunity for patients to receive subsequent treatment. Most patients in the IMPACT study went on to receive other therapies.4 In patients with newly identified M1 disease, there is no urgency to begin treatment with cytoreductive treatment. For example, in a clinical trial of prechemotherapy abiraterone acetate, control patients had a median time of 23.7 months to opioid use for cancer-related pain.22 However, the treatment duration with abiraterone acetate and enzalutamide may be prolonged (> 15 mo) and patients may progress rapidly at treatment failure. Therefore, the optimal treatment window for sipuleucel-T is prior to treatment with such agents. However, a patient of ours with mCRPC had an undetectable PSA for 14 months with enzalutamide alone. The patient then received sipuleucel-T plus enzalutamide combined treatment, and achieved a complete radiographic response 6 months after sipuleucel-T plus enzalutamide treatment.23 This complete radiographic response may represent one of the first reported in the literature.
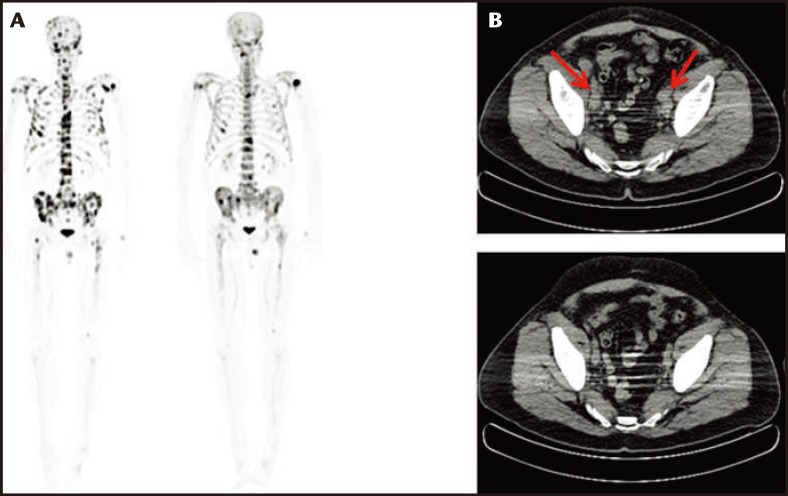
Enzalutamide was approved for use in the treatment of postchemotherapy mCRPC patients in August 2012, whereas prechemotherapy approval was granted in September 2014. The results of the IMPACT trial demonstrate that immunotherapy as a monotherapy is not sufficient to hinder or stop progression of mCRPC, especially in patients with a high baseline PSA level. The timing and sequencing of combination therapy is becoming important for the treatment of patients with mCRPC. Promising results were seen in a study of 19 patients in our practice who received sequential enzalutamide treatment after completion of sipuleucel-T. The average timing for the initiation of enzalutamide was 2.5 ± 1.6 months after completion of immune therapy. This cohort was unique in that all of the patients were treated with enzalutamide before chemotherapy (ie, off label). The median duration of enzalutamide therapy was 5.4 months (range, 1–13 mo). One patient showed radiographic full regression of metastatic lesions in bone, lung, and lymph nodes (Figure 2). One patient showed radiographic full regression of bony metastatic lesions. Two cases showed radiographic partial regression of bony metastatic disease; 13 cases showed stable disease according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria, and two patients died due to progression of mCRPC. The patients with full and partial responses were in the lowest quartile of PSA level (≤ 3.87 ng/mL). In contrast, all cases of progression and death were documented in the highest quartile of PSA (> 84 ng/mL). Our experience demonstrates a promising effect using sequential sipuleucel-T immunotherapy followed by enzalutamide.
Figure 2.
Sodium fluoride computed tomography/positron emission tomography (CT/PET) scans in a patient with bone metastases. (A) Sodium fluoride CT/PET scan showing diffuse metastatic bone disease before treatment with regression and many lesions 10 months after treatment with sipuleucel-T followed by enzalutamide. (B) CT scan of the pelvis showing pelvic lymph node enlargement (red arrows) before treatment with sipuleucel-T followed by enzalutamide with regression of nodes 10 months after treatment.
Finally, the mechanism of action (MoA) of sipuleucel-T does not overlap with any other currently approved treatment. Therefore, cross-resistance between sipuleucel-T and other available agents is not a concern. Also, because of the unique MoA, the AEs associated with sipuleucel-T treatment are primarily transient, low grade, and infusion related. Thus, sipuleucel-T would not preclude subsequent treatment with other therapies. This is supported by an exploratory study that found that sipuleucel-T was well tolerated in combination with abiraterone acetate plus prednisone, as either a concurrent or sequential therapy.14
Setting Patient Expectations
Management of patient expectations is another important consideration in the clinic. Physicians should explain to patients before therapy begins the leukapheresis and infusion processes, patient responsibilities, and expected treatment outcomes. This is particularly important with sipuleucel-T, because it is administered differently than other therapies and has a unique MoA. Importantly, patients should understand that sipuleucel-T treatment can achieve a long-term OS benefit but may not induce a PSA or radiographic response.8,24 Therefore, serial imaging examinations will need to be performed in the future to determine disease progression. Although an association between PSA response and OS may be anticipated with androgen receptor-targeted therapies, the same may not be true for therapies producing OS benefits via androgen receptor-independent mechanisms.25–27 Therefore, the predictive ability of PSA response for OS is dependent on MoA and should not be anticipated for immunotherapy.
Clinical Management of Infusion-related AEs
Infusion-related AEs, such as chills, fever, fatigue, nausea, and headache, are the most common with sipuleucel-T, although these are typically low grade and transient.10 In this integrated safety analysis of two phase 3 studies, no grade 3 or 4 AEs were reported in ≥ 5% of patients receiving sipuleucel-T. The majority of infusion-related, serious, and grade 3 or 4 AEs were ≤ 24 hours in duration, and all patients recovered. Less than 3% of patients were not able to receive all three infusions because of treatment-related AEs. In our clinic, we typically pretreat patients with acetaminophen, 1000 mg plus diphenhydramine, 25 mg, to minimize the risk of infusion reactions. For patients who develop rigors, pausing the infusion for a time and restarting it at a slower rate can be an effective strategy. Although we take care to keep intravenous meperidine (25 mg) on hand to treat infusion-related reactions, the above strategies have not so far rendered this necessary. Infusion-related AEs can generally be managed within the outpatient setting and most symptoms can be managed in line with their standard of care.1,2 However, sipuleucel-T treatment should be withdrawn if an anaphylactic reaction occurs.
On a separate point, a possible increased risk of cerebrovascular events has been observed with sipuleucel-T treatment. A combined analysis of four randomized, controlled trials of sipuleucel-T reported an incidence rate 3.5% (21 of 601 patients) for cerebrovascular events compared with 2.6% (8 of 303 patients) in control subjects.28 Accounting for the time from randomization, the number of events per 100 personyears of follow-up was 2.01 (95% CI, 1.25–3.08) for sipuleucel-T cases versus 1.50 (95% CI, 0.65–2.96) for control subjects. The ongoing, observational PROCEED study aims to quantify the risk of cerebrovascular events following sipuleucel-T treatment.
Conclusions
Experience with sipuleucel-T clinical trials is currently being translated into the clinic with new analyses guiding treatment decisions; for example, patients with lower PSA levels indicative of an early stage of mCRPC are associated with the greatest clinical benefit. Clinical trial data have also highlighted the importance of setting patient expectations upfront in the clinic. Immunotherapy may require a shift in mindset for patients who link their disease to their PSA level, and they should understand that sipuleucel-T treatment can extend life but that they may not see the reduction in PSA level that is the hallmark of other prostate cancer therapies. The availability in the clinic of baseline, on-treatment, and short-term post-treatment markers of clinical activity or benefit with immunotherapy would be useful to both physicians and patients. In this regard, the survival benefit with sipuleucel-T is correlated with APC activation and anti-PA2024 responses,29 and the identification of predictive markers is a key avenue of investigation.
We have found that most infusion-related side effects are avoidable or manageable with appropriate premedication and alteration of infusion rate. The optimal sequence and timing of mCRPC treatment options, including sipuleucel-T, remains a relatively unexplored area. In that respect, the case study that we have presented here, in which sipuleucel-T treatment was successfully followed by enzalutamide, suggests one possible sequencing strategy, and such sequencing strategies are the focus of multiple ongoing studies. Although clinical trial data can inform clinical practice, experience from community practice, as presented in this paper, can also provide useful guidance for physicians, and additionally may inform future clinical trials.
Main Points.
Sipuleucel-T is designed to target prostatic acid phosphatase, a protein almost exclusively expressed in prostate tissue; this treatment approach aims to specifically focus on the immune response to prostate cancer.
Sipuleucel-T treatment generates broad and durable systemic immune responses; these antitumor immune responses were reported to be sustained for at least 26 weeks following initial treatment. Evidence from a recent clinical trial of patients with localized prostate cancer prior to radical prostatectomy shows that sipuleucel-T induces infiltration of activated T cells to the site of the tumor.
Early screening and diagnosis are important to identify patients who may benefit most from sipuleucel-T treatment. Data have shown that the greatest magnitude of benefit occurs in patients with a lower disease burden, as they are likely to have a more robust immune system than those with more advanced disease, and as such will demonstrate a more effective immunologic response to sipuleucel-T.
The greater benefit in earlier metastatic castrate-resistant prostate cancer (mCRPC) that is seen with sipuleucel-T is in contrast to other mCRPC therapies, in which a larger treatment effect is observed with higher versus lower baseline prostate-specific antigen values. Therefore, patients with mCRPC should be identified as early as possible in their disease course in order for them to receive the maximum possible benefit from treatment.
The mechanism of action of sipuleucel-T does not overlap with any other currently approved treatment. Therefore, cross-resistance between sipuleucel-T and other available agents is not a concern. Also, because of the unique mechanism of action, the adverse events associated with sipuleucel-T treatment are primarily transient, low grade, and infusion related.
Footnotes
Dr. Pieczonka has received financial support from Astellas Pharma US (Northbrook, IL), Bayer HealthCare Pharmaceuticals (Berlin, Germany), Janssen Pharmaceuticals (Titusville, NJ), and Dendreon (Seattle, WA). Drs. Telonis, Mouraviev, and Albala report no real or apparent conflicts of interest.
Medical writing and coordination support was provided by Katherine St. John and Angela Rogers of Gardiner-Caldwell Communications (Macclesfield, United Kingdom), and funded by Dendreon (Seattle, WA). This article includes data from clinical trials that were sponsored by Dendreon.
References
- 1.PROVENGE [package insert] Seattle, WA: Dendreon Corporation; 2014. [Google Scholar]
- 2.PROVENGE [summary of product characteristics] London, UK: Dendreon UK Limited,; [Accessed May 19, 2015]. European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/document_librar y/EPAR_-_Product_Information/human/002513/WC500151099.pdf. [Google Scholar]
- 3.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, et al. IMPACT Study Investigators., authors Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigenloaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 6.Fong L, Ruegg CL, Brockstedt D, et al. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J Immunol. 1997;159:3113–3117. [PubMed] [Google Scholar]
- 7.Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong L, Weinberg VK, Chan SE, et al. Neoadjuvant sipuleucel-T in localized prostate cancer: effects on immune cells within the prostate tumor microenvironment. J Clin Oncol. 2012;30(suppl):2564. [Google Scholar]
- 10.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from two randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 11.Small EJ, Schellhammer PF, Higano CS, et al. Placebocontrolled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 12.Higano CS, Armstrong AJ, Cooperberg MR, et al. Impact of prior docetaxel (D) on sipuleucel-T (sipuleucel-T) product parameters in PROCEED patients (pts) J Clin Oncol. 2013;31(suppl):5034. [Google Scholar]
- 13.Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who receive sipuleucel-T (Provenge) followed by docetaxel derive greatest survival benefit; Presented at: The Chemotherapy Foundation Symposium 14th Annual Meeting; November 8–11, 2006; New York, NY. [Google Scholar]
- 14.Small EJ, Lance R, Gardner TA, et al. A phase 2 trial of sipuleucel-T in combination with concurrent or sequential abiraterone acetate in patients with metastatic castrate-resistant prostate cancer (mCRPC) Eur J Cancer. 2013;49(suppl 2):2860. [Google Scholar]
- 15.Kantoff PW. The Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial: lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T. [Accessed May 19, 2015]. Published October 28, 2013. UroToday website. http://www.urotoday.com/prostate-cancerimmunotherapy/65590-the-immunotherapyfor-prostate-adenocarcinoma-treatment-impacttrial-lower-baseline-prostate-specific-antigen-isassociated-with-a-greater-overall-survival-benefitfrom-sipuleucel-t-beyond-the-abstract-by-philip-wkantoff-md.html. [DOI] [PubMed]
- 16.Schellhammer PF, Chodak G, Whitmore JB, et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81:1297–1302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 17.Töpfer K, Kempe S, Müller N, et al. Tumor evasion from T cell surveillance. J Biomed Biotechnol. 2011;2011:918471. doi: 10.1155/2011/918471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooperberg M, Sartor O, Pieczonka C, et al. Treatment practice patterns in metastatic castration-resistant prostate cancer (mCRPC) patients prior to receiving sipuleucel-T: data from PROCEED; Presented at: American Urology Association Annual Meeting; May 4–8, 2013; San Diego, CA. Abstract 972. [Google Scholar]
- 20.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormonerefractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prostate Cancer. Guidelines for Patients. Version 1.2015. Updated October 24, 2014. National Comprehensive Cancer Network website. [Accessed May 19, 2015]. Available at: http://www.nccn.org/patients/guidelines/prostate/index.html.
- 22.Ryan CJ, Smith MR, de Bono JS, et al. COU-AA-302 Investigators., authors Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graff JN, Drake CG, Beer TM. Complete biochemical (prostate-specific antigen) response to sipuleucel-T with enzalutamide in castration-resistant prostate cancer: a case report with implications for future research. Urology. 2013;81:381–383. doi: 10.1016/j.urology.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Lorenzo G, Ferro M, Buonerba C. Sipuleucel-T (Provenge_) for castration-resistant prostate cancer. BJU Int. 2012;110:E99–E104. doi: 10.1111/j.1464-410X.2011.10790.x. [DOI] [PubMed] [Google Scholar]
- 25.Fleming MT, Morris MJ, Heller G, Scher HI. Posttherapy changes in PSA as an outcome measure in prostate cancer clinical trials. Nat Clin Pract Oncol. 2006;3:658–667. doi: 10.1038/ncponc0664. [DOI] [PubMed] [Google Scholar]
- 26.Tannock IF, de Wit R, Berry WR, et al. TAX 327 Investigators., authors Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong AJ, Garrett-Mayer E, de Wit R, et al. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–211. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- 28.Hall SJ, Klotz L, Pantuck AJ, et al. Integrated safety data from 4 randomized, double-blind, controlled trials of autologous cellular immunotherapy with sipuleucel-T in patients with prostate cancer. J Urol. 2011;186:877–881. doi: 10.1016/j.juro.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 29.Sheikh NA, Wesley JD, Perdue N, et al. Evaluation of immune activation following neoadjuvant sipuleucel-T in subjects with localized prostate cancer. J Clin Oncol. 2012;30(suppl):2563. [Google Scholar]