Abstract
Matrine is a naturally occurring alkaloid extracted from the Chinese herb Sophora flavescens. It has been demonstrated to exhibit antiproliferative properties, promote apoptosis and inhibit cell invasion in a number of cancer cell lines. It has also been shown to improve the efficacy of chemotherapy when it is combined with other chemotherapy drugs. However, the therapeutic efficacy of matrine for prostate cancer remains poorly understood. In the present study, we showed that matrine inhibited the proliferation, migration and invasion of both DU145 and PC-3 cells in a dose- and time-dependent manner. It also reduced the cell population at S phase and increased the cell population at sub-G1 phase. The increases in both the apoptotic cell population and cell population at S and sub-G1 phases consistently indicated a pro-apoptotic effect of matrine. Decreases in levels of P65, p-P65, IKKα/β, p-IKKα/β, IKBα and p-IKBα as detected by immunoblot analysis in the matrine-treated DU145 and PC-3 cells suggested an involvement of the NF-κB signaling pathway. Therefore, it is a novel promising addition to the current arsenal of chemotherapy drugs for the treatment of androgen-independent prostate cancer.
Keywords: matrine, androgen-independent prostate cancer, P65, IKBα, NF-κB signaling pathway
Introduction
Prostate cancer is the most common cancer and the second leading cause of cancer-related mortality among men in the US (1). Rates of prostate cancer are high in Western countries and low in Asian countries. Lifespan expansion, environmental changes and improvement of diagnostic techniques have contributed to the recent increases in prostate cancer incidence in China. Prostate cancer has now become the number one cancer of the urogenital system in China (2).
For patients with early-stage prostate cancer, androgens are the major regulators of cellular proliferation, and the growth of tumors is androgen-dependent. The treatment for patients who are not suitable for surgical intervention is by androgen deprivation (3). Long-term treatment with androgen deprivation was reported to have adverse effects. Nevertheless, there is no curative treatment for 70–80% of androgen-independent prostate cancer patients (4). Therefore, developing novel therapeutic modalities for the treatment of androgen-independent prostate cancer has now become the focus of research.
Sophora flavescens Aiton is a type of leguminous plant growing in China, Japan and some European countries. Its dry root is widely used in Traditional Chinese Medicine for the treatment of viral hepatitis (5–7), cardiac (8,9) and skin diseases (10). Matrine, an alkaloid extracted from the dry root of Sophora flavescens Aiton with low toxicity, has a molecular formula of C15H24N2O (10–12). It has been shown that matrine can inhibit proliferation and induce apoptosis of cancer cells developed from breast (13,14), pancreatic (15), liver (16–18), lung (19), gastric (20) and colon cancer (21,22), acute myeloid leukemia (23), multiple myeloma (24), retinoblastoma (25,26), esophageal cancer (27), nasopharyngeal carcinoma (28), osteosarcoma (29), harboring melanoma (30), rhabdomyosarcoma (31) and cervical cancer (32). In addition, matrine was shown to enhance the immune functions of patients and thus improve the quality of life of patients (33). Although matrine has been used clinically to treat a number of types of cancers in recent years, the therapeutic efficacy of matrine for prostate cancer remains poorly understood.
In the present study, we investigated the impact of matrine on the proliferation, migration, invasion, cell cycle and apoptosis of androgen-independent human prostate cancer cell lines DU145 and PC-3, and explored the mechanisms underlying the antitumor activity of matrine on these androgen-independent prostate cancer cells. Our aim was to develop new strategies for the treatment of androgen-independent prostate cancer.
Materials and methods
Cell lines and cell culture
Matrine (chemical formula, C15H24N2O; molecular weight, 248.36) was purchased from Sun Yat-sen University (Guangzhou, China). Human prostate cancer cell lines DU145 and PC-3 were purchased from the Center for Experiment Animals of Sun Yat-sen University (Guangzhou, China), and cultured at 37°C in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified CO2 incubator.
Cell proliferation assay
The cell proliferation rate was assessed using the MTS assay (Promega, Biosciences, USA) according to the manufacturer's protocols. Briefly, 10,000 cells were seeded in a well into 96-well plates (Corning, New York, NY, USA) containing 100 µl culture medium plus different concentrations of matrine and were grown at 37°C for 24 h. Then 20 µl MTS reagent was added to each well, cells were continuously incubated at 37°C for 4 h, and optical densities (ODs) at 490 nm (OD490) were determined using a microplate reader (Multiskan MK3; Thermo Scientific, Shanghai, China).
Cell invasion assay
In vitro invasion assays were performed with a BD Bio-Coat Matrigel invasion assay system according to the manufacturer's protocol. Cells were seeded 24 h after treatment with different concentrations of matrine for 48 h. Cells suspended in serum-free DMEM-F12 medium (c11330500bt; Invitrogen, Life Technologies) were seeded into the upper chamber, and fetal bovine serum (10%) was added to the bottom chamber. After an incubation for 48 h at 37°C in the presence of 5% CO2, the cells on the upper side were removed with a cotton swab, and the cells on the bottom side of the filter were fixed, stained and counted.
Cell migration assay
Cells suspended in serum-free RPMI-1640 medium were seeded into the upper chamber of a Transwell® well (BD, USA) for 24 h after treatment with different concentrations of matrine for 48 h. The lower chamber of each well was filled with 600 µl of RPMI-1640 medium with 10% fetal bovine serum and incubated for 48 h at 37°C in the presence of 5% CO2. Cells were fixed and stained, non-migratory cells in the upper chamber were removed, and migrated cells were counted in 10 random high-power fields.
Analysis of cell cycle
The cell cycle was evaluated using a KeyGen kit from BD. At first, cells were treated with different concentrations of matrine for 48 h, harvested, fixed in 70% pre-chilled ethanol (−20°C) and were set at 4°C overnight. Cells were then re-suspended in propidium iodide (PI) buffer (50 g/ml PI and 100 µg/ml RNase) and incubated at room temperature for 30 min in the dark. Cells were then washed twice (3 min each wash) with 1X PBS and subjected to flow cytometry (BD Calibur, USA). The excitation wavelength was 488 nm and the emitted red fluorescence was collected through a 630 nm long-pass filter. DNA analysis was performed with ModFit software (BD).
Detection of apoptotic cells
Apoptosis was evaluated using the Annexin V/FITC apoptosis detection kit from BD. At first, cells were treated with different concentrations of matrine for 48 h and harvested by twice centrifugation at 1,000 rpm (5 min each spin). Cells were then washed twice (3 min each wash) in binding buffer, 1×106 cells were resuspended in 1 ml of binding buffer containing 1.25 µl of Annexin V-FITC (BD Pharmingen, San Diego, CA, USA) and 10 µl of PI, and incubated for 15 min at room temperature in the dark. Finally, cell cycle analysis was performed by flow cytometry. Scatter plots were performed against the intensities of the FITC fluorescence and PI fluorescence. The scatter plot was divided into four quadrants: the left lower quadrant [Annexin V-FITC (−) and PI (−)] representing viable cells, the left upper quadrant [(Annexin V-FITC (−) and PI (+)] necrotic cells, right lower quadrant [Annexin V-FITC (+) and PI (−)] early apoptotic cells, and right upper quadrant [Annexin V-FITC (+) and PI (+)] late apoptotic cells.
Immunoblot analysis
Protein extracts from cells treated with different concentrations of matrine for 48 h were separated on SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Membranes were blocked and then probed with antibodies against P65 (8242), p-P65 (3033), IKKα (11930), IKKβ (8943), p-IKKα/β (2697), IKBα (4812) and p-IKBα (2859) (1:1,000; Cell Signaling Technology, Beverly, MA, USA) and GAPDH (1:1,000; Kangcheng Biology, Shanghai, China). The blots were then washed with tris-buffered saline/Tween-20 solution and incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (1:20,000; Cell Signaling Technology) at room temperature. After washing, the blots were exposed, stained with ECL Plus (Millipore) and visualized on X-ray film. The mean normalized ODs of protein bands relative to the ODs of the GAPDH bands from the same condition were calculated.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD) and analyzed using one-way ANOVA. Data concerning cell counts were analyzed by Fisher's exact test (SPSS 17.0; SPSS, Inc., USA). A probability value of <0.05 was considered to indicate a statistically significant result.
Results
Matrine suppresses the growth of DU145 and PC-3 cells
After treatment with different concentrations of matrine for different times, both DU145 and PC-3 cells exhibited a dose- and time-dependent growth inhibition (Fig. 1A and B). The growth inhibition rates of both types of cells reached as high as 88% when cells were treated with 6.0 g/l matrine for 72 h (Fig. 1C and D). The effectiveness of matrine to inhibit cell growth increased with treatment times (Fig. 1E and F). Therefore, matrine effectively suppressed the growth of both androgen-independent prostate cancer cell lines DU145 and PC-3.
Figure 1.
Matrine reduces the proliferation of prostate cancer cells. (A and B) Plots of the proliferation rates of prostate DU145 (A) and PC-3 cells (B) incubated with increasing concentrations of matrine for increasing time periods. Values of OD490 represent the number of viable cells. Data here or throughout are the average ± standard deviation of at least three repeats. Statistical significance was determined by the Student's t-test. *P≤0.05. (C and D) Plots of the rates of growth inhibition for increasing concentrations of matrine for increasing time periods in DU145 (C) or PC-3 cells (D). (E and F) Plots of rates of growth inhibition for increasing concentrations of matrine in DU145 (E) or PC-3 cells (F).
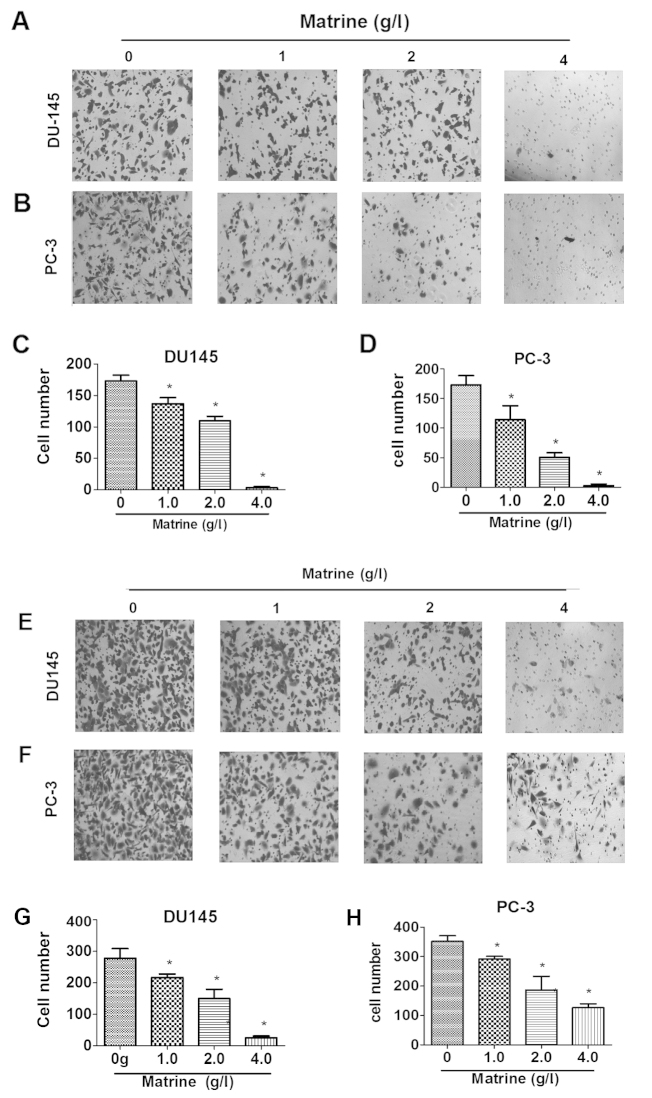
Matrine inhibits the migration and invasion of both DU145 and PC-3 cells
After treatment with different concentrations of matrine for 48 h, both DU145 and PC-3 cells exhibited significantly reduced abilities of migration and invasion in a dose-dependent manner (P<0.05, ANOVA analysis) (Fig. 2). The inhibitory effects of matrine on the migration of both DU145 and PC-3 cells were near 100% when the cells were treated with 4 g/l matrine for 48 h (Fig. 2A–D). The inhibitory impacts of matrine on the invasion of both DU145 and PC-3 cells were also close to 100% when the cells were treated with 4 g/l matrine for 48 h (Fig. 2E–H). Thus, matrine effectively impaired the migration and invasion of both DU145 and PC-3 cells.
Figure 2.
Matrine impairs the migration and invasion of prostate cancer cells. (A–D) Representative images (A and B) and plots of the number (C and D) of invaded DU145 (A and C) and PC-3 cells (B and D) per 10,000 seeded cells. (E–H) Representative images (E and F) and plots of the number (G and H) of migrated DU145 (E and G) and PC-3 cells (F and H) per 10,000 seeded cells in the presence of increasing concentrations of matrine for 48 h.
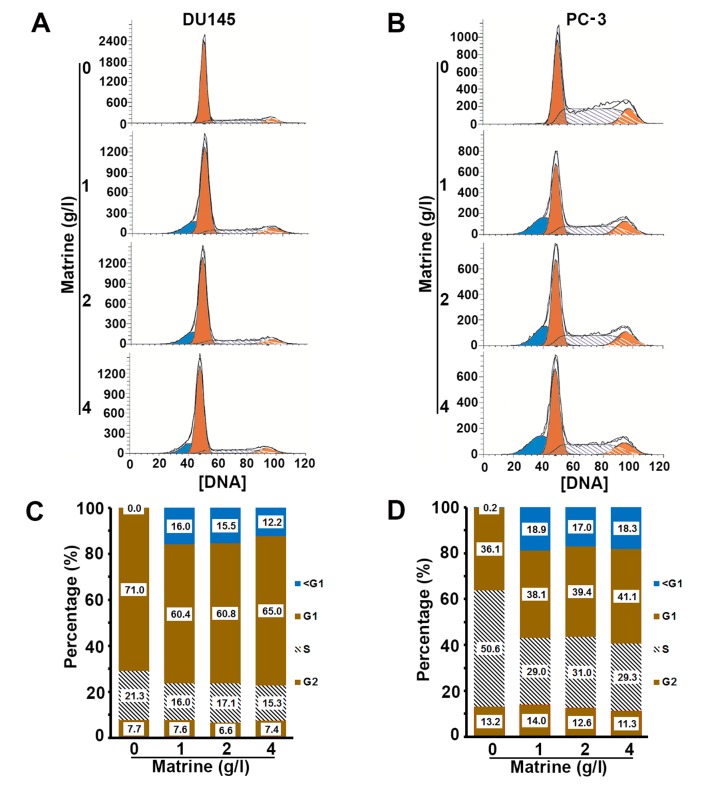
Matrine decreases the cell population at S phase but increases the apoptotic cell population
Both DU145 and PC-3 cells treated with matrine showed significantly lower percentages of cells at the S phase and higher percentages of cells at the sub-G1 phase than the untreated controls (Fig. 3). Matrine displayed no significant impact on the percentage of cells at both the G1 and G2 phases (Fig. 3). Therefore, matrine accelerated the process of DNA synthesis and promoted DNA damage and genome instability. The cell population at the sub-G1 phase consists of apoptotic cells. We further analyzed the impact of matrine on cell apoptosis by flow cytometry. Both DU145 and PC-3 cells exhibited significant increases in the apoptotic cell population upon exposure to matrine (Fig. 4). Therefore, matrine suppresses DNA replication and enhances apoptosis.
Figure 3.
Matrine delays the progress of the cell cycle of prostate cancer cells. (A and B) Representative histograms showing the results from flow cytometric analyses of the DNA content of DU145 (A) and PC-3 cells (B) in the presence of different concentrations of matrine. (C and D) Plots of the percentages of DU145 (C) and PC-3 cells (D) at the sub-G1, G1, S and G2 phases in the presence of increasing concentrations of matrine. The inserted numbers are the percentage of cells at the corresponding phase.
Figure 4.
Matrine promotes the apoptosis of prostate cancer cells. (A and B) Representative dot plots of apoptotic cells detected in the DU145 (A) and PC-3 cells (B) treated with increasing concentrations of matrine by flow cytometry. (C–H) Plots of the percentages of early (C and D), late (E and F) and total apoptotic cells (G and H) in the DU145 (C, E and G) and PC-3 cells (D, F and H) treated with increasing concentrations of matrine.
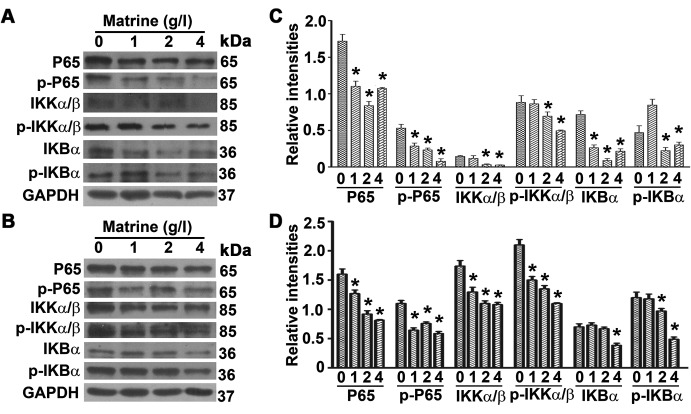
Matrine inhibits the NF-κB signaling pathway
To determine the impact of matrine on the expression levels of protein involved in the NF-κB signaling pathway, DU145 and PC-3 cells were treated with different concentrations of matrine for 48 h. The levels of P65, p-P65, IKKα/β, p-IKKα/β, IKBα and p-IKBα in both DU145 and PC-3 cells were significantly reduced upon exposure to matrine (Fig. 5). The results suggest that matrine inhibits the proliferation and enhances the apoptosis of androgen-independent prostate cancer cells by regulating the NF-κB signaling pathway.
Figure 5.
Matrine suppresses the NF-κB signaling pathway in prostate cancer cells. (A and B) Representative immune-blot results showing the intensities of P65, p-P65, IKKα/β, p-IKKα/β, IKBα and p-IKBα in DU145 (A) or PC-3 cells (B) treated with matrine at the concentrations as indicated. (C and D) Plots of the relative intensities of proteins shown in panel A and B as representatives against increasing concentrations of matrine in DU145 (C) and PC-3 cells (D). Levels of proteins are the ratios to GAPDH levels.
Discussion
Although matrine has been clinically used in recent years to treat a number of types of cancer, the therapeutic efficacy of matrine in androgen-independent prostate cancer and the exact mechanisms remain poorly understood. In the present study, we evaluated the antitumor effect of matrine on androgen-independent prostate cancer cells DU145 and PC-3. We showed in the present study that matrine inhibited the proliferation, migration and invasion of both DU145 and PC-3 cells, in a dose- and time-dependent manner. Matrine also inhibited DNA synthesis and reduced the cell population at the S phase but enhanced DNA damage and cell apoptosis. Therefore, it may be effective to use matrine to treat androgen-resistant prostate cancer.
The antitumor effects of matrine have been previously demonstrated in a number of types of cancers. Matrine was previously reported to inhibit the proliferation of tumor cells by causing cell cycle arrest at the G0/G1 or S phase (13,16,21,24,27,30,34). It was also suggested that matrine enhanced the anticancer activity by inducing apoptosis of tumor cells (13–22,24–31,35). Our data showed that matrine mainly functioned by inhibiting DNA synthesis and enhancing apoptosis, but did not markedly change the cell population at the G1 and G2 phases. It appears that matrine promotes the DNA replication-active cells to commit apoptosis.
NF-κB has been emphasized as a pleiotropic transcription factor in the regulation of diverse biological processes, including the inflammatory response, cell apoptosis and development, as well as tumorigenesis (36). Our previous studies based on traditional gene ontology (GO) and KEGG pathways showed that the highest differential expression in the matrine-treated androgen-independent prostate cancer cells DU145 and PC-3 was IKBα (unpublished data), suggesting that matrine likely interferes with the NF-κB signaling pathway. Thus, we investigated whether the suppression of the cancer cell proliferation and invasion by matrine is through the NF-κB signaling pathway. We found that matrine significantly decreased levels of proteins involving the NF-κB signaling pathway. Therefore, matrine may be a promising reagent for treating androgen-independent prostate cancer by inducing NF-κB signaling pathway-mediated apoptosis.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81472382), the National Natural Science Foundation of China for Young Scientists Grant (no. 81101947), the Guangdong Province Natural Science Foundation (no. 2014A030313079), the Fundamental Research Funds for the Central Universities (no. 14ykpy19), the Guangdong Province Science and Technology for Social Development Project (no. 2013B021800107), and the Guangzhou City 2015 Scientific Research Projects (7415600066401) to H.H.; and NCI R01CA142862 to L.L.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. 2005;41:834–845. doi: 10.1016/j.ejca.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 3.Quon H, Loblaw DA. Androgen deprivation therapy for prostate cancer-review of indications in 2010. Curr Oncol. 2010;17(Suppl 2):S38–S44. doi: 10.3747/co.v17i0.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gioeli D. Signal transduction in prostate cancer progression. Clin Sci. 2005;108:293–308. doi: 10.1042/CS20040329. [DOI] [PubMed] [Google Scholar]
- 5.Long Y, Lin XT, Zeng KL, Zhang L. Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2004;3:69–72. [PubMed] [Google Scholar]
- 6.Liu J, Zhu M, Shi R, Yang M. Radix Sophorae flavescentis for chronic hepatitis B: A systematic review of randomized trials. Am J Chin Med. 2003;31:337–354. doi: 10.1142/S0192415X03001107. [DOI] [PubMed] [Google Scholar]
- 7.Ma ZJ, Li Q, Wang JB, Zhao YL, Zhong YW, Bai YF, Wang RL, Li JY, Yang HY, Zeng LN, et al. Combining oxymatrine or matrine with lamivudine increased its antireplication effect against the hepatitis B virus in vitro. Evid Based Complement Alternat Med. 2013;2013:186573. doi: 10.1155/2013/186573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Wang X, Guo Y, Deng N, Zheng P, Xu Q, Wu Y, Dai G. Regulation of endothelial nitric oxide synthase and asymmetric dimethylarginine by matrine attenuates isoproterenol-induced acute myocardial injury in rats. J Pharm Pharmacol. 2012;64:1107–1118. doi: 10.1111/j.2042-7158.2012.01502.x. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Wang B, Zhou C, Bi Y. Matrine induces apoptosis in angiotensin II-stimulated hyperplasia of cardiac fibroblasts: Effects on Bcl-2/Bax expression and caspase-3 activation. Basic Clin Pharmacol Toxicol. 2007;101:1–8. doi: 10.1111/j.1742-7843.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu JY, Hu JH, Zhu QG, Li FQ, Wang J, Sun HJ. Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int Immunopharmacol. 2007;7:816–823. doi: 10.1016/j.intimp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Lai JP, He XW, Jiang Y, Chen F. Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal Bioanal Chem. 2003;375:264–269. doi: 10.1007/s00216-002-1675-2. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Mao J, Liu X, Wang Y, Sun Y, He Z. Separation and mechanism elucidation for six structure-like matrine-type alkaloids by micellar liquid chromatography. J Sep Sci. 2009;32:2043–2050. doi: 10.1002/jssc.200900066. [DOI] [PubMed] [Google Scholar]
- 13.Yu P, Liu Q, Liu K, Yagasaki K, Wu E, Zhang G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology. 2009;59:219–229. doi: 10.1007/s10616-009-9225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao H, Yang B, Hu R, Wang Y. Matrine effectively inhibits the proliferation of breast cancer cells through a mechanism related to the NF-κB signaling pathway. Oncol Lett. 2013;6:517–520. doi: 10.3892/ol.2013.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Song Y, Chen H, Pan S, Sun X. Matrine inhibits proliferation and induces apoptosis of pancreatic cancer cells in vitro and in vivo. Biol Pharm Bull. 2010;33:1740–1745. doi: 10.1248/bpb.33.1740. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JQ, Li YM, Liu T, He WT, Chen YT, Chen XH, Li X, Zhou WC, Yi JF, Ren ZJ. Antitumor effect of matrine in human hepatoma G2 cells by inducing apoptosis and autophagy. World J Gastroenterol. 2010;16:4281–4290. doi: 10.3748/wjg.v16.i34.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong X, Gao Y, Guo G, Vondran FW, Schwartlander R, Efimova E, Pless G, Sauera IM, Neuhaus P. Effect of matrine on primary human hepatocytes in vitro. Cytotechnology. 2015;67:255–265. doi: 10.1007/s10616-013-9680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Gao C, Yao S, Xie B. Blocking autophagic flux enhances matrine-induced apoptosis in human hepatoma cells. Int J Mol Sci. 2013;14:23212–23230. doi: 10.3390/ijms141223212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan H, Luan G, Yagasaki K, Zhang G. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology. 2009;59:191–200. doi: 10.1007/s10616-009-9211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song S, Zhu S, Zhang Z, Mo Z, Ke Q, Luo Z. A study on the inhibitory effect of matrine on gastric cancer SGC-7901 cells. Afr J Tradit Complement Altern Medicines. 2013;10:435–438. doi: 10.4314/ajtcam.v10i6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Cheng B, Li H, Xu W, Zhai B, Pan S, Wang L, Liu M, Sun X. Matrine inhibits proliferation and induces apoptosis of human colon cancer LoVo cells by inactivating Akt pathway. Mol Biol Rep. 2014;41:2101–2108. doi: 10.1007/s11033-014-3059-z. [DOI] [PubMed] [Google Scholar]
- 22.Chang C, Rao DL, Qiu XM, Wang H, Xiong L. The inhibitory effect of matrine on the growth of human colorectal cancer HT29 cells: An experimental observation. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:62–65. In Chinese. [PubMed] [Google Scholar]
- 23.Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J, Zhang S, Wu J, Yu K, Han Y. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS One. 2012;7:e46853. doi: 10.1371/journal.pone.0046853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Q, Chen B, Zhang X, Qian W, Ye B, Zhou Y. Arsenic trioxide-enhanced, matrine-induced apoptosis in multiple myeloma cell lines. Planta Med. 2013;79:775–781. doi: 10.1055/s-0032-1328554. [DOI] [PubMed] [Google Scholar]
- 25.Shao Q, Zhao X, Yao L. Matrine inhibits the growth of retinoblastoma cells (SO-Rb50) by decreasing proliferation and inducing apoptosis in a mitochondrial pathway. Mol Biol Rep. 2014;41:3475–3480. doi: 10.1007/s11033-014-3209-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhao B, Li B, Bai S, Shen L, Ren R, Jonas JB, Xu X, Lu Q, Liu Q. Effects of matrine on proliferation and apoptosis of cultured retinoblastoma cells. Graefes Arch Clin Exp Ophthalmol. 2012;250:897–905. doi: 10.1007/s00417-011-1751-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Du H, Geng G, Zhou H, Xu M, Cao H, Zhang B, Song G, Hu T. Matrine inhibits proliferation and induces apoptosis via BID-mediated mitochondrial pathway in esophageal cancer cells. Mol Biol Rep. 2014;41:3009–3020. doi: 10.1007/s11033-014-3160-3. [DOI] [PubMed] [Google Scholar]
- 28.Xie M, He G, Wang R, Shi S, Chen J, Ye Y, Xie L, Yi X, Tang A. Matrine-induced apoptosis of human nasopharyngeal carcinoma cells via in vitro vascular endothelial growth factor-A/extracellular signal-regulated kinase1/2 pathway inactivation. Horm Metab Res. 2014;46:556–560. doi: 10.1055/s-0034-1367077. [DOI] [PubMed] [Google Scholar]
- 29.Liang CZ, Zhang JK, Shi Z, Liu B, Shen CQ, Tao HM. Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother Pharmacol. 2012;69:317–331. doi: 10.1007/s00280-011-1699-4. [DOI] [PubMed] [Google Scholar]
- 30.Jin H, Sun Y, Wang S, Cheng X. Matrine activates PTEN to induce growth inhibition and apoptosis in V600EBRAF harboring melanoma cells. Int J Mol Sci. 2013;14:16040–16057. doi: 10.3390/ijms140816040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo L, Xue TY, Xu W, Gao JZ. Matrine promotes G0/G1 arrest and down-regulates cyclin D1 expression in human rhabdomyosarcoma cells. Panminerva Med. 2013;55:291–296. [PubMed] [Google Scholar]
- 32.Zhang L, Wang T, Wen X, Wei Y, Peng X, Li H, Wei L. Effect of matrine on HeLa cell adhesion and migration. Eur J Pharmacol. 2007;563:69–76. doi: 10.1016/j.ejphar.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Mei Q, Xu YC, Du J, Wei Y, Xu ZM. Effects of matrine injection on T-lymphocyte subsets of patients with malignant tumor after gamma knife radiosurgery. Zhong Xi Yi Jie He Xue Bao. 2006;4:78–79. doi: 10.3736/jcim20060121. In Chinese. [DOI] [PubMed] [Google Scholar]
- 34.Chang C, Liu SP, Fang CH, He RS, Wang Z, Zhu YQ, Jiang SW. Effects of matrine on the proliferation of HT29 human colon cancer cells and its antitumor mechanism. Oncol Lett. 2013;6:699–704. doi: 10.3892/ol.2013.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang HH, Liu X, Liang DS, Lu YJ, Shan HL, et al. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol Biochem. 2012;30:631–641. doi: 10.1159/000341444. [DOI] [PubMed] [Google Scholar]
- 36.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]