Abstract
A 13-year-old female Lhasa Apso was presented for blepharospasm and conjunctival hyperemia of the right eye. Ophthalmic examination revealed an anterior stromal ulcer associated with a raised yellow corneal plaque. In vivo confocal microscopy and cytology of the cornea identified neutrophilic inflammation and yeast cells. Malassezia pachydermatis was isolated from a corneal scraping. Treatment with topical voriconazole ophthalmic solution resolved the keratitis.
Keywords: Cornea, Dog, Fungal keratitis, Keratomycosis, Malassezia pachydermatis
1. Introduction
Fungal keratitis is reported infrequently in the dog and can represent a diagnostic and therapeutic challenge in this species [1], [2], [3]. The clinical appearance of canine fungal keratitis is highly variable between cases and corneal lesions can be either ulcerative or nonulcerative in nature. Fungal infections of the canine cornea may be associated with substantial ocular morbidity and result in vision or globe loss [1], [2], [3], [4], [5], [6], [7], [8], [9]. Genera of fungi reported to be associated with canine fungal keratitis include Acremonium, Alternaria, Aspergillus, Candida, Cephalosporium, Chrysosporium, Cladosporium, Curvularia, Fusarium, Hormographiella, Penicillium, Phialemonium, Pseudallescheria, and Scedosporium [1], [2], [3], [4], [5], [6], [7], [8], [9].
Malassezia pachydermatis is a saprophytic yeast that frequently colonizes the skin and mucosa of healthy dogs and is a common etiologic agent of opportunistic canine dermatologic and otic infections [10]. A precursory publication that describe the clinical application of in vivo corneal confocal microscopy during the course of canine fungal keratitis in a series of dogs included a single case of M. pachydermatis keratomycosis, but did not provide extensive historical, clinical examination, therapeutic, or microbiologic details regarding individual cases [11]. Prior to that report, Malassezia species had not been described as pathogens of the canine cornea and only rare descriptions of Malassezia corneal infections in human patients are published [11]. The purpose of the present case report is to provide a detailed clinical and mycological description of the previously reported case of canine keratomycosis associated with M. pachydermatis infection.
2. Case
A 13-year-old female spayed Lhasa Apso was presented (day 0) for a 5 day history of blepharospasm and conjunctival hyperemia in the right eye. The dog had a chronic history of medically-controlled diabetes mellitus and keratoconjunctivitis sicca in both eyes. With the exception of a subcutaneous lipoma that was surgically excised from the dog's right axillary region 7 years prior to presentation, the dog had no history of dermatologic disease. Elective phacoemulsification was performed 4 years prior and the dog was pseudophakic in both eyes. Cyclosporine 2% ophthalmic solution, tacrolimus 0.03% ophthalmic solution, and flurbiprofen 0.03% ophthalmic solution were being administered twice daily in both eyes. The dog had received this medication combination for approximately 5 years prior to presentation.
During ophthalmic examination, multifocal corneal pigmentation and peripheral corneal vascularization were present in the right eye. An anterior stromal ulcer associated with a mild leukocyte infiltrate was present in the axial cornea of the right eye. There was marked miosis of the right pupil and the remainder of the ophthalmic examination was unremarkable. Gram positive cocci and neutrophils were identified during cytological evaluation of a corneal swab specimen collected from the region of ulceration. Staphylococcus schleiferi was isolated from a corneal swab specimen and corneal anaerobic bacterial culture was negative. Initial treatment included the addition of ofloxacin 0.3% ophthalmic solution (1 drop q6h), cefazolin 5% ophthalmic solution (1 drop q6h), and atropine 1% ophthalmic ointment (1/4” strip q24h) to the treatment regimen of the right eye with discontinuation of the flurbiprofen solution. The corneal appearance was unchanged during recheck ophthalmic examinations performed on days 3 and 8.
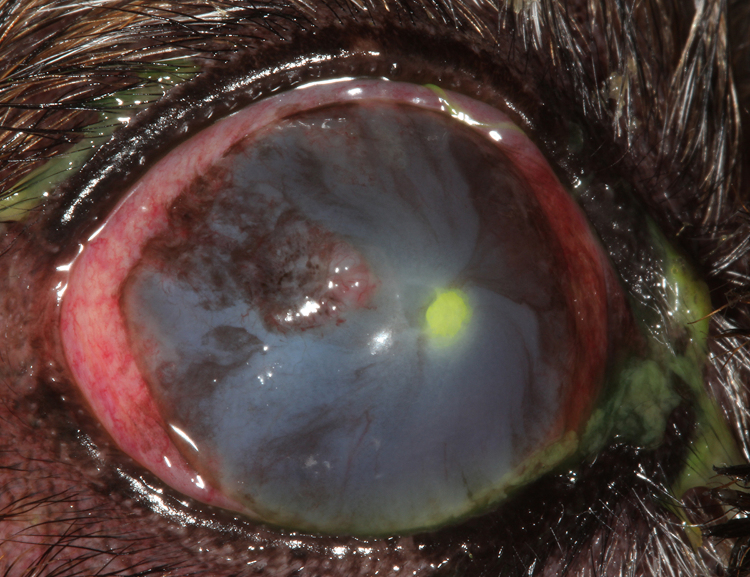
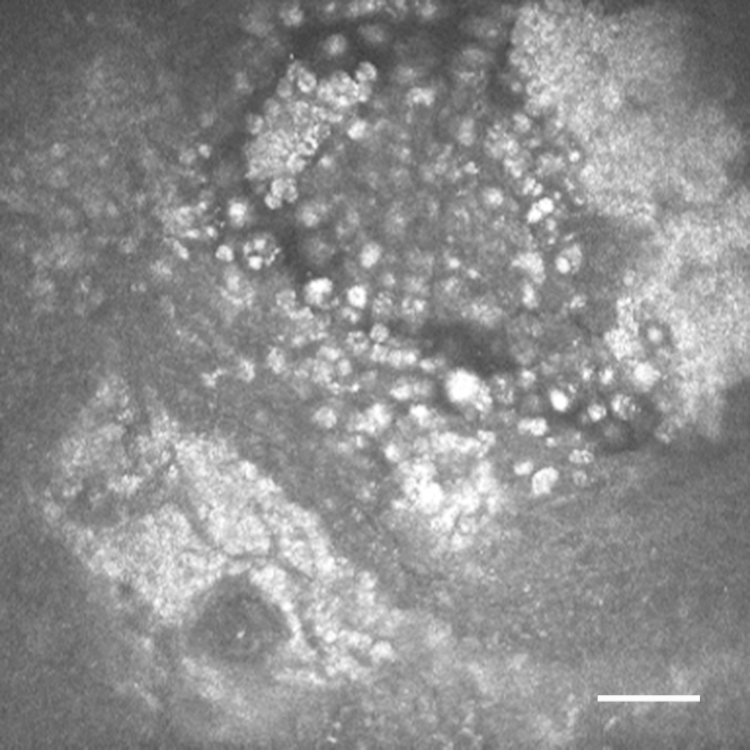
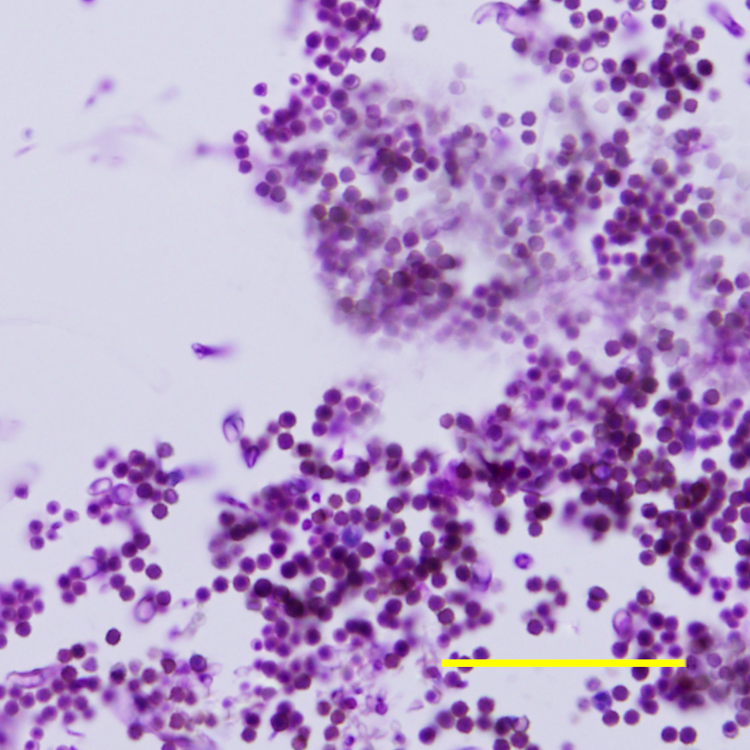
During recheck ophthalmic examination performed on day 14, progressive centripetal migration of the corneal blood vessels was noted with diffuse and marked corneal edema present. The anterior stromal ulcer was now associated with a dense leukocyte infiltrate and raised yellow plaque (Fig. 1). In vivo confocal microscopy of the cornea revealed the corneal plaque was composed of numerous leukocytes and circular and oval structures that measured 2.5–7.5 µm in diameter. Clumps of these structures were present on the surface of the exposed corneal stroma and intermixed within epithelial cells on the periphery of the corneal ulcer, but did not appear to invade deeper into the stromal tissue (Fig. 2). Marked leukocyte and Langerhans cell infiltrates were present in the epithelium surrounding the corneal plaque. A corneal scraping was collected from the lesion with a Kimura spatula for cytological evaluation, aerobic bacterial culture, and fungal culture. The majority of the grossly visible yellow corneal plaque was removed by gentle manual scraping with the Kimura spatula. Neutrophilic inflammation with numerous yeast cells were observed on cytology (Fig. 3) and M. pachydermatis was isolated during fungal culture. Aerobic bacterial culture was negative for growth.
Fig. 1.
Clinical photograph of a fluorescein stained anterior stromal corneal ulcer associated with a superficial yellow fungal plaque.
Fig. 2.
In vivo corneal confocal photomicrograph displaying numerous circular and oval fungal structures intermixed with corneal epithelial cells. Bar: 50 µm.
Fig. 3.
Cytological photomicrograph of a corneal scraping stained with Diff-Quik® (modified Giemsa stain) and demonstrating numerous yeast cells. Bar: 50 µm.
The M. pachydermatis isolate was observed macroscopically as cream to buff colored colonies that become darker with age and had a dull, matte texture. The colonies were convex with margins that were entirely or slightly lobed. Microscopically, the yeast cells were ovoid with unipolar budding on a broad base with almost no constriction between the mother cell and its bud, leaving a prominent scar forming a collarette. These characteristics together produced a distinctive foot-print shape or peanut shape appearance. Pseudohyphae and true hyphae were absent or sparse. The M. pachydermatis isolate was able to grow on Sabouraud agar and other commercial media at 30–37 °C, unlike other members of the Malassezia genus, which are lipophilic and require the addition of lipid supplementation to grow on agar.
Voriconazole 1% ophthalmic solution (1 drop q6h) was added to the treatment regimen and administration of cyclosporine and tacrolimus ophthalmic solutions was suspended. Recheck clinical examination was performed on days 28 and 49 with progressive improvement of the corneal ulcer. On day 28, the corneal ulcer was beginning to re-epithelialize and the corneal leukocyte infiltrate was decreased. On day 49, the corneal lesion was epithelialized with dense stromal fibrosis, pigmentation, and vascularization. Repeat confocal microscopic examination was performed and no leukocytes or fungal structures were identified. Voriconazole and antimicrobial ophthalmic medications were discontinued and therapy with cyclosporine, tacrolimus, and flurbiprofen ophthalmic solutions was restarted. There were no additional corneal lesions identified during clinical examinations performed periodically over the subsequent 12 months (up to day 414).
3. Discussion
M. pachydermatis is a lipophilic yeast that frequently colonizes the skin and mucosa of healthy dogs [10]. M. pachydermatis can be cultured from the conjunctival microflora of a low percentage (3.8%) of dogs without overt clinical ocular disease [12]. In one study, the frequency of M. pachydermatis detection in the conjunctival microflora was significantly higher in samples collected from the eyes of dogs with corneal ulcers (23% of samples culture positive) than dogs with apparently normal eyes (3% of samples culture positive) [13]. The authors of that report speculated that alterations in the local ocular microenvironment associated with inflammation or immunodeficiency contributed to the higher incidence of M. pachydermatis in the conjunctival microflora of the dogs with corneal ulcers [13]. Conjunctival flora overgrowth with M. pachydermatis might have created a situation permissible to development of keratomycosis in the present case, as bacterial ulcerative keratitis preceded the detection of fungal keratitis in the described dog. In addition to direct effects of the keratitis, aggressive treatment with broad spectrum topical antimicrobials in the dog might have altered the ocular microflora and created an environment conducive to M. pachydermatis overgrowth. Additionally, long-term use of ophthalmic immunosuppressive agents and pre-existing ocular surface disease associated with keratoconjunctivitis sicca and diabetes mellitus may have contributed to development of this unusual corneal infection.
M. pachydermatis is a common opportunistic pathogen associated with canine dermatitis and otitis [10]. M. pachydermatis dermatologic infections frequently exhibit a chronic or recurrent clinical course and their treatment may be complicated by the ability of the yeast to produce biofilm [14]. Other fungal virulence factors that contribute to the pathogenesis of M. pachydermatis dermatitis include the production of proteinase, phospholipase, hyaluronidase, and chondroitin-sulphatase [15]. These extracellular enzymes might also contribute to corneal damage during ocular infection.
Prior to the present report, Malassezia species were not described as pathogens of the canine cornea and only rare descriptions of human patients with Malassezia corneal infections were published [16], [17]. Malassezia furfur was identified in corneal samples from a patient with infectious crystalline keratopathy and blepharitis who was receiving long-term topical corticosteroid treatment [16]. Malassezia restricta was cultured from a diabetic farm worker with infectious ulcerative keratitis that developed following soil contamination of the ocular surface [17]. In both human cases, lipid-dependent species of Malassezia that lacked fatty acid synthase activity were cultured from the corneal specimens. In contrast, the Malassezia species isolated from the described dog was lipophilic, but not lipid-dependent, and is the only species in the genus that does not exhibit an absolute external lipid requirement for growth.
In the reported dog, in vivo confocal microscopy suggested that the fungal structures were restricted to a plaque on the corneal surface intermixed with epithelial cells and leukocytes, but the yeast cells did not appear to extend deeper in the corneal stroma. The tear film was previously suggested as a source of lipids for Malassezia organisms and localization to the corneal surface may have resulted from the greater contact with tears afforded by this anatomic position [13]. An alternative explanation for this finding is that M. pachydermatis may not possess the necessary virulence factors for invading deeper into intact corneal stroma. The ability to invade corneal tissues is a key virulence factor contributing to the pathogenesis of keratitis for many other fungal organisms [18]. If M. pachydermatis is a less invasive corneal pathogen than some other fungal species, this may partially explain why it is infrequently associated with keratitis despite its common presence in the canine cutaneous microflora.
Antifungal susceptibility testing was attempted, but the Malassezia isolate in the present study was too fastidious for assay completion. Voriconazole was empirically selected for the treatment of the dog as it is commonly used as an ophthalmic formulation for dogs with fungal keratitis and previous in vitro studies suggest canine M. pachydermatis isolates are likely to be susceptible to this antifungal medication [19], [20]. Treatment with voriconazole was successful in the described dog, with resolution of the corneal infection without any clinical episodes of recurrence after antifungal discontinuation.
Conflict of interest
None.
Acknowledgment
None.
References
- 1.Marlar A.J., Miller P.E., Canton D.D., Scagliotti R., Murphy C.J. Canine keratomycosis: a report of eight cases and literature review. J. Am. Anim. Hosp. Assoc. 1994;30:331–340. [Google Scholar]
- 2.Scott E.M., Carter R.T. Canine Keratomycosis in 11 dogs: a case series (2000–2011) J. Am. Anim. Hosp. Assoc. 2014;50:112–118. doi: 10.5326/JAAHA-MS-6012. [DOI] [PubMed] [Google Scholar]
- 3.Nevile J.C., Hurn S.D., Turner A.G. Keratomycosis in five dogs. Vet. Ophthalmol. 2015 doi: 10.1111/vop.12313. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza L., Donato A., Padhye A. Canine mycotic keratoconjuntivitis caused by Acremonium kiliense. Sabouraudia. 1985;23:447–450. [PubMed] [Google Scholar]
- 5.Smedes S.L., Miller P.E., Dubielzig R.R. Pseudallescheria boydii keratomycosis in a dog. J. Am. Vet. Med. Assoc. 1992;200:199–202. [PubMed] [Google Scholar]
- 6.Rampazzo A., Kuhnert P., Howard J., Bornand V. Hormographiella aspergillata keratomycosis in a dog. Vet. Ophthalmol. 2009;12:43–47. doi: 10.1111/j.1463-5224.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Shlomo G., Plummer C., Barrie K., Brooks D. Curvularia keratomycosis in a dog. Vet. Ophthalmol. 2010;13:126–130. doi: 10.1111/j.1463-5224.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 8.Newton E.J. Scedosporium apiospermum keratomycosis in a dog. Vet. Ophthalmol. 2012;15:417–420. doi: 10.1111/j.1463-5224.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- 9.Pucket J.D., Allbaugh R.A., Rankin A.J. Treatment of dematiaceous fungal keratitis in a dog. J. Am. Vet. Med. Assoc. 2012;240:1104–1108. doi: 10.2460/javma.240.9.1104. [DOI] [PubMed] [Google Scholar]
- 10.Cafarchia C., Otranto D. The pathogenesis of Malassezia yeasts. Parassitologia. 2008;50:65–67. [PubMed] [Google Scholar]
- 11.Ledbetter E.C., Norman M.L., Starr J.K. In vivo confocal microscopy for the detection of canine fungal keratitis and monitoring of therapeutic response. Vet. Ophthalmol. 2015 doi: 10.1111/vop.12287. [DOI] [PubMed] [Google Scholar]
- 12.Prado M.R., Brilhante R.S., Cordeiro R.A., Monteiro A.J., Sidrim J.J., Rocha M.F. Frequency of yeasts and dermatophytes from healthy and diseased dogs. J. Vet. Diagn. Investig. 2008;20:197–202. doi: 10.1177/104063870802000208. [DOI] [PubMed] [Google Scholar]
- 13.Prado M.R., Brito E.H., Girao M.D., Monteiro A.J., Sidrim J.J., Rocha M.F. Higher incidence of Malassezia pachydermatis in the eyes of dogs with corneal ulcer than in healthy dogs. Vet. Microbiol. 2004;100:115–120. doi: 10.1016/j.vetmic.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Jerzsele A., Gyetvai B., Csere I., Galfi P. Biofilm formation in Malassezia pachydermatis strains isolated from dogs decreases susceptibility to ketoconazole and itraconazole. Acta Vet. Hung. 2014;62:473–480. doi: 10.1556/AVet.2014.019. [DOI] [PubMed] [Google Scholar]
- 15.Coutinho S.D., Proteinase Paula C.R. phospholipase, hyaluronidase and chondroitin-sulphatase production by Malassezia pachydermatis. Med. Mycol. 2000;38:73–76. doi: 10.1080/mmy.38.1.73.76. [DOI] [PubMed] [Google Scholar]
- 16.Roodhooft J., van Rens G., Bogaerts M., Vermander J.M. Infectious crystalline keratopathy: a case report. Bull. Soc. Belge. d’Ophtalmol. 1998;268:121–126. [PubMed] [Google Scholar]
- 17.Suzuki T., Hori N., Miyake T., Hori Y., Mochizuki K. Keratitis caused by a rare fungus, Malassezia restricta. Jpn. J. Ophthalmol. 2007;51:292–294. doi: 10.1007/s10384-007-0447-0. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell B.M., Wu T.G., Jackson B.E., Wilhelmus K.R. Candida albicans strain-dependent virulence and Rim13p-mediated filamentation in experimental keratomycosis. Investig. Ophthalmol. Vis. Sci. 2007;48:774–780. doi: 10.1167/iovs.06-0793. [DOI] [PubMed] [Google Scholar]
- 19.Grundon R.A., O’Reilly A., Muhlnickel C., Hardman C., Stanley R.G. Keratomycosis in a dog treated with topical 1% voriconazole solution. Vet. Ophthalmol. 2010;13:331–335. doi: 10.1111/j.1463-5224.2010.00799.x. [DOI] [PubMed] [Google Scholar]
- 20.Cafarchia C., Iatta R., Immediato D., Puttilli M.R., Otranto D. Azole susceptibility of Malassezia pachydermatis and Malassezia furfur and tentative epidemiological cut-off values. Med. Mycol. 2015;53:743–748. doi: 10.1093/mmy/myv049. [DOI] [PubMed] [Google Scholar]