Abstract
Readily available natural α-amino acids are one of nature’s most attractive and versatile building blocks in synthesis of natural products and biomolecules. Peptides and N-heterocycles exhibit various biological and pharmaceutical functions. Conjugation of amino acids or peptides with N-heterocycles provides boundless potentiality for screening and discovery of diverse biologically active molecules. However, it is a great challenge to install amino acids or peptides on N-heterocycles through formation of carbon-carbon bonds under mild conditions. In this article, eighteen N-protected α-amino acids and three peptides were well assembled on phenanthridine derivatives via couplings of N-protected α-amino acid and peptide active esters with substituted 2-isocyanobiphenyls at room temperature under visible-light assistance. Furthermore, N-Boc-proline residue was successfully conjugated with oxindole derivatives using similar procedures. The simple protocol, mild reaction conditions, fast reaction, and high efficiency of this method make it an important strategy for synthesis of diverse molecules containing amino acid and peptide fragments.
Amino acids are one of nature’s most attractive and versatile building blocks in the synthesis of natural products and biomolecules1, and peptides are of great importance in present day drug discovery programs2. On the other hand, N-heterocycles are ubiquitous in natural products and biologically active molecules3, and they have been assigned as privileged structures in drug discovery because N-heterocyclic moieties often exhibit improved solubility and can facilitate salt formation property, both of which are important for oral absorption and bioavailability4,5,6. Conjugations of amino acids or peptides with N-heterocyclic compounds provide great opportunity for screening and discovery of diverse biologically active substances. Among the previous strategies available, common conjugations are usually performed through formation of amides (Fig. 1a), carbon-heteroatom bonds (Fig. 1b). The conjugations via the formation C-C bonds have been developed by using olefin metathesis7 and transition metal-catalyzed cross-coupling8 strategies. However, connections by C-C bonds still are a great and pressing challenge for chemists because of inherent structural characteristics of amino acids and peptides. On the other hand, the transition metal-catalyzed decarboxylative strategy for the formation of C-C bonds has provided some valuable reactions in organic synthesis9,10,11,12, such as Heck-type reactions13,14, allylations15, redox-neutral cross-coupling reactions16,17, and oxidative arylations18,19. However, the reactions usually need high temperatures and the stronger bases, which are intolerant for amino acids and peptides. Recently, visible light photoredox catalysis has attracted much attention, and it has emerged as a powerful activation protocol in new chemical transformations20,21,22,23,24,25,26,27. Furthermore, some decarboxylative couplings to the formation C-C bonds have been developed28,29,30,31,32,33,34,35,36,37,38,39. To the best of our knowledge, conjugation between amino acids or peptides and N-heterocycles is limited via formation of C-C bond under mild conditions and visible-light assistance38. Herein, we report an efficient installation of amino acids and peptides on phenanthridine and oxindole derivatives at room temperature under visible-light assistance (Fig. 1c).
Figure 1. Design on installing amino acids and peptides on N-heterocycles.
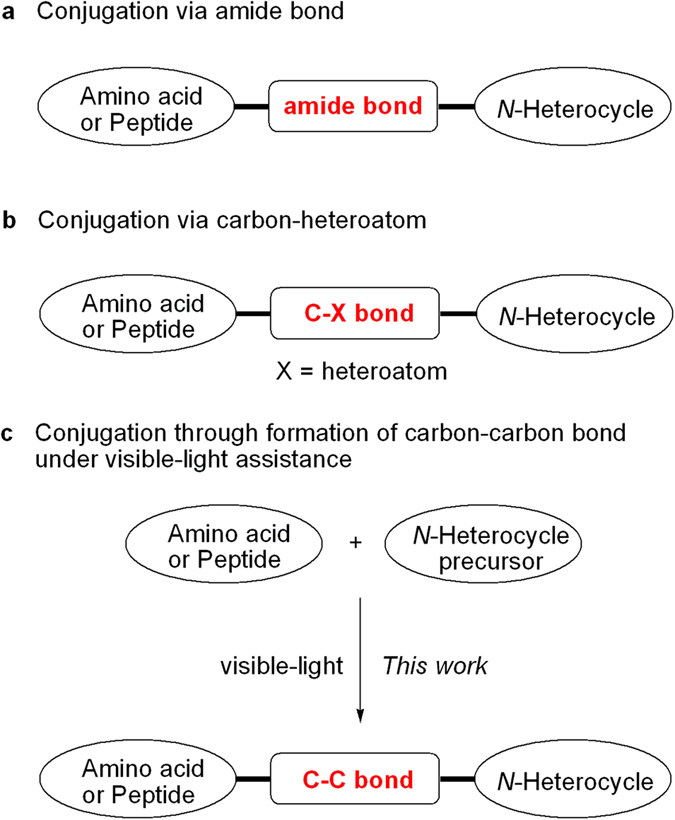
(a) The previous conjugation via amide bond. (b) The previous conjugation via carbon-heteroatom bond. (c) Our conjugation through formation of carbon-carbon bond under visible-light assistance.
Results and Discussion
Since 2-isocyanobiphenyls are effective radical acceptors40,41,42, we first selected them as the partners of N-protected amino acid active esters under the visible-light photoredox catalysis. As shown in Table 1, our investigation for optimized conditions began by using model reaction of N-Boc-Pro-OPht (Pht = phthalimide) (1r) with isonitrile 2a. The reaction was performed by using 1.0 mol% [Ru(bpy)3]Cl2 as the photoredox catalyst, one equiv of diisopropylethylamine (DIPEA) (relative to amount of 2a) as the reductant, DMF as the solvent at room temperature under argon atmosphere for 2 h (entry 1), and 3r was produced in 86% conversion yield (determined by 1H NMR using trichloroethylene as the internal standard). When 0.4 equivalent of DIPEA was used, yield decreased (entry 2), but addition of 1.2 equiv of K2CO3 as the base greatly promoted their reactivity (entry 3). Yields declined in the presence of 0.2 equiv of DIPEA (entry 4) or in the absence of DIPEA (entry 5). Other tertiary amines were screened (entries 6 and 7), and they were inferior to DIPEA. We investigated Na2CO3, Cs2CO3 and NaHCO3 as the bases (entries 8–10), and the results showed that K2CO3 was a suitable base (compare entries 3, 8–10). Effect of solvents was explored, and DMF provided the best result (compare entries 3, 11–13). The reaction in aqueous media (DMF/H2O = 9:1) was also attempted, and a 92% yield was provided (entry 14). When [fac-Ir(ppy)3] was used as the photoredox catalyst, only 79% conversion yield was found (entry 15). No reaction was observed when the system was exposed in air (entry 16). The reaction was performed well under irradiation of blue LED (entry 17). The reaction did not work in the absence of light (entry 18).
Table 1. Development of a method for installing N-Boc proline residue on 8-methylphenanthridine*.
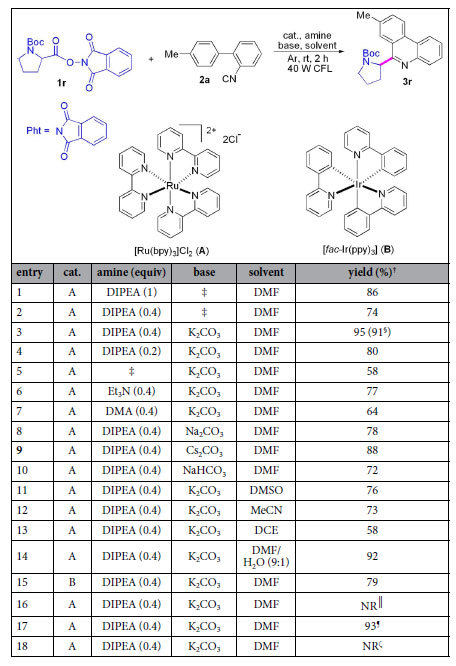
*Reaction conditions: under Ar atmosphere and irradiation of visible light, N-Boc-Pro-OPht (1r) (0.30 mmol), 1-isocyano-(p-phenyl)-benzene (2a) (0.15 mmol), catalyst (1.5 μmol), amine (0.03–0.15 mmol), base (0.18 mmol), solvent (2.0 mL), temperature (rt, ~25 oC), time (2 h) in a sealed Schlenk tube. †Conversion yield by 1H NMR determination using trichloroethylene as the internal standard. ‡No addition of reagent. §Isolated yield. ║Under air. ¶Under irradiation of blue LED. ζNo light. DIPEA = diisopropylethylamine. DCE = 1,2-dichloroethane. DMA = N, N- Dimethylaniline. CFL = compact fluorescent light.
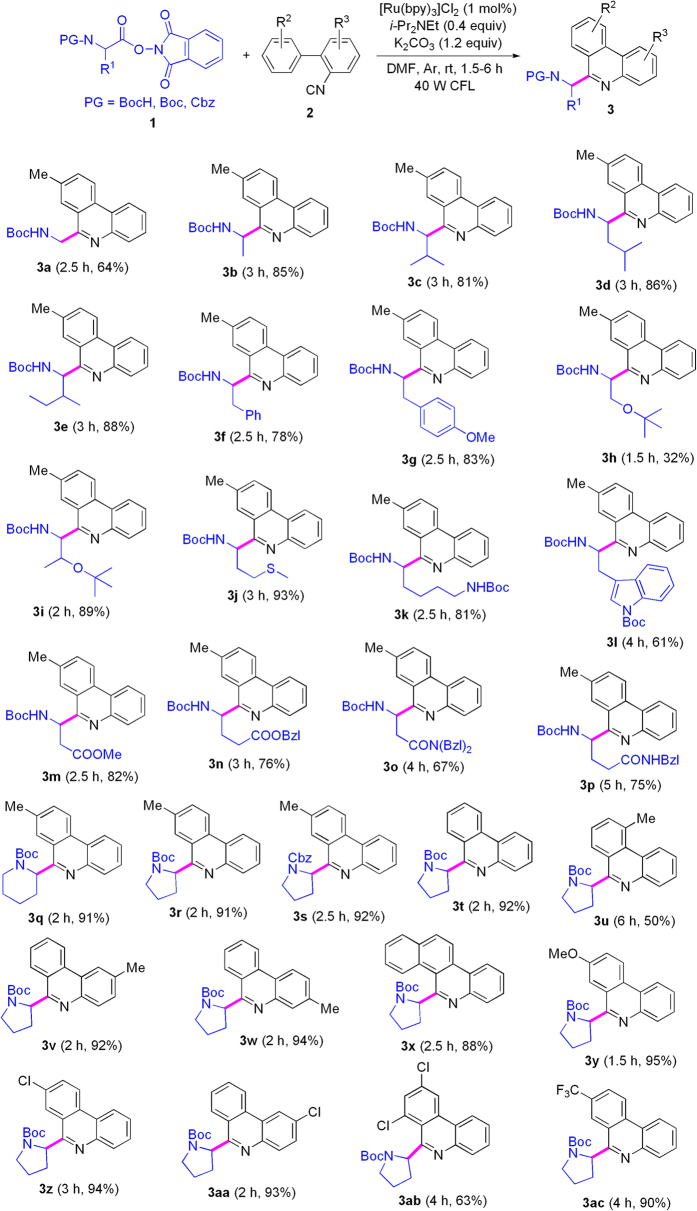
After getting the optimized conditions under visible-light assistance, we investigated the substrate scope of this reaction by testing decarboxylative couplings of various N-protected amino acid active esters (1) with substituted 2-isocyanobiphenyls (2). As shown in Figure 2, active esters of six neutral amino acids including glycine, alanine, valine, leucine, isoleucine and phenylalanine provided high yields (see 3a–f). N,O-Protected amino acid active esters with hydroxyl on the side chains were tested (see 3g–i), N-Boc-Ser(OBut)-OPht gave lower yield for occurrence of unknown by-products (see 3h), and N-Boc-Tyr(OMe)-OPht and N-Boc-Thr(OBut)-OPht provided satisfactory results (see 3g and 3i). N-Boc-Met-OPht was a good substrate, and it afforded the target product 3j in 93% yield. N, N’-Bis(Boc)-protected Lys-OPht and Trp-OPht displayed high reactivity (see 3k and 3l). Active esters of two acidic amino acids (aspartic acid and glutamic acid) donated good yields after their carboxyls on the side chains were esterized with methanol or phenylmethanol (see 3m and 3n). N, N’-Protected asparagine and glutamine active esters afforded 3o and 3p in 67 and 75% yields, respectively. N-Boc pipecolinic acid active ester was used as the substrate, and it showed good result (see 3q). We attempted another N-protective group, benzyloxycarbonyl (Cbz), and N-Cbz-Pro-OPht exhibited similar reactivity to N-Boc-Pro-OPht (see 3r and 3s). Unfortunately, active esters of three natural amino acids including cysteine, histidine and arginine gave some by-products because of side reaction on side chains of the amino acids under the present photoredox conditions. We also explored the scope of substituted 2-isocyanobiphenyls, and isonitriles with steric hindrance provided lower yields (see 3u and 3ab). The visible-light photoredox decarboxylicative couplings showed tolerance of some functional groups including amides, ether (see 3g, 3h, 3i and 3y), thioether (3j), esters (see 3m and 3n), C-Cl bond (see 3z, 3aa and 3ab), and CF3 (see 3ac). It is worthwhile to note that the obtained products contain amino acid residues, and further derivatization is an easy task after the protective groups on the amino acid residues are removed. Therefore, the results above provide opportunity for construction of diverse molecules. In addition, phenanthridines are found in a wide variety of naturally occurring alkaloids43,44,45 and display diverse biological and pharmaceutical activities46,47. The present method affords a convenient, efficient and practical protocol for synthesis of phenanthridines.
Figure 2. Substrate scope on conjugations of N-protected amino acids and phenanthridines*.
*Reaction conditions: under Ar atmosphere and irradiation of visible light, N-protected amino acid-OPht (1) (0.45 mmol for synthesis of 3a–p; 0.30 mmol for synthesis of the others), substituted 2-isocyanobiphenyl (2) (0.15 mmol), [Ru(bpy)3]Cl2 (1.5 μmol), DIPEA (0.06 mmol), K2CO3 (0.18 mmol), DMF (2.0 mL), temperature (rt, ~25 oC), time (1.5–6 h) in a sealed Schlenk tube. †Isolated yield. Boc = tert-butyloxycarbonyl. Bzl = benzyl. Cbz = benzyloxycarbonyl.
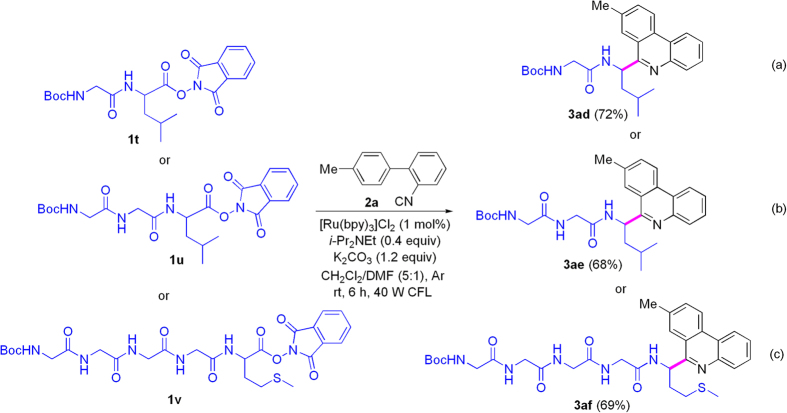
Inspired by the excellent results above, we extended scope of substrates to N-Boc-peptide active esters. As shown in Fig. 3, reaction of dipeptide derivative Boc-Gly-Leu-OPht (1t) with 2a provided the target product (3ad) in 72% yield under photoredox catalysis in mixed solvent of CH2Cl2 and DMF (2.0 mL, CH2Cl2/DMF = 5:1) (note: the active ester was not dissolved well in complete DMF) (Fig. 3a). Further, we attempted decarboxylative coupling of tripeptide Boc-Gly-Gly-Leu-OPht (1u) and pentapeptide Boc-Gly-Gly-Gly-Gly-Met-OPht (1v) with 2a under the same conditions, and 3ae and 3af were obtained in 68% and 69% yields, respectively (Fig. 3b,c). The results above exhibited that Boc-protected peptide active esters also were effective radical precursors for the photoredox catalysis.
Figure 3. Conjugations of N-Boc peptides and 8-methylphenanthridine under visible-light assistance.
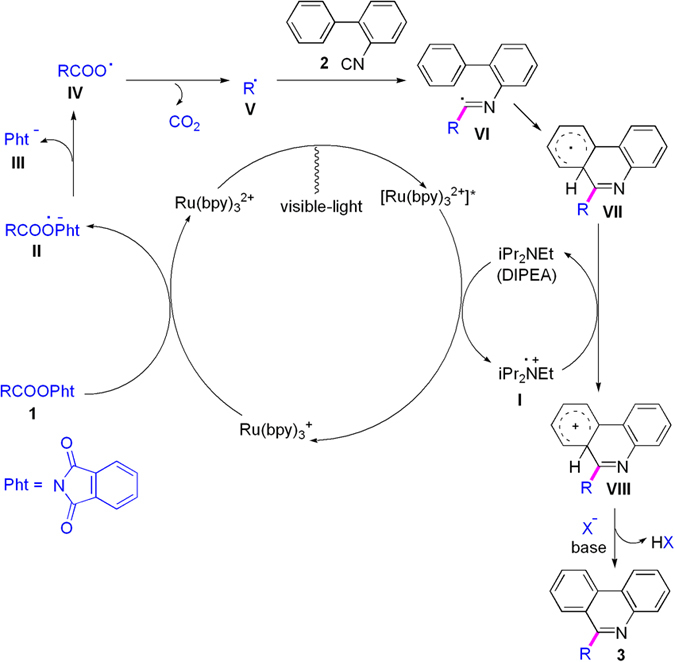
To explore the mechanism on this conjugation, a radical-trapping agent, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), was added to the reaction system of N-Boc-Pro-OPht (1r) and isonitrile 2a, and the reaction was completely inhibited, which shows that a free-radical intermediate process can be involved in the reaction. Therefore, a plausible mechanism on the visible-light photoredox synthesis of phenanthridines is suggested in Fig. 4 according to the results above and the previous references20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39. Irradiation of Ru(bpy)32+ with visible light gives the excited-state [Ru (bpy)32+]*, and the photoexcited catalyst was reduced by DIPEA to give Ru(bpy)3+, in which DIPEA forms I. Treatment of 1 with Ru(bpy)3+ produces II regenerating catalyst Ru(bpy)32+, subsequent elimination of phthalimide anion (III) from II provides carboxyl radical IV, and release of carbon dioxide in IV yields α-amino radical V. Addition of V to substituted 2-isocyanobiphenyl (2) affords imidoyl radical VI, intramolecular hemolytic aromatic substitution of VI gives radical intermediate VII, and oxidation of VII with I affords cation VIII regenerating DIPEA. Finally, deprotonation of VIII in the presence of base leads to the target product (3).
Figure 4. A plausible mechanism on installing N-protected amino acids and peptides on phenanthridines under visible-light assistance.
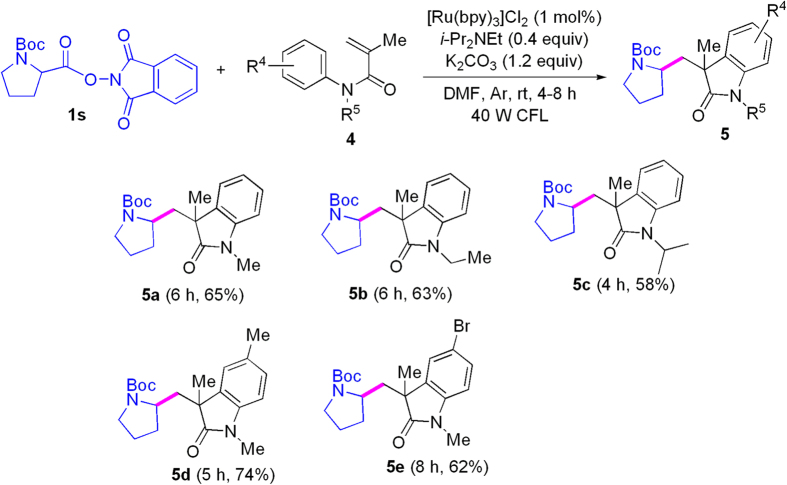
We next explored synthesis of oxindole derivatives under visible-light photoredox catalysis by using N-protected amino acid active esters. As shown in Fig. 5, decarboxylative couplings of N-Boc-Pro-OPht with N-alkyl-N-phenylalkacrylamides under the optimized reaction conditions provided the corresponding oxindoles in moderate yields. It is known that oxindoles widely occur in natural products with unique biological activity, and they are the privileged scaffolds for design and discovery of drugs48,49,50. Therefore, the present method affords a novel protocol for synthesis of oxindole derivatives.
Figure 5. Installing N-Boc proline residue on oxindoles under visible-light assistance.
In conclusion, we have developed an efficient mode of installing N-protected α-amino and peptide residues on N-heterocycles through the formation of C-C bonds with the assistant of the photocatalyst [Ru(bpy)3]Cl2 and visible-light, in which active esters of eighteen N-protected amino acids and three peptides were used. The photoredox-generated α-amino or peptide radicals were trapped with substituted 2-isocyanobiphenyls or N-alkyl-N-phenylalkacrylamides at room temperature, and the phenanthridine and oxindole derivatives with biological and pharmaceutical activity were prepared in good yields. The generation of reactive radicals under the mild photocatalytic conditions may reduce the incidence of undesired side reactions from N-protected amino acid and peptide derivatives. Most importantly, the obtained products contain amino acid and peptide fragments, and their further modification can provide diverse molecules after the protective groups on the amino acid and peptide fragments. The present findings pave the way for future synthesis of biological and pharmaceutical molecules containing amino acid and peptide fragments, and we believe that the present strategy will find wide applications in organic synthesis.
Methods
General procedure for synthesis of compounds 3a–af and 5a–e
[Ru(bpy)3]Cl2 (1.5 μmol, 1.2 mg), 1 (0.45 mmol for synthesis of 3a–p; 0.30 mmol for synthesis of the others), 2 (0.15 mmol) or 4 (0.15 mmol), DIPEA (0.06 mmol, 10 μL) and K2CO3 (0.18 mmol, 25 mg) were added to a 25-mL Schlenk tube with DMF (2.0 mL) or mixed solvent of CH2Cl2 and DMF (2.0 mL, CH2Cl2/DMF (5:1)), and the tube was degassed by argon sparging for over 5 min. The tube was sealed, and then irradiated with a 40 W fluorescent lamp (approximately 2 cm away from the light source). After the complete conversion of the substrates (monitored by TLC), the reaction mixture was diluted with 20 L of EtOAc, and the solution was filtered by flash chromatography. The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the desired product (3a–af and 5a–e).
Additional Information
How to cite this article: Jin, Y. et al. Installing amino acids and peptides on N-heterocycles under visible-light assistance. Sci. Rep. 6, 20068; doi: 10.1038/srep20068 (2016).
Supplementary Material
Acknowledgments
Financial support for this work was provided by the National Natural Science Foundation of China (Grant No. 21172128, 21372139 and 21221062), the Ministry of Science and Technology of China (Grant No. 2012CB722605) and Shenzhen Municipal Government (Grant No. SZSITIC CXB201104210014A).
Footnotes
Author Contributions Y.J. and H.F. conceived this subject, Y.J., M.J. and H.W. conducted the experimental work, Y.J. and H.F. analysed the results, Y.J. and H.F. co-wrote the manuscript.
References
- Blaskovich M. A. Handbook on Syntheses of Amino Acids: General Routes for the Syntheses of Amino Acids, 1st ed.; Oxford University Press: New York (2010). [Google Scholar]
- Noisier A. F. M. & Brimble M. A. C−H Functionalization in the synthesis of amino acids and peptides. Chem. Rev. 114, 8775–8806 (2014). [DOI] [PubMed] [Google Scholar]
- DeSimone R. W., Currie K. S., Mitchell S. A., Darrow J. W. & Pippin D. A. Privileged structures: applications in drug discovery. Comb. Chem. High Throughput Screen. 7, 473–493 (2004). [DOI] [PubMed] [Google Scholar]
- Leeson P. D. & Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discovery 6, 881–890 (2007). [DOI] [PubMed] [Google Scholar]
- Meanwell N. A. Improving drug candidates by design: a focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol. 24, 1420–1456 (2011). [DOI] [PubMed] [Google Scholar]
- Ritchie T. J., Macdonald S. J. F., Young R. J. & Pickett S. D. The impact of aromatic ring count on compound developability: further insights by examining carbo- and hetero-aromatic and -aliphatic ring types. Drug Discov. Today 16, 164–171 (2011). [DOI] [PubMed] [Google Scholar]
- Lin Y. A., Boutureira O., Lercher L., Bhushan B., Paton R. S. & Davis B. G. Rapid cross-metathesis for reversible protein modifications via chemical access to se-allyl-selenocysteine in proteins. J. Am. Chem. Soc. 135, 12156–12159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Gouverneur V. & Davis B. G. Enhanced aqueous suzuki- Miyaura coupling allows site- specific polypeptide 18F- labeling. J. Am. Chem. Soc. 135, 13612–13615 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J. D., Recio A. III, Grenning A. J. & Tunge J. A. Transition metal-catalyzed decarboxylative allylation and benzylation reactions. Chem. Rev. 111, 1846–1913 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez N. & Gooβen L. J. Decarboxylative coupling reactions: a modern strategy for C–C-bond formation. Chem. Soc. Rev. 40, 5030–5048 (2011). [DOI] [PubMed] [Google Scholar]
- Dzik W. I., Lange P. P. & Gooβen L. J. Carboxylates as sources of carbon nucleophiles and electrophiles: comparison of decarboxylative and decarbonylative pathways. Chem. Sci. 3, 2671–2678 (2012). [Google Scholar]
- Goonβen L. J., Rodríguez N. & Gooβenn K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem., Int. Ed. 47, 3100–3120 (2008). [DOI] [PubMed] [Google Scholar]
- Myers A. G., Tanaka D. & Mannion M. R. Development of a decarboxylative palladation reaction and its use in a Heck-type olefination of arene carboxylates. J. Am. Chem. Soc. 124, 11250–11251 (2002). [DOI] [PubMed] [Google Scholar]
- Forgione P., Brochu M. C., Stonge M., Thesen K. H., Bailey M. D. & Bilodeau F. Unexpected intermolecular Pd-catalyzed cross-coupling reaction employing heteroaromatic carboxylic acids as coupling partners. J. Am. Chem. Soc. 128, 11350–11351 (2006). [DOI] [PubMed] [Google Scholar]
- Rayabarapu D. K. & Tunge J. A. Catalytic decarboxylative sp-sp3 coupling. J. Am. Chem. Soc. 127, 13510–13511 (2005). [DOI] [PubMed] [Google Scholar]
- Gooβen L. J., Deng G. & Levy L. M. Synthesis of biaryls via catalytic decarboxylative coupling. Science 313, 662–664 (2006). [DOI] [PubMed] [Google Scholar]
- Gooβen L. J., Rodríguez N., Melzer B., Linder C., Deng G. & Levy L. M. Biaryl synthesis via Pd-catalyzed decarboxylative coupling of aromatic carboxylates with aryl halides. J. Am. Chem. Soc. 129, 4824–4833 (2007). [DOI] [PubMed] [Google Scholar]
- Wang C., Piel I. & Glorius F. Palladium-catalyzed intramolecular direct arylation of benzoic acids by tandem decarboxylation/C−H activation. J. Am. Chem. Soc. 131, 4194–4195 (2009). [DOI] [PubMed] [Google Scholar]
- Goonβen L. J., Rodríguez N., Lange P. P. & Linder C. Decarboxylative cross-coupling of aryl tosylates with aromatic carboxylate salts. Angew. Chem., Int. Ed. 49, 1111–1114 (2010). [DOI] [PubMed] [Google Scholar]
- Prier C. K., Rankic D. A. & MacMillan D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T. P., Ischay M. A. & Du J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2, 527–532 (2010). [DOI] [PubMed] [Google Scholar]
- Narayanam J. M. R. & Stephenson C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011). [DOI] [PubMed] [Google Scholar]
- Zeitler K. Photoredox catalysis with visible light. Angew. Chem., Int. Ed. 48, 9785–9789 (2009). [DOI] [PubMed] [Google Scholar]
- Shi L. & Xia W. Photoredox functionalization of C-H bonds adjacent to a nitrogen atom. Chem. Soc. Rev. 41, 7687–7697 (2012). [DOI] [PubMed] [Google Scholar]
- Xuan J. & Xiao W.-J. Visible-light photoredox catalysis. Angew. Chem., Int. Ed. 51, 6828–6838 (2012). [DOI] [PubMed] [Google Scholar]
- Hari D. P. & König B. The photocatalyzed meerwein arylation: classic reaction of aryl diazonium salts in a new light. Angew. Chem., Int. Ed. 52, 4734–4743 (2013). [DOI] [PubMed] [Google Scholar]
- Chemical Photocatalysis (Ed: König, B.), De Gruyter, Stuttgart (2013).
- DiRocco D. A., Dykstra K., Krska S., Vachal P., Conway D. V. & Tudge M. Late-stage functionalization of biologically active heterocycles through photoredox catalysis. Angew. Chem., Int. Ed. 53, 4802–4806 (2014). [DOI] [PubMed] [Google Scholar]
- Lang S. B., O’Nele K. M. & Tunge J. A. Decarboxylative allylation of amino alkanoic acids and esters via dual catalysis. J. Am. Chem. Soc. 136, 13606–13609 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann M. J. & Overman L. E. A concise synthesis of (−)-aplyviolene facilitated by a strategic tertiary radical conjugate addition. Angew. Chem., Int. Ed. 51, 9576–9580 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner G. L., Quasdorf K. W. & Overman L. E. Direct construction of quaternary carbons from tertiary alcohols via photoredox-catalyzed fragmentation of tert-alkyl N–phthalimidoyl oxalates. J. Am. Chem. Soc. 135, 15342–15345 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang J., Qi L., Hu C. & Chen Y. Visible-light-induced chemoselective reductive decarboxylative alkynylation under biomolecule-compatible conditions. Chem. Commun. 51, 5275–5278 (2014). [DOI] [PubMed] [Google Scholar]
- Leung J. C. T., Chatalova-Sazepin C., West J. G., Rueda-Becerril M., Paquin J.-F. & Sammis G. M. Photo-fluorodecarboxylation of 2-aryloxy and 2-aryl carboxylic acids. Angew. Chem., Int. Ed. 51, 10804–10807 (2012). [DOI] [PubMed] [Google Scholar]
- Liu J., Liu Q., Yi H., Qin C., Bai R., Qi X., Lan Y. & Lei A. Visible-light-mediated decarboxylation/oxidative amidation of α-keto acids with amines under mild reaction conditions using O2. Angew. Chem., Int. Ed. 53, 502–506 (2014). [DOI] [PubMed] [Google Scholar]
- Rueda-Becerril M., Mahé O., Drouin M., Majewski M. B., West J. G., Wolf M. O., Sammis G. M. & Paquin J.-F. Direct C−F Bond Formation Using Photoredox Catalysis. J. Am. Chem. Soc. 136, 2637–2641 (2014). [DOI] [PubMed] [Google Scholar]
- Noble A. & MacMillan D. W. C. Photoredox α–vinylation of α–amino acids and N–aryl amines. J. Am. Chem. Soc. 136, 11602–11605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L., Ohta C., Zuo Z. & MacMillan D. W. C. Carboxylic acids as a traceless activation group for conjugate additions: a three-step synthesis of (±)-pregabalin. J. Am. Chem. Soc. 136, 10886–10889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z. & MacMillan D. W. C. Decarboxylative arylation of α–amino acids via photoredox catalysis: a one-step conversion of biomass to drug pharmacophore. J. Am. Chem. Soc. 136, 5257–5260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z., Ahneman D. T., Chu L., Terrett J. A., Doyle A. G. & MacMillan D. W. C. Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 345, 437–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu I., Sonoda N. & Curran D. P. Tandem radical reactions of carbon monoxide, isonitriles, and other reagent equivalents of the geminal radical acceptor radical precursor synthon. Chem. Rev. 96, 177–194 (1996). [DOI] [PubMed] [Google Scholar]
- Tobisu M., Koh K., Furukawa T. & Chatani N. Modular Synthesis of Phenanthridine Derivatives by Oxidative Cyclization of 2-Isocyanobiphenyls with Organoboron Reagents. Angew. Chem., Int. Ed. 51, 11363–11366 (2012). [DOI] [PubMed] [Google Scholar]
- Jiang H., Cheng Y., Wang R., Zheng M., Zhang Y. & Yu S. Synthesis of 6-alkylated phenanthridine derivatives using photoredox neutral somophilic isocyanide insertion. Angew. Chem., Int. Ed. 52, 13289–13292 (2013). [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Masuda A., Suwa M., Akiyama Y., Hoshino-Abe N. & Suzuki M. Synthesis of derivatives of NK109, 7-OH benzo[c]phenanthridine alkaloid, and evaluation of their cytotoxicities and reduction-resistant properties. Bioorg. Med. Chem. Lett. 10, 2321–2323 (2000). [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Suzuki M., Saimoto A. & Kabasawa T. Structural considerations of NK109, an antitumor benzo[c]phenanthridine alkaloid. J. Nat. Prod. 62, 864–867 (1999). [DOI] [PubMed] [Google Scholar]
- Nakanishi T. & Suzuki M. Revision of the structure of fagaridine based on the comparison of UV and NMR data of synthetic compounds. J. Nat. Prod. 61, 1263–1267 (1998). [DOI] [PubMed] [Google Scholar]
- Denny W. A. Acridine derivatives as chemotherapeutic agents. Curr. Med. Chem. 9, 1655–1665 (2002). [DOI] [PubMed] [Google Scholar]
- Ishikawa T. Benzo[c]phenanthridine bases and their antituberculosis activity. Med. Res. Rev. 21, 61–72 (2001). [DOI] [PubMed] [Google Scholar]
- Galliford C. V. & Scheidt K. A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem., Int. Ed. 46, 8748–8758 (2007). [DOI] [PubMed] [Google Scholar]
- Le Tourneau C., Raymond E. & Faivre S. Sunitinib: a novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors (GIST). Ther. Clin. Risk Manag. 3, 341–348 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H., Kato A. & Esaki T. AG-041R, a novel indoline-2-one derivative, induces systemic cartilage hyperplasia in rats. Eur. J. Pharmacol. 418, 225–230 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.