Abstract
A vitamin K-dependent carboxylase has recently been purified from bovine liver microsomes and candidate cDNA clones have been isolated. Definitive identification of the carboxylase remains circumstantial since expression of candidate carboxylase cDNAs in mammalian cells is confounded by the presence of endogenous carboxylase activity. To overcome this problem, a recombinant strain of baculovirus (Autographa california nuclear polyhedrosis virus, AcMNPV) encoding a putative carboxylase (vbCbx/AcMNPV) was used to infect Sf9 insect cells, which we demonstrate have no endogenous carboxylase activity. Infection with vbCbx/AcMNPV conferred vitamin K-dependent carboxylase activity to Sf9 insect cells. Carboxylase activity was demonstrated to peak 2-3 days after infection with vbCbx/AcMNPV. Metabolic radiolabeling with L-[35S]methionine revealed that the 90-kDa recombinant protein is the major protein synthesized at the time of peak activity after infection. An anti-peptide antibody directed against residues 86-99 reacted with bovine liver carboxylase on Western blot analysis and immunoprecipitated recombinant carboxylase from infected Sf9 microsomal protein preparations. Since Sf9 insect cells lack endogenous vitamin K-dependent carboxylase activity, expression of carboxylase activity in Sf9 insect cells with recombinant baculovirus demonstrates that the protein encoded by this cDNA is a vitamin K-dependent gamma-glutamyl carboxylase.
Full text
PDF


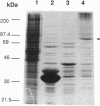
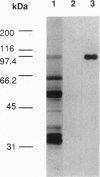
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L., Harbeck M., Lingenfelter S., Bailey C., Sanders-Hinck C. M., Suttie J. W. Purification and identification of bovine liver gamma-carboxylase. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6242–6246. doi: 10.1073/pnas.89.14.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle T. L., Suttie J. W. Vitamin K dependent carboxylase: subcellular location of the carboxylase and enzymes involved in vitamin K metabolism in rat liver. Biochemistry. 1980 Mar 18;19(6):1161–1167. doi: 10.1021/bi00547a019. [DOI] [PubMed] [Google Scholar]
- Crawford A. M., Miller L. K. Characterization of an early gene accelerating expression of late genes of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1988 Aug;62(8):2773–2781. doi: 10.1128/jvi.62.8.2773-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Sadowski J. A., Suttie J. W. A new carboxylation reaction. The vitamin K-dependent incorporation of H-14-CO3- into prothrombin. J Biol Chem. 1975 Jun 25;250(12):4744–4748. [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Hubbard B. R., Ulrich M. M., Jacobs M., Vermeer C., Walsh C., Furie B., Furie B. C. Vitamin K-dependent carboxylase: affinity purification from bovine liver by using a synthetic propeptide containing the gamma-carboxylation recognition site. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6893–6897. doi: 10.1073/pnas.86.18.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber P., Schmitz T., Griffin J., Jacobs M., Walsh C., Furie B., Furie B. C. Identification of amino acids in the gamma-carboxylation recognition site on the propeptide of prothrombin. J Biol Chem. 1990 Jul 25;265(21):12467–12473. [PubMed] [Google Scholar]
- Jorgensen M. J., Cantor A. B., Furie B. C., Brown C. L., Shoemaker C. B., Furie B. Recognition site directing vitamin K-dependent gamma-carboxylation resides on the propeptide of factor IX. Cell. 1987 Jan 30;48(2):185–191. doi: 10.1016/0092-8674(87)90422-3. [DOI] [PubMed] [Google Scholar]
- Knobloch J. E., Suttie J. W. Vitamin K-dependent carboxylase. Control of enzyme activity by the "propeptide" region of factor X. J Biol Chem. 1987 Nov 15;262(32):15334–15337. [PubMed] [Google Scholar]
- Kuliopulos A., Cieurzo C. E., Furie B., Furie B. C., Walsh C. T. N-bromoacetyl-peptide substrate affinity labeling of vitamin K dependent carboxylase. Biochemistry. 1992 Oct 6;31(39):9436–9444. doi: 10.1021/bi00154a016. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. Signals important for high-level expression of foreign genes in Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1988 Nov;167(1):56–71. doi: 10.1016/0042-6822(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Magnusson S., Sottrup-Jensen L., Petersen T. E., Morris H. R., Dell A. Primary structure of the vitamin K-dependent part of prothrombin. FEBS Lett. 1974 Aug 25;44(2):189–193. doi: 10.1016/0014-5793(74)80723-4. [DOI] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Nelsestuen G. L., Zytkovicz T. H., Howard J. B. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J Biol Chem. 1974 Oct 10;249(19):6347–6350. [PubMed] [Google Scholar]
- Pan L. C., Price P. A. The propeptide of rat bone gamma-carboxyglutamic acid protein shares homology with other vitamin K-dependent protein precursors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6109–6113. doi: 10.1073/pnas.82.18.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A., Roth D. A., Wasley L. C., Kuliopulos A., Walsh C. T., Furie B., Furie B. C., Kaufman R. J. In vitro and in vivo functional characterization of bovine vitamin K-dependent gamma-carboxylase expressed in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4611–4615. doi: 10.1073/pnas.90.10.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford D. G., Kanagy C., Sudmeier J. L., Furie B. C., Furie B., Bachovchin W. W. Structure of the propeptide of prothrombin containing the gamma-carboxylation recognition site determined by two-dimensional NMR spectroscopy. Biochemistry. 1991 Oct 15;30(41):9835–9841. doi: 10.1021/bi00105a004. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Fernlund P., Egan W., Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie J. W., Lehrman S. R., Geweke L. O., Hageman J. M., Rich D. H. Vitamin K-dependent carboxylase: requirements for carboxylation of soluble peptide and substrate specificity. Biochem Biophys Res Commun. 1979 Feb 14;86(3):500–507. doi: 10.1016/0006-291x(79)91742-x. [DOI] [PubMed] [Google Scholar]
- Suttie J. W. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- Ulrich M. M., Furie B., Jacobs M. R., Vermeer C., Furie B. C. Vitamin K-dependent carboxylation. A synthetic peptide based upon the gamma-carboxylation recognition site sequence of the prothrombin propeptide is an active substrate for the carboxylase in vitro. J Biol Chem. 1988 Jul 15;263(20):9697–9702. [PubMed] [Google Scholar]
- Wu S. M., Cheung W. F., Frazier D., Stafford D. W. Cloning and expression of the cDNA for human gamma-glutamyl carboxylase. Science. 1991 Dec 13;254(5038):1634–1636. doi: 10.1126/science.1749935. [DOI] [PubMed] [Google Scholar]
- Wu S. M., Morris D. P., Stafford D. W. Identification and purification to near homogeneity of the vitamin K-dependent carboxylase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2236–2240. doi: 10.1073/pnas.88.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]