Abstract
Large numbers of the small brown planthopper Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae) occur in temperate regions, causing severe losses in rice, wheat, and other economically important crops. The planthoppers enter diapause in the third- or fourth-instar nymph stage, induced by short photoperiods and low temperatures. To investigate the geographic variation in L. striatellus diapause, we compared the incidence of nymphal diapause under various constant temperature (20 and 27°C) and a photoperiod of 4:20, 8:16, 10:14, 12:12, 14:10, and 16:8 (L:D) h regimes among three populations collected from Hanoi (21.02° N, 105.85° E, northern Vietnam), Jiangyan (32.51° N, 120.15° E, eastern China), and Changchun (43.89° N, 125.32° E, north-eastern China). Our results indicated that there were significant geographic variations in the diapause of L. striatellus. When the original latitude of the populations increased, higher diapause incidence and longer critical photoperiod (CP) were exhibited. The CPs of the Jiangyan and Changchun populations were ∼12 hr 30 min and 13 hr at 20°C, and 11 hr and 11 hr 20 min at 27°C, respectively. The second- and third-instar nymphs were at the stage most sensitive to the photoperiod. However, when the fourth- and fifth-instar nymphs were transferred to a long photoperiod, the diapause-inducing effect of the short photoperiod on young instars was almost reversed. The considerable geographic variations in the nymphal diapause of L. striatellus reflect their adaptation in response to a variable environment and provide insights to develop effective pest management strategies.
Keywords: diapause, geographic variation, Laodelphax striatellus, sensitive stage
Diapause is an important physiological response and an adaptation for insects to survive under adverse environmental or climatic conditions (Tauber et al. 1986; Danks 1987). The diapause characteristics related to fitness often result from adaptive evolution. Geographic variations in diapause are especially interesting for us to study to determine the climatic adaptation of organisms because climate varies with geography (Danilevsky et al. 1970). Diapause variations among populations have been frequently reported in insects, including hemipterans such as the red firebug, Pyrrhocoris apterus (Socha 2001), Dicyphus hesperus (Gillespie and Quiring 2005), the big-eyed bug, Geocoris punctipes (Ruberson et al. 2001), Orius sauteri (Ito and Nakata 2000; Shimizu and Kawasaki 2001; Musolin and Ito 2008), and Nesidiocoris tenuis (Pazyuk et al. 2014), and these results support the hypothesis of local adaptation or adaptation to environmental stress. Knowledge of geographic variations in the diapause of insects clarifies not only the life history traits of insects, but also their adaptive mechanisms to environments, which is important to develop new strategies for pest management (Denlinger 2008).
The small brown planthopper Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae) occurs mainly in the temperate zone and is one of the most destructive agricultural pests (Kisimoto 1958, 1989). Adults and nymphs suck the sap from a large number of economically important crops, which results in reduced yields and poor grain quality (Liu et al. 2006), and the species causes further damage by transmitting plant viruses (Nault 1994; Nemoto et al. 1994; Wang et al. 2014). L. striatellus overwinters as second to fifth-instar nymphs in weeds and wheat fields in temperate and cold temperate zones (Kisimoto 1958, 1989; Cai et al. 1964; Noda 1992; Wang et al. 2014). Adults can migrate over a long distance in early summer (Sanada-Morimura et al. 2001; Syobu et al. 2011; Wang et al. 2011; Zhang et al. 2011; He et al. 2012).
The diapause of L. striatellus has been investigated in previous studies. During the development of third- or fourth-instar nymphs, the planthoppers might enter diapause that is induced by short photoperiods at low temperatures. The photoperiodic response curves indicated a typical long-day response type (Kisimoto 1958, 1989; Noda 1992; Wang et al. 2014). As temperature decreases, L. striatellus shows a higher diapause incidence with a longer critical photoperiod (CP: the photoperiod in which 50% of individuals enter diapause). However, short photoperiods hardly induce diapause at all at high temperatures (Noda 1992; Wang et al. 2014). In Nanchang, e.g., the induction of photoperiodic diapauses is nearly completely inhibited at temperatures under 28°C (Wang et al. 2014). Meanwhile, a long photoperiod or high temperature acted as a diapause-terminating stimulus for L. striatellus nymphs (Noda 1992; Wang et al. 2014), and 26–28°C has been found to be the optimal temperature for diapause termination (Wang et al. 2014).
L. striatellus is widely distributed in temperate, tropical, and subtropical zones, although it is rampant mainly in the temperate zone (Kisimoto 1989). Therefore, it is critical to uncover the characteristics of diapause in different geographic populations. Geographic variation in the diapause of L. striatellus has, so far, only been reported by Noda (1992), who identified significant differences in the diapause patterns of nine local populations in Japan. However, in other important rice-planting regions including China and Southeast Asian areas, geographic variations have not been fully understood.
In this study, we compared the diapause characteristics of three L. striatellus populations collected from three regions of Vietnam and China by measuring diapause incidence under various constant temperature and photoperiod regimes, aiming to reveal the geographical variations in diapause in this insect.
Materials and Methods
Insect
Three populations of L. striatellus were collected from Hanoi, Vietnam, in the tropical zone (21.02° N, 105.85° E, called ‘Hanoi population, HN’); Jiangyan, Jiangsu Province, China, in the subtropical zone (32.51° N, 120.15° E, called ‘Jiangyan population, JY’); and Changchun, Jilin Province, China, in the temperate zone (43.89° N, 125.32° E, called ‘Changchun population, CC’) for this study (Table 1). The insects were reared on rice in the laboratory at 25°C with a long photoperiod of 16:8 (L:D) h for one generation before use.
Table 1.
Geographic populations of L. striatellus included in the study
| Population (abbreviation) | Location of collection (latitude, longitude, altitude) | Climatic condition | Mean annual temperature | Collection date (year–month) |
|---|---|---|---|---|
| HN | Hanoi, Vietnam (21.02°N, 105.85°E, 10 m) | Tropical zone | 21°C | 2011 April |
| JY | Jiangyan, Jiangsu Province, China (32.51° N, 120.15°E, 3 m) | Subtropical zone | 14.6°C | 2011 May |
| CC | Changchun, Jilin Province, China (43.89°N, 125.32°E, 236 m) | Temperate zone | 5.2°C | 2011 July |
Data of mean annual temperature (from 1979 to 2012) were obtained from China Meteorological Data Sharing Service System (http://cdc.cma.gov.cn/).
Effect of Photoperiod and Temperature on Nymphal Development
One-day-old male and female adults were paired in a 500-ml transparent cup with rice seedlings for feeding and oviposition. The cups were transferred to six photoperiodic conditions of 4:20, 8:16, 10:14, 12:12, 14:10, and 16:8 (L:D) h at both 20 and 27°C, respectively. Each nymph hatching within 12 hr was reared in a glass test tube (1.5 cm in diameter and 20 cm in length) with a fresh rice seedling until eclosion. The seedling and the glass tube were renewed every 2 d to avoid wilting of the rice. Molting was checked daily to confirm the nymphal instar; 53–108 individuals were included in each of three replicates for all treatments.
All of the experiments were conducted in illuminated incubators with four 30-W fluorescent tubes controlled by an automatic time switch (Bic-300, Shanghai Boxun Industry & Commerce Co., Ltd, Medical Equipment Factory, Shanghai, China). The light intensity was held between 500 and 600 lx, and the temperature variation was ±0.5°C.
Criterion for Diapause
The diapause of a nymph was defined as its failure to grow into an adult at a specific age, which was considered as the maximum time taken for the development from egg to adult under a diapause-suppressing photoperiodic regime (Vinogradova 1974; Rock et al. 1983; Rock and Shaffer 1983). This age varies with temperature and population. As the nymphal diapause of L. striatellus is induced by short photoperiods, long photoperiods would serve as a diapause-suppressing photoperiodic regime for this planthopper. In this study, we set up two long photoperiods of 16:8 and 14:10 (L:D) h, and preliminary experiments provided evidence that the nymphs developed more quickly under a long photoperiod of 16:8 (L:D) h than under 14:10 (L:D) h. Therefore, to more precisely identify the diapause of individuals from each of the three populations, we compared the average development time under the two long photoperiods at 20 and 27°C, respectively. If the differences were significant, the age was determined as the maximum development time (MDT) of nymphs under 16:8 (L:D) h; otherwise, the age was the MDT of nymphs under the two long photoperiods.
Determination of the Sensitive Stage to Diapause Induction
To determine the sensitive stage to diapause induction, experiments were performed for the JY and CCs following the method described by Eizaguirre et al. (1994) and Spieth (1995). The HN was excluded here because its diapause response to photoperiods was very weak. Nymphs from the Changchun and JYs were transferred from long photoperiod of (16:8) h to short photoperiod of (10:14) h or vice versa at various nymphal stages at 20°C. Two photoperiods of 16:8 and 10:14 (L:D) h were selected to detect the sensitive stage of L. striatellus, because none of the nymphs entered diapause under the long photoperiod of 16:8 (L:D) h, while most nymphs entered diapause under the short photoperiod of 10:14 (L:D) h at 20°C. The development time and diapause incidence of these nymphs under the treated conditions were examined; 57–102 individuals were included in each of three replicates for all treatments.
Statistical Analysis
The development times of nymphs under the two long photoperiods of 16:8 and 14:10 (L:D) h were compared using a Student’s t-test. The diapause incidences were statistically examined using Tukey’s honestly significant difference (HSD) test with arcsin square-root transformation. Logistic regression analysis (Sokal and Rohlf 1995) was conducted to decide whether the photoperiod, temperature, and population significantly affected the incidence of diapause. In this study, the dependent variables were assumed as 0 and 1 in the cases of non-diapause and diapause, respectively. All statistical analyses were conducted using the SAS statistical program (SAS Institute Inc. 2002).
Results
Criterion for Diapause
No significant differences were observed for the average development time between the two long photoperiods of 14:10 and 16:8 (L:D) h at both 20 and 27°C for the Hanoi and JYs as well as for the CC at 27°C (Student’s t-test: HN, 20°C, P = 0.207; 27°C, P = 0.0786; JY, 20°C, P = 0.1415; 27°C, P = 0.7320; CC, 27°C, P = 0.1455; Table 2). For the CC, however, a significant difference was observed between the development times under 14:10 and 16:8 (L:D) h at 20°C (CC, 20°C, P < 0.0001). Therefore, the criterion for diapause in nymphs of the Hanoi and JYs was the MDT under the two long photoperiods. For the CC, at 27°C, the criterion was also the MDT at each photoperiod and, at 20°C, it was the MDT under a photoperiod of 6:8 (L:D) h. As shown in the results, at 20°C, the nymphs not growing into adults after 47, 49, and 72 d from birth were considered diapause for the Hanoi, Jiangyan, and CCs, respectively; at 27°C, the diapause criteria were 21, 28, and 32 d, respectively.
Table 2.
Nymphal average and MDT of L. striatellus in the three geographic populations under 2 long-day photoperiods at 20 and 27°C
| Temperature | Population | Development time (d) |
|||
|---|---|---|---|---|---|
| Average |
Maximum |
||||
| Day length—14hr | Day length—16 hr | Day length—14 hr | Day length—16 hr | ||
| 20°C | HY | 31.52 ± 0.44 | 32.20 ± 0.40 | 47 | 45 |
| JY | 38.02 ± 0.63 | 37.78 ± 0.43 | 49 | 47 | |
| CC | 48.46 ± 1.99a | 43.53 ± 0.79 | 80 | 72 | |
| 27°C | HY | 14.49 ± 0.20 | 13.98 ± 0.37 | 21 | 18 |
| JY | 16.51 ± 0.40 | 16.28 ± 0.54 | 28 | 27 | |
| CC | 22.45 ± 0.69 | 21.51 ± 0.67 | 32 | 30 | |
Data of average development time in the table are means ± SE. aThe significant difference of average development time between 2 long-day photoperiods of 14:10 and 16:8 (L:D) h within the same population at the same temperature in by Student’s t-test (P < 0.05). The sample size for each treatment varied from 268 to 315. HN, Hanoi population; JY, Jiangyan population; CC, Changchun population.
Geographic Variation in Diapause Induction
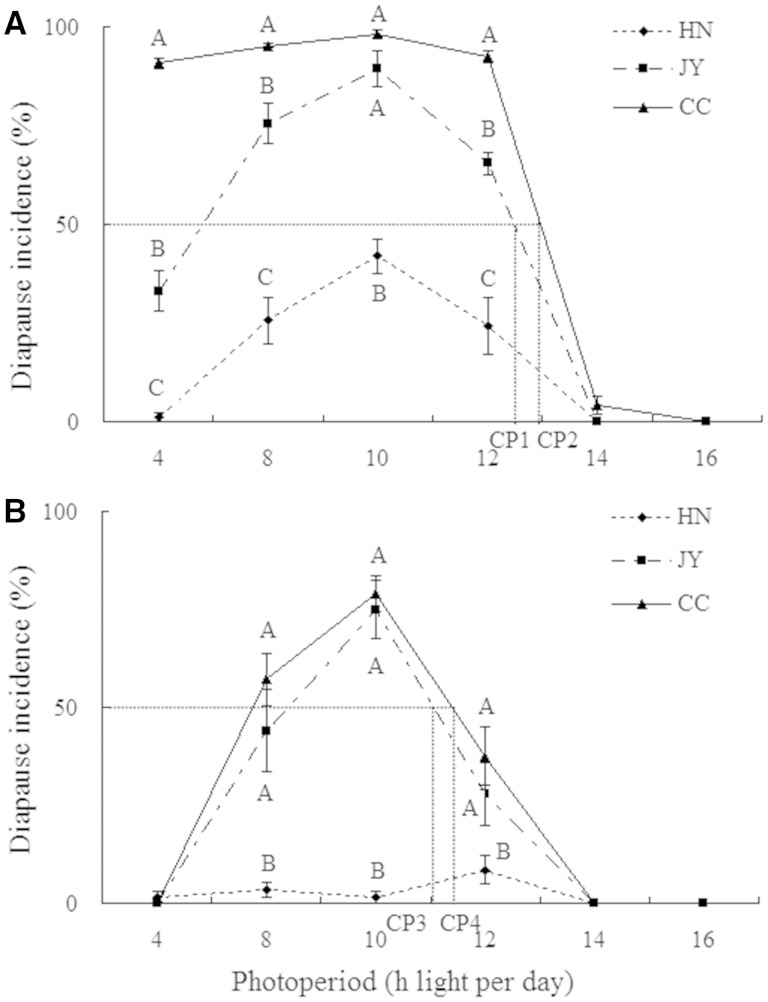
The typical long photoperiodic response was found in all three populations at 20 and 27°C, except for the HN at 27°C (Fig. 1). Significant differences in diapause incidence were observed among the three populations under the same diapause-inducing photoperiod [Tukey’s HSD test: 20°C, 4:20 (L:D) h, df = 2, 6, F = 165.99, P < 0.0001; 8:16 (L:D) h, df = 2, 6, F = 68.74, P < 0.0001; 10:14 (L:D) h, df = 2, 6, F = 63.04, P < 0.0001; 12:12 (L:D) h, df = 2, 6, F = 56.37, P < 0.0001; 27°C, 4:20 (L:D) h, df = 2, 6, F = 1.00, P = 0.42; 8:16 (L:D) h, df = 2, 6, F = 14.78, P = 0.0048; 10:14 (L:D) h, df = 2, 6, F = 72.75, P < 0.0001; 12:12 (L:D) h, df = 2, 6, F = 4.91, P = 0.0055; Fig. 1]. The most northern population (Changchun) exhibited the highest diapause incidence; it is worth noting that the diapause incidence reached nearly 100% at 20°C when the day length was ≤12 hr. Even under the long photoperiod of 14:10 (L:D) h, a small number of nymphs entered diapause. The diapause incidence of the JY was lower than that of the CC under the same conditions, which was only 33.14% at 20°C under the photoperiod of 4:20 (L:D) h. The HN had a very weak response to photoperiods, with fewer than 50% of the individuals entering diapause under all photoperiods, and they even displayed no photoperiodic response at 27°C. Also, as the temperature increased to 27°C, the diapause incidences decreased in each population. Logistic regression analysis indicated that the photoperiod, temperature, population origin, and the interactions of these factors significantly affect the incidence of diapause (Table 3).
Fig. 1.
Photoperiodic response curves for nymphal diapause induction in L. striatellus for three populations at 20°C (A) and 27°C (B). The CPs were ∼12 hr 30 min (CP1) at 20°C and 11 hr (CP3) at 27°C in the JY, and 13 hr (CP2) at 20°C and 11 hr 20 min (CP4) at 27°C in the CC. Different uppercase letters indicate significant differences in the diapause incidences among the three populations at the same photoperiod and temperature. Diapause incidences were arcsin square-root transformed prior to analysis. The sample size for each treatment varied from 174 to 315. HN, Hanoi population; JY, Jiangyan population; CC, Changchun population.
Table 3.
Logistic regression results indicating the effects of photoperiod, population and temperature on diapause induction of L. striatellus
| Effect | DF | χ2 | P |
|---|---|---|---|
| Photoperiod | 1 | 67.18 | <0.0001 |
| Population | 2 | 116.75 | <0.0001 |
| Temperature | 1 | 94.11 | <0.0001 |
| Photoperiod * Population | 2 | 25.77 | <0.0001 |
| Photoperiod * Temperature | 1 | 34.55 | <0.0001 |
| Population * Temperature | 2 | 47.72 | <0.0001 |
| Photoperiod * Population * Temperature | 2 | 31.52 | <0.0001 |
The CP became longer as the latitude increased and the temperature decreased (Fig. 1). The results showed that the CPs were ∼13 hr at 20°C and 11 hr 20 min at 27°C in the northern-most population (Changchun). For the more southern population (Jiangyan), the CPs were ∼12 hr 30 min at 20°C and 11 hr at 27°C. The CP of the HN cannot be estimated in this.
Determination of the Sensitive Stage to Diapause Induction
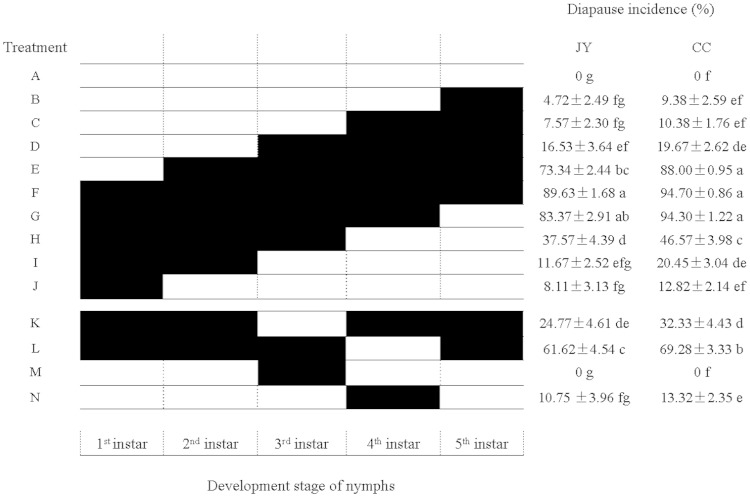
The results showed that the diapause incidences were significantly different among the nymphs that experienced a short photoperiod at different ages (Tukey’s HSD test: JY, df = 13, 28, F = 109.32, P < 0.0001; CC, df = 13, 28, F = 203.49, P < 0.0001; Fig. 2). The experiments in which the nymphs reared under the long photoperiod were then transferred to the short photoperiod mimicked the natural light conditions in late autumn. In these experiments, only a few nymphs entered diapause when they were transferred to the diapause-inducing photoperiod of 10:14 (L:D) h after completing their second instar under a diapause-suppressing photoperiod of 16:8 (L:D) h (JY and CC: diapause incidence <20%; Fig. 2; treatments B, C, and D). However, >70% of the nymphs entered diapause when they were transferred to the diapause-inducing photoperiod of 10:14 (L:D) h in the second instar (JY: 73.34%; CC: 88.00%; Fig. 2; treatment E). Therefore, the second instar was considered to be the most sensitive to the photoperiod. Furthermore, there were high diapause incidences when the nymphs were constantly exposed to the short photoperiod of 10:14 (L:D) h (JY: 89.63%; CC: 94.70%; Fig. 2, treatment F). The diapause incidences remained high when the nymphs reared under the long photoperiod only in the fourth instars (JY: 61.62%; CC: 69.28%; Fig. 2, treatment L). However, when the diapause-inducing photoperiod of 10:14 (L:D) h was interrupted by a long photoperiod of 16:8 (L:D) h only in the third instar, the diapause incidences decreased sharply (JY: 24.77%; CC: 32.33%; Fig. 2, treatment K). Thus, the third instar was also a photoperiodically sensitive stage in the JY and CCs.
Fig. 2.
The nymphal diapause incidences in populations of Jiangyan and Changchun that were transferred from the short photoperiod of 10:14 (L:D) h to the long photoperiod of 16:8(L:D) h vice versa at various nymphal stages. Diapause incidences in figure are means ± SE. Means followed by different lowercase letters within the same column are significantly different by Tukey’s HSD test (P < 0.05) among different treatments. Diapause incidences were arcsin square-root transformed prior to analysis. The sample size for each treatment varied from 163 to 226. White bars represent the long photoperiod of 16:8 (L:D) h and black bars represent the short photoperiod of 10:14 (L:D) h. JY, Jiangyan population; CC, Changchun population.
When the nymphs reared under the short photoperiod were transferred to the long photoperiod, the incidence of diapause exceeded 50% only when the nymphs experienced the short photoperiod from the first to the fourth instar (JY: 83.37%; CC: 94.30%; Fig. 2, treatment G). There were low diapause incidences when nymphs were transferred from the short to the long photoperiod in the fourth-instar stage (JY: 37.57%; CC: 46.57%; Fig. 2; treatment H), and low incidences of diapause also occurred when only the third- or fourth-instar nymphs were under the short photoperiod (the third instar: JY, 0%; CC, 0%; the fourth instar: JY, 10.75%; CC, 13.32 %; Fig. 2, treatments M and N). The results suggest that the diapause incidence of nymphs also depended on the duration of the short photoperiod that the nymph experienced, although the sensitive stages of the Jiangyan and CCs are the second and third instars.
Discussion
The photoperiod is a reliable indicator that can be used to predict unfavorable conditions for insects. Temperature is a less dependable factor but it can modify the photoperiodic response in several insects (Saunders 1982; Hodkova and Socha 1995; Christiansen-Weniger and Hardie 1999). In this study, the results clearly showed that both the photoperiod and the temperature can significantly affect diapause in L. striatellus, although the diapause incidence and intensity varied among populations (Fig. 1, Table 3). Our results were consistent with those of Kisimoto (1958), Noda (1992), and Wang et al. (2014), but these studies showed that the diapause incidence was 100% under the photoperiod of 10:14 (L:D) h. We found that only the CC had a high diapause incidence (nearly 100%) at 20°C, and the Jaingyan and HNs had lower incidences. Therefore, the diapause traits are different among geographic populations of L. striatellus.
Variation in photoperiod-inducing diapause along with latitude is well known in many insect species. Generally, there are higher diapause incidences and longer CPs in northern populations than in southern ones (Danilevskii 1965; Danilevsky et al. 1970; Tauber and Tauber 1972; Beck 1980; Wang et al. 2012), and this phenomenon was also found in L. striatellus in this study. The most northern population (Changchun) showed the highest diapause incidence and a longer CP, where even some nymphs entered diapause under the long photoperiod of 14:10 (L:D) h. However, the most southern population (Hanoi) showed the lowest diapause incidence and displayed no photoperiodic response at 27°C. Noda (1992) reported that a similar phenomenon also emerged in the diapause pattern of nine local populations in Japan. Northern populations overwinter for a longer period than southern ones, so greater diapause intensity is required by populations in northern areas. Additionally, relative to southern areas, winter arrives earlier in northern areas and is accompanied by longer day lengths, so northern L. striatellus populations have a longer CP (Danilevsky et al. 1970; Tauber et al. 1986; Umsalama et al. 2007; Bradshaw and Holzapfel 2010; Wang et al. 2012). The annual minimum temperature experienced by the most southern population (Hanoi) in this study is ∼13°C (data set for 1979–2012; China Meteorological Data Sharing Service System, 2015: http://cdc.cma.gov.cn/), which is close to the threshold temperature for the development of L. striatellus (∼15°C) (Zhang et al. 2008). As a result, diapause is not a necessary trait in the HN for overwinter. Thus, based on the earlier studies, we infer that such variability in diapause among L. striatellus populations can be attributed to an adaptation to each of the local environmental conditions.
Wang et al. (2014) found that the CP of the L. striatellus population in Nanchang, China (28.8° N, 115.90° E) was 12 hr at 20°C and, here, the CPs of the Jiangyan (32.51° N, 120.15° E) and Changchun (43.89° N, 125.32° E) populations were ∼12 hr 30 min and 13 hr at 20°C, respectively. An obvious gradual clinal type with latitudinal variation in the CP of L. striatellus was found. A similar diapause pattern with latitudinal variation was also found in Japan by Noda (1992). Specifically, the CPs of nine populations in Japan were positively correlated with the original latitude and gradually decreased toward the south at a rate of about 1.1 hr for each 5 degrees of latitude, which was in accordance with the relationship found in many long-day species (Danilevsky et al. 1970; Beck 1980; Tauber et al. 1986; Danks 1987; Ito and Nakata 2000; Suwa and Gotoh 2006; Timer et al. 2010; Wang et al. 2012). However, in China, the CPs for diapause induction in the Nanchang, Jiangyan, and CCs of L. striatellus only varied by about 1 hr under a range of 14 degrees of latitude. The possible factor underlying the difference in CPs with latitudinal variation between China and Japan was analyzed as follows. Several reports have proved that extensive gene flow can reduce the genetic variation in diapause characteristics of different populations (Danilevsky et al. 1970; Mcwatters and Saunders 1996; Suwa and Gotoh 2006), and even lead to a lack of clinal variation in CP (Kimura et al. 1993). In Japan, significant differentiation among the genetic structure of populations of L. striatellus was revealed based on three allozyme loci, which suggests that long-range dispersal of L. striatellus does not regularly occur over the main lands (Hoshizaki 1997). Moreover, although the overseas migration of L. striatellus from the East China Sea and south-eastern Asia to western Japan occurs (Otuka et al. 2010; Sanada-Morimura 2001; Syobu et al. 2011), the offspring of the immigrants have low adaptability to the local climate conditions at the landing site (Hoshizaki 1997). From the earlier studies, we infer that gene flow among the L. striatellus populations in Japan is low. In China, however, L. striatellus can undertake long-distance migration and intercross between domestic populations, suggesting extensive gene flow (Wang et al. 2011; Zhang et al. 2011; He et al. 2012; Sun et al. 2015). As a result, L. striatellus populations lack significant genetic differences in China (Sun et al. 2015). Therefore, we infer that the different degrees of variation in diapause with latitude between Japan and China are due to the different levels of gene flow among the domestic populations in the two countries.
Our results indicated that the sensitive stage to photoperiods in the Jiangyan and CCs was the third instar, which was consistent with that of the Nanchang population as reported by Wang et al. (2014). Additionally, the results in the experiment of transferring nymphs from the long day length of 16:8 (L:D) h to the short day length of 10:14 (L:D) h proved that the second instar was also a photoperiodically sensitive stage in the two populations. Like many other insect species, the sensitive stage of L. striatellus tends to be the age immediately preceding the diapause stage (Danks 1987), offering obvious advantages of preparation for the upcoming winter and conferring better adaptation to overwintering in the field. However, although the second and third instars were the sensitive stages to the photoperiod in L. striatellus, the diapause incidences depended on the duration of the short photoperiod that the nymph experienced. We conferred that these phenomena would result from two factors. The first is that L. striatellus, like many other insects (Danks 1987), could terminate diapause under long day lengths (Noda 1992; Wang et al. 2014). The second is that the fourth- and fifth-instar periods were also under the control of photoperiodism in addition to the young instars (Kisimoto 1958). Thus, when the nymphs were transferred to a long photoperiod during the later nymphal stages, the diapause-inducing effect of a short photoperiod on young instars was almost reversed.
Entering diapause actually confers costs as well as benefits. During the period of diapause, the individuals might miss chances to reproduce and will consume energy reserves, or be killed by predators or parasitic animals. Under these circumstances, a risk-spreading strategy, which is expressed as the existence of both diapausing and non-diapausing individuals under short photoperiod here, may be selected (Kurota and Shimada 2001; Cáceres and Tessier 2004a,b; Umsalama et al. 2007). In our experiments, the response of diapause induction to photoperiods is not an ‘all-or-nothing’ reaction; no treatment under short day lengths resulted in a 100% diapause incidence. We infer that, in the field, when the CP occurs with a shortened day length, some nymphs will grow slowly and enter diapause. However, other nymphs will grow continuously to adulthood, and these adults will migrate, disperse, and reproduce. This is a risk-spreading strategy formed during long-term evolution to ensure the survival of L. striatellus populations.
In conclusion, although L. striatellus migrates over long distances, populations maintain their particular diapause characteristics to adapt to the local environment. This conservatism appears conducive to advantageous selections in planthoppers, through which they have adapted to survive the cold winters. Our results provide important insights into one possible mechanism underlying adaptation of pests to the complicated and volatile climate conditions. Understanding the geographic variation in diapause characteristics of L. striatellus may be useful for improving strategies to pest management, according to which the outbreak of L. striatellus can be predicted more precisely.
Acknowledgments
This study was supported by a grant from the National Basic Research Program of China Program (2010CB126200) and the Special Research Fund for the Doctoral Program of Higher Education (20120097110037).
References Cited
- Beck S. D. 1980. Insect photoperiodism. 2nd ed Academic Press, Inc., New York. [Google Scholar]
- Bradshaw W. E., Holzapfel C. M. 2010. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 72: 147–166. [DOI] [PubMed] [Google Scholar]
- Cai B. H., Huang F. S., Feng W. X., Fu Y. R., Dong Q. F. 1964. Study on Delphacodes striatellus Fallén (Hemiptera: Delphacidae) in North China. Acta Entomol. Sinica 13: 552–571 (in Chinese). [Google Scholar]
- Cáceres C. E., Tessier A. J. 2004a. Incidence of diapause varies among populations of Daphnia pulicaria. Oecologia 141: 425–431. [DOI] [PubMed] [Google Scholar]
- Cáceres C. E., Tessier A. J. 2004b. To sink or swim: variable diapause strategies among Daphnia species. Limnol. Oceanogr. 49: 1333–1340. [Google Scholar]
- Christiansen-Weniger P., Hardie J. 1999. Environmental and physiological factors for diapause induction and termination in the aphid parasitoid, Aphidius ervi (Hymenoptera: Aphidiidae). J. Insect Physiol. 45: 357–364. [DOI] [PubMed] [Google Scholar]
- Danilevskii A. S. 1965. Photoperiodism and seasonal development of insects. Oliver and Boyd, Edinburgh. [Google Scholar]
- Danilevsky A. S., Goryshin N. I., Tyschenko V. P. 1970. Biological rhythms in terrestrial arthropods. Annu. Rev. Entomol. 15: 201–244. [Google Scholar]
- Danks H.V. 1987. Insect dormancy: an ecological perspective. Biological Survey of Canada, Ottawa, Canada. [Google Scholar]
- Denlinger D.L. 2008. Why study diapause. Entomol. Res. 38: 1–9. [Google Scholar]
- Eizaguirre M., LÓpez C., AsÍn L., Albajes R. 1994. Thermoperiodism, photoperiodism and sensitive stage in the diapause induction of Sesamia nonagrioides (Lepidoptera: Noctuidae). J. Insect Physiol. 40: 113–119. [Google Scholar]
- Gillespie D. R., Quiring D.M.J. 2005. Diapause induction under greenhouse condition in two populations of Dicyphus hesperus (Hemiptera: Miridae). Biocontrol Sci. Techn. 15: 571–583. [Google Scholar]
- He Y., Zhu Y. B., Hou Y. Y., Yao S. T., Lu Z. J., Jin Z. H., Zhang X. X., Zhai B. P. 2012. Fluctuation and migration of spring population of small brown planthopper (Laodelphax striatellus) on wheat in Jiangsu and Zhejiang provinces. Chinese J. Rice Sci. 26: 109–117 (in Chinese with English summary). [Google Scholar]
- Hodkova M., Shoca R. 1995. Effect of temperature on photoperiodic response in a selected ‘non-diapause’ strain of Pyrrhocoris apterus (Heteroptera). Physiol. Entomol. 20: 303–308. [Google Scholar]
- Hoshizaki S. 1997. Allozyme polymorphism and geographic variation in the small brown planthopper, Laodelphax striatellus (Homoptera: Delphacidae). Biochem. Genet. 35: 383–393. [DOI] [PubMed] [Google Scholar]
- Ito K., Nakata T. 2000. Geographical variation of photoperiodic reponse in the females of a predatory bug, Orius sauteri (Poppius) (Heteroptera: Anthocoridae) from northern Japan. Appl. Entomol. Zool. 35: 101–105. [Google Scholar]
- Kisimoto R. 1958. Studies on the diapause in the planthoppers, effect of photoperiod on the induction and the completion of diapause in the fourth larval stage of the small brown planthopper, Delphacodes striatella Fallén. Japanese J. Appl. Entomol. Zool. 2: 128–134. [Google Scholar]
- Kisimoto R. 1989. Flexible diapause response to photoperiod of a laboratory selected line in the small brown planthopper, Laodelphax striatellus Fallén. Appl. Entomol. Zool. 24: 157–159. [Google Scholar]
- Kimura M. T., Bessho A., Dai Z. H. 1993. The influence of gene flow on latitudinal clines of photoperiodic adult diapause in the Drosophila auraria species-complex. Biol. J. Linn. Soc. 48: 335–341. [Google Scholar]
- Kurota H., Shimada M. 2001. Photoperiod- and temperature-dependent induction of larval diapause in a multivoltine bruchid, Bruchidius dorsalis. Entomol. Exp. Appl. 99: 361–369. [Google Scholar]
- Liu X. D., Zhai B. P., Liu C. M. 2006. Outbreak reasons of Laodelphax striatellus population. Chinese B. Entomol. 43: 141–146 (in Chinese with English summary). [Google Scholar]
- Mcwatters H. G., Saunders D. S. 1996. The influence of each parent and geographic origin on larval diapause in the blow fly, Calliphora vicina. J. Insect physio. 42: 721–726. [Google Scholar]
- Musolin D. L., Ito K. T. 2008. Photoperiodic and temperature control of nymphal development and induction of reproductive diapause in two predatory Orius bug: interspecific and geographic differences. Physiol. Entomol. 33: 291–301. [Google Scholar]
- Nault L. R. 1994. Transmission biology, vector specificity and evolution of planthopper-transmitted plant viruses, pp. 429–448. In Denno R. E., Perfect T. J. (eds.), Planthopper, their ecology and management. Chapman & Hall, New York. [Google Scholar]
- Nemoto H., Ishikawa K., Shimura E. 1994. The resistances to rice stripe virus and small brown planthopper in rice variety, IR 50. Breeding Sci. 44: 13–18. [Google Scholar]
- Noda H. 1992. Geographic-variation of nymphal diapause in the small brown planthopper in Japan. Japan Agr. Res. Q. 26: 124–129. [Google Scholar]
- Otuka A., Matsumura M., Sanada-Morimura S., Takeuchi H., Watanabe T., Ohstu R., Inoue H. 2010. The 2008 overseas mass migration of the small brown planthopper, Laodelphax striatellus, and subsequent outbreak of rice stripe disease in western Japan. Appl. Entomol. Zool. 45: 259–266. [Google Scholar]
- Pazyuk I. M. D. L., Musolin ., Reznik S. Y. 2014. Geographic variation in thermal and photoperiodic effects on development of zoophytophagous plant bug Nesidiocoris tenuis. J. Appl. Entomol. 138: 36–44. [Google Scholar]
- Rock G. C., Shaffer P. L., Shaltout A. D. 1983. Tufted apple budmoth (Lepidoptera: Tortricidae): photoperiodic induction of larval diapause and stages sensitive to induction. Environ. Entomol. 12: 69–70. [Google Scholar]
- Rock G. C., Shaffer P. L. 1983. Tufted apple budmoth (Lepidoptera: Tortricidae): Effects of constant daylengths and temperatures on larval diapause. Environ. Entomol. 12: 71–75. [Google Scholar]
- Ruberson J. R., Yeargan K. V., Newton B. L. 2001. Variation in diapause responses between geographic populations of the predator Geocoris punctipes (Heteroptera: Geocoridae). Ann. Entomol. Soc. Am. 94: 116–122. [Google Scholar]
- Sanada-Morimura S., Sakumoto S., Ohtsu R., Otuka A., Huang S., Thanh D. V., Matsumura M. 2001. Current status of insecticide resistance in the small brown planthopper, Laodelphax striatellus, in Japan, Taiwan, and Vietnam. Appl. Entomol. Zool. 46: 65–73. [Google Scholar]
- Saunders D. S. 1982. Insect clocks. Pergamon, Oxford. [Google Scholar]
- Shimizu T., Kawasaki K. 2001. Geographic variability in diapause response of Japanese Orius species. Entomol. Exp. Appl. 98: 303–316. [Google Scholar]
- Socha R. 2001. Latitudinal gradient in response of wing polymorphism to photoperiod in a flightless bug, Pyrrhocoris apterus (Heteropera: Pyrrhocoris). Eur. J. Entomol. 98: 167–169. [Google Scholar]
- Sokal R. R., Rohlf F. J. 1995. Biometry: the principles and practice of statistics in biological research, 3rd ed W. H. Freeman & Company, New York. [Google Scholar]
- Spieth H. R. 1995. Change in photoperiodic sensitivity during larval development of Pieris brassicae. J. Inscet Physiol. 41: 77–83. [Google Scholar]
- Suwa A., Gotoh T. 2006. Geographic variation in diapause induction and mode of diapause inheritance in Tetranychus pueraricola. J. Appl. Entomol. 130: 329–335. [Google Scholar]
- Syobu S., Otuka A., Matsumura M. 2011. Trap catches of the small brown planthopper, Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae), in northern Kyushu district, Japan in relation to weather conditions. Appl. Entomol. Zool. 46: 41–50. [Google Scholar]
- Sun J. T., Wang M. M., Zhang Y. K., Chapuis M. P., Jiang X. Y., Hu G., Yang X. M., Ge C., Xue X. F., Hong X. Y. 2015. Evidence for high dispersal ability and mito-nuclear discordance in the small brown planthopper, Laodelphax striatellus. Sci Rep-UK. 5: 8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber M. J., Tauber C. A. 1972. Geographic variation in critical photoperiod and in diapause intensity of chrysopa carnea (Neuroptera). J. Inscet Physiol. 18: 25–29. [Google Scholar]
- Tauber M. J., Tauber C. A., Masaki S. 1986. Seasonal adaptations of insects. Oxford University Press, New York. [Google Scholar]
- Timer J., Tobin P. C., Saunders M. C. 2010. Geographic variation in diapause induction: the grape berry moth (Lepidoptera: Tortricidae). Popul. Ecol. 39: 1751–1755. [DOI] [PubMed] [Google Scholar]
- Umsalama A.M.A., Shi Z. H., Guo Y. L., Zou X. F., Hao Z. P., Pang S. T. 2007. Maternal photoperiod effect on and geographic variation of diapause incidence in Cotesia plutellae (Hymenoptera: Braconidae) from China. Appl. Entomol. Zool. 42: 383–389. [Google Scholar]
- Vinogradova E. B. 1974. The pattern of reactivation of diapausing larvae in the blowfly, Calliphora vicina. J. Insect Physiol. 20: 2487–2496. [DOI] [PubMed] [Google Scholar]
- Wang L., Hang C., Xu Y. B., Cai G. C., Sun Y. W., Hu X. Y., Zhang X. X., Zhai B. P. 2011. Migration and dispersal of the small brown planthopper Laodelphax striatellus (Fallén) in the Jianghuai region: Case studies in Fengtai, Anhui Province in spring of 2009 and 2010. Chinese J. Appl. Entomol. 48: 1288–1297 (in Chinese with English summary). [Google Scholar]
- Wang L. F., Lin K. J., Chen C., Fu S., Xue F. S. 2014. Diapause induction and termination in the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae). PLoS One, 9, e107030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. P., Yang Q. S., Dalin P., Zhou X. M., Luo Z. W., Lei C. L. 2012. Geographic variation in photoperiodic diapause induction and diapause intensity in Sericinus montelus (Lepidoptera: Papilionidae). Insect Sci. 19: 295–302. [Google Scholar]
- Zhang A. M., Liu X. D., Zhai B. P., Gu X. Y. 2008. Influences of temperature on biological characteristics of the small brown planthopper, Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae). Acta Entomol. Sinica. 51: 640–645 (in Chinese with English summary). [Google Scholar]
- Zhang H. Y., Diao Y. G., Yang H. B., Zhao Y., Zhang X. X., Zhai B. P. 2011. Population dynamics and migration characteristics of the small brown planthopper in spring in Jining, Shangdong Province. Chinese J. Appl. Entomol. 48: 1298–1308 (in Chinese with English summary). [Google Scholar]