Abstract
Electrical and chemical stimulation have been studied as potent mechanisms of enhancing nerve regeneration and wound healing. However, it remains unclear how electrical stimuli affect nerve growth, particularly in the presence of neurotrophic factors. The objective of this study was to explore (1) the effect of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) supplementation to support neurite outgrowth in a 3D scaffold, and (2) the effect of brief, low voltage, electrical stimulation (ES) on neurite outgrowth prior to neurotrophin supplementation. Dissociated E11 chick dorsal root ganglia (DRG) were seeded within a 1.5 mg/mL type-I collagen scaffold. For neurotrophin treatments, scaffolds were incubated for 24 hrs in culture media containing nerve growth factor (NGF, 10 ng/mL) or BDNF (200 ng/mL), or both. For ES groups, scaffolds containing neurons were stimulated for 10 min at 8–10 V/m DC, then incubated for 24 hrs with neurotrophin. Fixed and labeled neurons were imaged to measure neurite growth and directionality. BDNF supplementation was not as effective as NGF at supporting DRG neurite outgrowth. ES prior to NGF supplementation improved DRG neurite outgrowth compared to NGF alone. This combination of brief ES with NGF treatment was the most effective treatment compared to NGF or BDNF alone. Brief ES had no impact on neurite directionality in the 3D scaffolds. These results demonstrate that ES improves neurite outgrowth in the presence of neurotrophins, and could provide a potential therapeutic approach to improve nerve regeneration when coupled with neurotrophin treatment.
Keywords: Neurite growth, collagen scaffold, electrical stimulation, nerve growth factor, brain-derived neurotrophic factor, dorsal root ganglion
1. Introduction
Tissue engineering is a rapidly growing field that seeks to utilize principles of human development and wound healing to create strategies for repair of damaged tissue function. Many strategies include growth factors to provide signals for cells to function, i.e., to proliferate, migrate, or differentiate. Nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), and neurotrophins (NT-3 or NT-4) are four of the growth factors that provide instructive signals by binding with cells via tropomyosin-receptor-kinase (Trk) receptors. There are 3 receptors: TrkA receptors activated by NGF and NT-3, TrkB receptors activated by BDNF and NT-4, as well as NT-3, but with less affinity, and TrkC receptors activated by NT-3 (for review, see refs34). A promiscuous, low-affinity receptor, p75NTR, has been shown to support and supplement high-affinity Trk receptor binding of growth factors, but has also been shown to activate growth inhibition and death pathways when exposed to high levels of neurotrophins. 8, 35, 38 Therefore, these specific growth factor cues are considered critical in many tissue-engineering strategies and are utilized in scaffolds developed for nerve regeneration.4, 14, 15, 17, 20
While it is generally agreed that appropriate growth factor supplementation enhances nerve regeneration, the influence of electrical stimulation (ES) on nerve growth is less understood. The relative impact of ES in previous studies depends upon the stimulation protocol, as increased survival and axonal growth has been found to be dependent upon the ES parameters applied. ES has been applied for a range of time, hours to weeks, with alternating current that varied from 0.2–3.0 V or 100–500 μA, a range of frequencies (10–300 Hz pulses using both monophasic and biphasic waveforms), and pulses or trains with varying durations.10 One laboratory has shown that low frequency (20 Hz) pulsed ES (1 hr to weeks) improved regeneration of peripheral sensory axons in vivo.16
Direct current (DC) ES at 25 V/m for 10 min duration increased dorsal root ganglia (DRG) neurite outgrowth on 2D collagen and laminin surfaces in vitro.42, 43 Furthermore, environmental changes such as media composition, levels of Ca2+, temperature, and stimulation duration (10 min vs. 100 min) had minimal impact on the neurite outgrowth results, where the application of ES resulted in a 40% increase in neurite length compared to non-stimulated controls.43 These results after short (10 min) duration DC ES gave similar neurite length improvement as previously studied longer duration DC or pulsed ES (1 hr to 2 weeks).18, 22, 25 Therefore, in this in vitro study, we chose to use the same short duration, low voltage ES as reported by Wood, 2006. 42
The mechanism of ES on growth enhancement of neurons has not been clearly elucidated, although it has been proposed that its action may be through Trk receptors. ES caused an upregulation of intrinsic neuronal growth factor and their membrane surface receptors.1, 32 Specifically, ES for 1 hr at 20 Hz caused an upregulation of BDNF and TrkB receptor expression in rat motor neurons, and an increase in expression of BDNF in rat sensory neurons in vivo.1, 16, 40 From these previous studies, we hypothesized that a 10 min ES protocol would similarly upregulate TrkB receptor expression. NGF, however, is the typical growth factor supplemented in vitro.25, 36 Therefore, in this study we sought to explore the relationships between neurite outgrowth, BDNF (TrkB), NGF (TrkA), and ES isolated in a three-dimensional environment.
To accomplish this, embryonic chick DRG were used as a primary cell model for neuronal growth, and were cultured with and without NGF, BDNF, or NGF+BDNF in a 3D collagen gel. ES was applied for 10 min at 25 V/m. After 24 hr, the length and directionality of the neurites were measured. Here, we show that ES augmented the response of NGF or BDNF alone, but not when NGF and BNDF were combined. Overall, these results show that short duration ES enhances neuronal growth in 3D scaffolds.
2. Materials & Methods
2.1 Materials
Phosphate buffered saline, sodium hydroxide, sodium bicarbonate, bovine serum albumin, F12 Ham’s media, and Hanks’ Balanced Salt Solution Modified were purchased from Sigma-Aldrich (St. Louis, MO). Collagen Type I rat tail and mouse 2.5S NGF were purchased from BD Bioscience. Neurobasal Media, penicillin/streptomycin/L-glutamine, B27 supplement, and trypsin-EDTA were all purchased from Gibco/Life Technologies. Paraformaldehyde and Triton X-100 were purchased from Fisher Scientific. HEPES was purchased from Roche. Fetal bovine serum was purchased from Thermo Scientific/Hyclone. Fertilized white leghorn Specific Pathogen Free (SPF) eggs were purchased from Sunrise Farms. The mouse anti-chick neurofilament monoclonal antibody was obtained from Developmental Studies Hybridoma Bank. Anti-mouse AlexaFluor secondary antibody was purchased from Life Technologies/Molecular Probes. Vectashield Mounting Medium was purchased from Vector Laboratories. Experimental dishes were prepared from 35 mm tissue culture dishes with round holes drilled through the bottom of the plastic. Glass coverslips were sealed underneath the dish with a silicone sealant, and sterilized with 70% ethanol.
2.2 Neuron Isolation
DRG were extracted from E11 chick (which prior to E16 are not considered animals and do not fall under animal regulation) and dissociated in 1x trypsin-EDTA (Gibco/Life Technologies) for 30 minutes. Trypsin dissociation was terminated by addition of fetal bovine serum. Dissociated cells were resuspended in B27/penicillin/streptomycin/L-glutamine supplemented neurobasal media prior to suspending them in the collagen gel solution. In separate experiments, it was determined that ~28% of the total live cells after this isolation were neurons.
2.3 Collagen Gel Fabrication
Collagen gels were fabricated at physiological pH using a standard protocol. 30 The following components were mixed in order, on ice: sodium bicarbonate (0.5 mg/ml in gel), sterile water, NaOH (0.1 M), collagen (1.5 mg/mL rat tail type I in 0.02 M acetic acid), 10X F12K media, and HEPES (10 mM in gel). Cells were added as the final component of the suspension prior to gelation. Gel solutions (18 μL) were added to the imaging well seeded with cells at 5 × 105 cells/mL, gelled at 37°C within 15 min, and had a pH range of 7.2–7.6. For gels that were not electrically stimulated, the gels were cultured immediately in supplemented neurobasal media and growth factor for 24 hours prior to fixing for experimentation. 5, 26 Growth factor conditions were as follows: NGF only (10 ng/mL); BDNF only (200 ng/mL); or NGF (10 ng/mL) and BDNF (200 ng/mL).
2.4 Electrical Stimulation
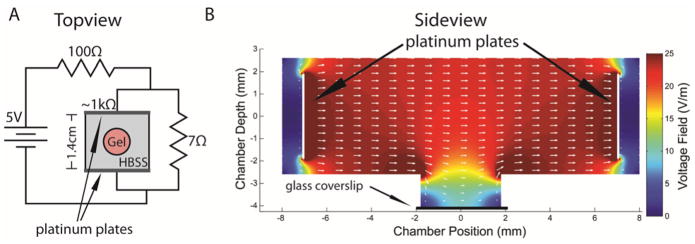
Immediately after gelling, a dish was placed in an electrical stimulation chamber and gels were submerged in HBSS as the electrical conducting media. The imaging dish was made by drilling a small hole through the bottom of the dish, and attaching a coverslip to the underneath surface with 100% silicone sealant. Collagen gel solution (1.5 mg/mL) seeded with dissociated DRG neurons was added to the “well” of the culture dish formed from the hole and glass coverslip. The collagen gel was allowed to set before placing a stimulation chamber within the circumference of the culture dish (Fig 1). The stimulation chamber was a molded PDMS rectangle with platinum plates lining opposite sides. The culture dish was stimulated for 10 min with a low voltage electrical field set to 24 V/m (0.324 μA). A schematic of the circuit for ES and the orientation of the dish is shown in Fig 1A (Topview). The circuit (shown in Fig 1A) was turned on and a voltmeter was used to establish that 24V/m was applied across the two platinum conducting plates for the duration of the ES. A current of 0.324 μA was calculated between the conducting plates based on a measured resistance of ~1kΩ although there could be a change of resistance at the platinum-solution interface that would effectively change the current through the media. ES was performed for 10 min, and pH of the HBSS solution was measured in preliminary experiments before and after ES to confirm that no pH changes occurred in the conducting solution. Immediately following ES or sham ES, HBSS was removed and replaced with B27-supplemented neurobasal media that contained either no neurotrophins (null), NGF or BDNF alone, or both neurotrophins at the concentrations described in Section 2.3.
Figure 1.
Electrical modeling for the ES chamber. (A) Topview schematic of the electrical stimulation chamber and circuit. Gel with cells, HBSS solution, and chamber are not drawn to scale. (B) Sideview of ES chamber with platinum plates (black arrows) and a 2D FEA simulation with showing electrical current (white arrows). Drilled hole in plastic culture dish has a glass coverslip epoxied underneath (labeled).
2.5 Scanning Electron Microscopy (SEM) of Gel Scaffolds
To perform SEM, the gels were formed as described in Section 2.3 except the cell suspension was not added. Gels were either fixed immediately without exposure to ES, or electrically stimulated as described in Section 2.3 and then fixed with 2% glutaraldehyde for 1 hr and washed 3X in PBS. Samples were prepared as described previously by ethanol gradient dehydration, critically point dried (using CO2 as the exchange medium), and sputter coated with Au/Pd before imaging with a scanning electron microscope. 23
2.6 Confocal Microscopy Imaging
After 24 hrs of incubation, scaffolds with neurons were fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 2 mg/mL bovine serum albumin. Primary antibody against chick neurofilament (1:200) was incubated overnight at 4°C while rocking. Scaffolds were washed and incubated with a secondary AlexaFluor-conjugated antibody (1:200) for 4 hrs, and stored with Vectashield Mounting Medium that contained the nuclei stain, DAPI, at a dilution of 1:1000. Gels were stored at 4 °C for no longer than 4 days until imaging. Confocal images were acquired using a confocal microscope (Olympus FluoView 1000) with a 4x zoom on a 20x lens (NA 1.42) to detect the fluorescently labeled neurons. Z-series of images were acquired for each gel by imaging from the glass coverslip surface through the gel until visual clarity was lost (about 530 μm above the coverslip). Each z-series was comprised of approximately 250 images taken in 2.17 μm increments, the optimal increment in confocal imaging for 3D reconstruction. Image dimensions of 630 μm × 630 μm were acquired at 1024 × 1024 pixels.
2.7 Neurite Extension
Images were analyzed using FIJI 33 Simple Neurite Tracer (a plugin for FIJI, 24) to reconstruct the z-series into 3-dimensional stacks of images with neurons and their extending neurites. Starting in the center of the image, every cell body was labeled working from the center to the edge until 10 neurons with neurites at least 2 times longer than the neuron soma diameter were acquired for that gel.41 Typically, the soma of the neurons were ~10 μm in diameter. No neuron was counted that was situated within 100 μm of the edge of the stacked image. The longest length neurite was traced and measured for each of the individual neurons. If a neurite left the image or touched the coverslip, it was not used in analysis. The neurite lengths were calculated in Simple Neurite Tracer from the scaling factor acquired when imported from the stacked confocal images. To evaluate whether growth conditions failed to support neurite extension, neurons with neurites less than 2 times the neuron soma diameter were also counted, but were considered not to produce useable neurites. Statistics were performed in OriginPro8 (OriginLab) or MATLAB (Mathworks). All data for each of the ES and growth conditions were nonparametric. The Kruskal-Wallis statistical test was used to determine whether ES and growth factors significantly affected neurite length, and was significant to p<1×10−6. To determine whether individual conditions were statistically significant, individual data sets were compared as shown in Fig 3 with a post-hoc Mann-Whitney test. 37, 41
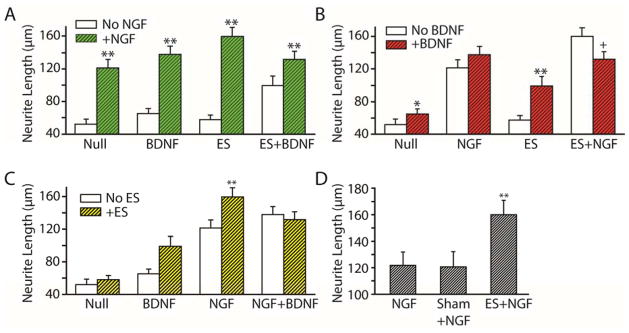
Figure 3.
Average neurite length from Table 1 is graphically shown from three perspectives: with and without NGF (A), with and without BDNF (B), and with and without ES (C). Mann-Whitney post-hoc significance test gave increased neurite length (*p<0.05 and **p<0.01), and decreased length (+p<0.05). (D) ES chamber without electrical current (sham+NGF) had no significant difference than NGF alone, and ES+NGF exhibited significantly longer neurites than both NGF alone and sham+NGF (**p<0.01).
2.8 Finite Element Modeling
The electrical stimulation chamber was designed to produce a uniform field between the two parallel platinum electrodes of 24 V/m. To accomplish this, we placed platinum electrodes 1.4 cm apart, represented in Fig 1A. A circuit was built with a 5V power supply that provided a constant 0.327 V potential between the platinum plates. This provided a theoretical electrical field between the plates of 23.4 V/m. However, due to the geometry of the well, and because the well was filled with collagen that has a reduced conductivity compared to a saline solution, the electric field was reduced in the well.
A finite element analysis (FEA) of the ES chamber was performed using MATLAB and the 2-dimensional simulation with Partial Differential Equation Toolbox 1.2 (MathWorks, Inc.). The FEA modeled the electrical field in the imaging well where neurons were suspended in a collagen scaffold. For the coordinates of the chamber, the X dimension describes the distance between the plates, and the Z dimension describes the depth of the chamber. Because the FEA is for 2 dimensional simulations only, the electric field was modeled from a side view, looking at an X-Z cross-section through the center of the well.
The 2D geometry of the conducting solution measured in the chamber is shown in Fig 1B. The chamber was modeled with the following boundaries: air on top, polydimethylsiloxane (PDMS) on the sides, polystyrene on the bottom of the chamber and sides of the well, and glass at the bottom of the well. All of these boundaries were set to have zero current conductance (using Neumann boundary conditions ∂V/∂n = 0, where n denotes the vector normal to the boundary), and the two plates (left and right) were set to have voltages of 0.327 V and 0 V, respectively (using Dirichlet boundary conditions V = 0.327 and V = 0 respectively). The conductance of the PBS buffer solution filling the chamber was set to the value for PBS, 1.4 S/m as stated in the technical specifications for PBS from Fisher Scientific (Catalog #BP399). The conductivity of a collagen hydrogel was reported to be 86% that of the solution that fills it 6, so we assumed a value of 1.2 S/m for the collagen type I gel. The gel-PBS boundary was assumed to be straight and continuous with the bottom of the chamber at ~2.7 mm. The mesh was generated by MATLAB refined to a total of 113,025 triangles. The initial voltage and current within the solution was set to 0, and the long-term stationary voltages and currents within the solution were solved for the elliptical Laplace equation −Δ · (σΔV) = 0, where σ denotes the conductivity of the media. The FEA solution is shown in Fig 1B.
2.9 Neurite Directionality Calculation
To determine if ES or either of the neurotrophins had an effect on directional growth of neurites, each 3D image, spanning 630 μm × 630 μm and roughly 550 μm deep was analyzed for directionality. The direction of each neurite was quantified by the vector measured for each neurite from the neurite origin at the neuron body to the end point at the tip of the longest neurite. Because the direction of the electrical field was not recorded during confocal imaging, the directionality of neurite growth was measured in relation to the other neurites within the image and not with respect to an external electrical field. Orientation of neurites was measured as the proportion of all neurite growth in the direction of the principal component axis of an image. The principle component of the neurite vectors was calculated using the built-in principle component analysis function in MATLAB (pca(X)). In order to compare the direction of neurite growth between multiple neurons within an image, at least three neurons with slightly longer neurites (an arbitrarily chosen threshold of at least 40 μm was used) needed to be present. Of the total 71 images acquired, 37 images met this criteria. The directionality in each of these images was deemed significant if the proportion of the neurite growth in the direction of the principle axis was less than 5% likely to happen by chance.
3. Results
3.1 Finite Element Model of Electrical Stimulation
The electrical field was modeled with an FEA to determine the electrical field through the collagen gel where the cells were situated (Fig 1A). This FEA provided a simulation of a 2D cross section of the stimulating electrical chamber and imaging well (Fig 1B). The FEA was simulated for the measured dimensions of the stimulation chamber, distance between platinum plates (1.4 cm), and voltage drop across the platinum plates (0.327 V). These results show that a relatively constant electrical field was established in the dish across the platinum plates, ranging from 22–24 V/m. However, within the imaging depth (below the dotted line in Fig 1B), the voltage field was closer to 8–10 V/m. Therefore, the FEA indicated that the voltage field applied to the neurons within the collagen gel was less than that measured between the platinum plates, but still within physiological electrical field ranges.29
3.2 ES and Neurotrophins Improve Neurite Growth From Neurons in 3D Scaffolds
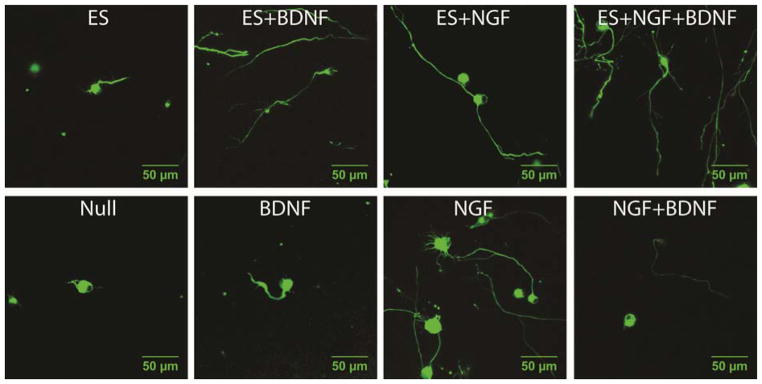
Neurons were exposed to growth conditions with NGF, BDNF, or both growth factors for 24 hours after scaffolds were formed. Separately, scaffolds imbedded with neurons were exposed to either ES or sham ES (electrical chamber placed in dish, but no ES applied) for 10 min, and then incubated for 24 hours in NGF, BDNF, or both growth factors. Sham controls for ES showed no differences than the conditions without any ES chamber. Images of the neurons are shown for each of the 8 conditions measured for ES and neurotrophins (Fig 2); each image is the third-quartile neurite measured in length. For example, for the ES+NGF conditions (n=117 neurons), the 88th longest neurite image is shown in Fig 2.
Figure 2.
Representative neurons from each of the eight growth conditions were stained for neurofilament and imaged after 24 hours of 3D growth suspended in collagen gels. Top images contain neurons grown with ES, and bottom images contain neurons grown with corresponding neurotrophins without ES. For each growth condition, a representative neuron at the third quartile of neurite length was used. [Note: electrical field directionality was not determined in this experiment.] Scale bars = 50 μm.
As expected, neurons had significantly longer neurite extensions when grown in NGF than neurons grown in conditions without NGF, either with or without ES (Fig 3A). The mean neurite length for NGF (121.3 μm ± 10.0 μm) was significantly increased compared to the neurite length grown in the absence of any growth factor, called the null condition (52.2 μm ± 6.5 μm; p<0.01). Similarly, the average neurite length for neurons grown in NGF and BDNF (137.7 μm ± 9.9 μm) was significantly longer compared to neurite length of neurons grown in BDNF alone (65.1 μm ± 6.0 μm; p<0.01). In conditions with ES, NGF improved neurite length either in the presence of BDNF (131.7 μm ± 9.7 μm) or the absence of BDNF (159.6 μm ± 11.0 μm) compared to neurite lengths with only BDNF+ES (99.1 μm ± 12.1 μm) or ES alone (57.7 μm ± 5.5 μm).
Without NGF, BDNF significantly improved average neurite length (65.1 μm ± 6.0 μm) compared to neurite length measured without any growth factor (52.2 μm ± 6.5 μm; p<0.05) (Fig 3B). When ES was applied in combination with BDNF, neurite length (99.1 μm ± 12.1 μm) was significantly increased over that with ES alone (57.7 μm ± 5.5 μm; p<0.01). However, for conditions with NGF and ES, neurite length (159.6 μm ± 11.0 μm) was significantly decreased with the addition of BDNF (131.7 μm ± 9.7 μm; p<0.05) (Fig 3B).
ES improved neurite length only when combined with single neurotrophin treatment (Fig 3C). Neurite lengths were significantly greater with ES and NGF (159.6 μm ± 11.0 μm) than with NGF alone (120.1 μm ± 11.4 μm; p<0.01); and neurite lengths were greater with ES and BDNF (99.1 μm ± 12.1 μm) than with BDNF alone (65.1 μm ± 6.0 μm) though the increase was not significant (p=0.08). In contrast, neurite length was not significantly changed when ES was applied with both NGF and BDNF (Fig 3C). Together, these data indicate that BDNF mitigates the enhancement effects of NGF.
ES alone (57.7 μm ± 5.5 μm) did not improve neurite outgrowth compared to the null condition (52.2 μm ± 6.5 μm) (Fig 3C). To determine that the ES chamber itself did not pose any deleterious effects on neurite growth, sham ES was performed and compared to neurite length with NGF alone and with NGF+ES. Sham ES (120.1 μm ± 11.4 μm) showed no differences in neurite length compared to NGF alone (121.3 μm ± 10.0 μm, Fig 3D), and the NGF+ES condition (159.6 μm ± 11.0 μm) was significantly greater than either NGF alone or NGF+shamES (p<0.01). These data show that the effects of the ES conditions are due to the electrical current, not the electrical chamber components or the 10 min that the neurons remained outside of the incubator while being stimulated.
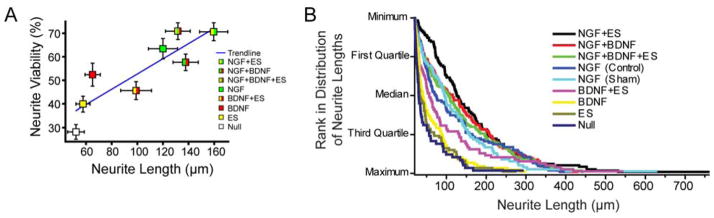
To further investigate the impact of ES on neurogenesis, the percentage of neurons labeled with neurofilament that produced neurites longer than twice the neuron soma diameter (Table I) relative to all labeled neurons were calculated. Mean values from Table I (and standard error of the mean) were plotted to show that this percentage of neurons exhibited a strong positive correlation (R2=0.76) with neurite length for all conditions (Fig 4A). However, the neurite length data are right skewed, and therefore nonparametric, as shown by the cumulative distribution plot (Fig 4B). For conditions without NGF (null, ES alone, and BDNF alone), the median neurite length is less than ~40 μm. Combining BDNF+ES caused a noticeable increase in neurite length. Specifically, the third quartile neurites increased from ~70 μm in null, ES, and BDNF to ~130 μm for ES+BDNF. All conditions with NGF (NGF alone, NGF+BDNF, NGF+ES, and NGF+BDNF+ES) had longer neurites than all conditions without NGF, compared at the first quartile, the median, and the third quartile neurite lengths. NGF+ES had the fewest, short neurites. Specifically, the first quartile neurite length was ~70 μm for NGF+ES compared to the next longest first quartile length of ~45 μm for NGF+BDNF. Together, these results show that brief, low voltage ES enhances neurite growth when used in combination with neurotrophins.
Table I.
Mean (± S.E.M.) of neurite lengths and percentage of neurons producing neurites are shown for each of the growth conditions of ES and growth factors. Data is graphically displayed in Fig 3.
| Growth Condition | Neurite Length | % of Neurons Producing Neurites |
|---|---|---|
|
| ||
| Mean (μm) ± S.E.M. (μm) | Percent (%) ± S.E.M. (%) | |
| ES+NGF | 159.6 ± 11.0 (N = 117) | 71 ± 4 (N = 143) |
| BDNF+NGF | 137.7 ± 9.9 (N = 113) | 58 ± 4 (N = 196) |
| ES+BDNF+NGF | 131.7 ± 9.7 (N = 117) | 71 ± 4 (N = 165) |
| NGF (Sham ES) | 120.1 ± 11.4 (N = 78) | 63 ± 4 (N = 123) |
| ES+BDNF | 99.1 ± 12.1 (N = 78) | 46 ± 4 (N = 171) |
| BDNF | 65.1 ± 6.0 (N = 89) | 52 ± 5 (N = 105) |
| ES | 57.7 ± 5.5 (N = 87) | 40 ± 3 (N = 218) |
| Null | 52.2 ± 6.5 (N = 58) | 28 ± 3 (N = 206) |
Figure 4.
Percentage of neurons with neurites are strongly correlated with neurite length (R2=0.76). (A) Mean percentage of neurons with neurites and neurite lengths are plotted with standard error of the means (horizontal and vertical bars) for each growth condition. (B) Cumulative distribution displays the nonparametric distribution of neurite lengths when ranked from minimum to maximum.
3.3 Impact of ES on Neurite Directionality
Neurons significantly orient in the same direction as other neurons within the imaging area regardless of ES or growth conditions (Fig 5A). Of the 37 images analyzed, 19 of the images had ES and 18 images did not have ES. 32% of images of neurons with ES showed significant directionality, and 33% of images without ES showed significant directionality (Fig 5A). Therefore, ES did not increase the directionality of neurites. None of the specific growth enhancements (NGF, BDNF or ES) caused a significant increase in the percent of images showing directionality compared to the percent of images showing directionality for all images analyzed (Fig 5A).
Figure 5.
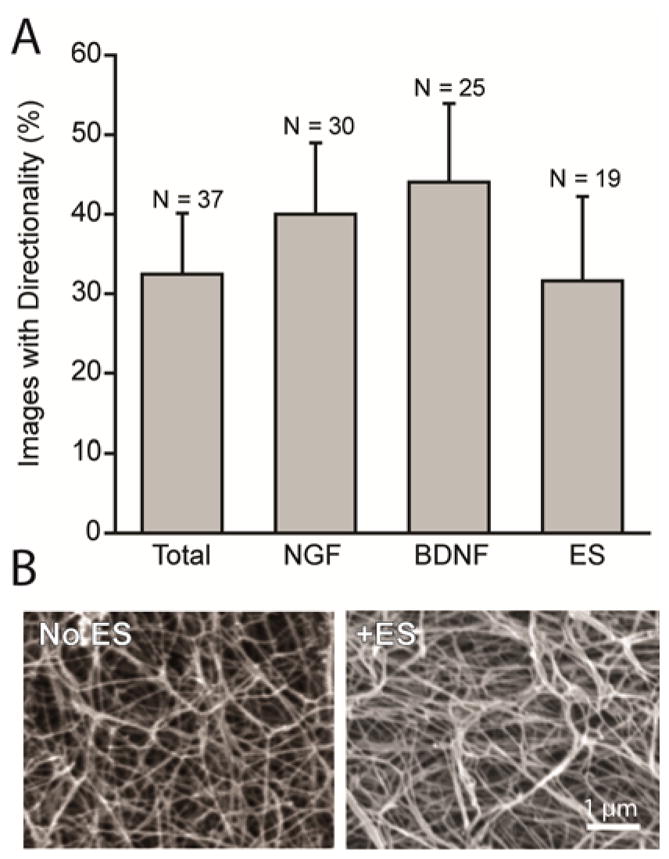
A significant number of neurites grow directionally regardless of growth conditions. (A) Percent of total images that exhibited directionality are compared to all conditions grown in NGF, in BDNF, and with ES. (B) Scanning electron micrographs of 3D collagen gels without ES and with ES reveals no apparent change in collagen fiber directionality.
Impact of ES on Collagen Scaffold
To determine whether the collagen fibers could contribute to neurons growing in an oriented manner, we considered whether collagen fibers become oriented after gelation either with or without ES. Scanning electron microscopy (SEM) was performed to consider whether ES affected alignment and/or change of orientation of the collagen fibers. From SEM images shown in Fig 5B, there is no apparent alignment or orientation of the collagen gels with or without ES.
4. Discussion
ES for nerve regeneration has been widely studied with varied results. Typically, ES has enhanced nerve regeneration, both in vivo and in vitro.28 The literature has indicated that growth factor receptors may be a potential mechanism for ES enhancement of neurite outgrowth.1, 7 However, data to support this hypothesis has been limited. The goal of this study was to examine the interplay of ES and growth factors in 3D scaffolds to more closely mimic that of the endogenous extracellular environment.
First, we demonstrated that in 3D collagen gels, ES alone did not significantly increase neurite length in conditions where neurotrophins were not exogenously added (Fig 3C). This finding is relatively difficult to compare, as most studies on ES have been conducted on 2D surfaces in vitro, where any autocrine and paracrine signaling molecules, such as growth factors, are not diffusion-limited. For example, previous work has demonstrated equivalent growth enhancement of dissociated chick DRG either with or without growth factors present on 2D collagen substrates.43 However, support cells, including fibroblasts and Schwann cells, were present within ~100 μm of the neurons, and able to supply neurons with growth factors.43 In our study, ES did not improve neurite growth without the addition of exogenous growth factors, yet it did increase the percentage of neurons producing neurites (Fig 4A).
Growth factor supplementation increased the length of neurites over that measured with no supplementation and without ES. The neurite lengths with NGF (no ES) in 3D collagen gels were similar to previous studies of dissociated DRG in collagen gels.5, 37, 41 The BDNF condition alone (13 μm increase) had significantly shorter neurites than NGF alone (68 μm increase; see Fig 3A and 3B). BDNF+NGF (85 μm increase) was increased over either BDNF or NGF alone, indicating that the effects of both growth factors are additive. Chick DRG have previously been noted to be more responsive to NGF than BDNF, requiring 1.25 ng/mL and >100 ng/mL, respectively, to have similar neurite outgrowth.11 In addition, neurite outgrowth from whole chick DRG was approximately 50% reduced with BDNF compared to NGF 21. Therefore, our results agree with the previous reports of reduced growth with BDNF compared to NGF. Similarly to what was found here, the addition of BDNF did not reduce viability of DRG neurons when cultured in optimal NGF conditions (>10 ng/mL) 3, but NGF was found to support neuron survival more than BDNF.21 Thus, our results without ES are in general agreement with previous findings.
Our ES protocol (10 min) did not alter the 3D collagen (1.5 mg/mL) environment, having no effect on the collagen structure (Fig 5B). Previous research has shown that diluted collagen fibers organized orthogonally to the field after long duration stimulation of 24 hr.27 The application of electric (2.5 kV/m) 9 or magnetic fields (~10 T) during gelation 12, 39 have also been found to induce organization. Therefore, we suspect that the duration was not long enough, nor the intensity high enough in our study to induce reorganization of the collagen fibrils, or that reorganization of fibrils requires sufficiently diluted gels (<0.25 mg/mL 27). Work to study these two options is underway. The significant directionality in 32% of the confocal images analyzed for directionality (Fig. 5A) was seen regardless of whether ES treatment was applied, further suggesting that our applied ES did not cause a reorganization of collagen, and did not affect the direction of neurite growth. The 32% directionality seen in the images analyzed may be due to subtle anisotropic organization of the collagen fibers due to the flow of the collagen mixture prior to scaffold formation. Collagen gels formed on a slanted surface were found to align in the direction of drainage.13 In our experiments, the mixing, pipetting, and filling of wells with collagen may cause an alignment in some areas of the well, causing directionality of neurites growing in these areas.
Low voltage DC stimulation combined with NGF performed as the optimal condition tested for neurite growth and neurogenesis (Fig 4A). ES increased mean length by 33%. The increase in mean neurite length from ES+NGF is caused primarily by an increase in length of the shortest neurites, and not by an increase in length of the longest neurites. Specifically, in Fig 4B the neurites in the fourth quartile treated with NGF alone or with ES+NGF have a similar length. The shorter 50% of neurites in the ES+NGF condition are longer than those of all other conditions. The effect of ES to improve the growth rate of slower growing neurites without increasing the growth rate of the fastest neurites has also been shown using motorneurons in vivo.2.
Brief ES increased growth significantly for NGF or BDNF alone, but not when NGF and BDNF were combined (Fig 3C); the reasons for this result are not understood but suggest an interesting interaction of neurotrophins and receptor signaling mechanisms. When electrical fields that are large enough to cause propagated depolarization, estimated to be roughly 1,000 V/m31, are applied to axons, the mechanism of ES appears to occur by conduction of a retrograde action potential to the neuronal body, where the signal may be propagated by opening voltage-dependent calcium channels.1 In the experiments reported here, a voltage field of 8–10 V/m was used, which is two orders of magnitude below the propagated depolarization threshold, therefore, this mechanism is unlikely. ES has been demonstrated to cause depolarization on the cathodic cell membrane and hyperpolarization on the anodic cell membrane that is proportional to the magnitude of the electrical field (for review see19). The depolarization and hyperpolarization of different areas of the membrane may physically alter transmembrane receptors or receptor interactions in those regions of the membrane. For example, the number of receptors may increase or decrease in response to the polarization, or the affinity of growth factor Trk receptors may be altered by the polarization. Another possibility is that the open probability of voltage-dependent ion channels is changed by the membrane polarizations, and that the conductance of ions caused by ES has intracellular effects on the neuron. Our work demonstrates a link between growth factor supplementation and ES. Future work will explore whether ES affects the growth factor receptor interaction, and whether ES has other intracellular effects that alter the cellular response to growth factors.
No authors have a financial or personal conflict. This work described complies with the Ethical Policies of the journal, and has been conducted under internationally accepted ethical standards after relevant ethical review.
Acknowledgments
All authors have made substantial contributions to the design, acquisition, and data analysis. Robert Adams, Rebecca Willits, and Amy Harkins drafted the paper and revised it critically. All have approved the submitted version of the manuscript. We acknowledge Dr. Jessica Wagensiel (Washington University in St. Louis) for helpful discussion and Susan Westerfield Foy for SEM collection. We acknowledge our funding for the project: NSF CBET Grant #1061834 for partial support of this work (RKW) and NIH Training Grant #5T 32 GM008306-24 (RDA). We appreciate the open source platform for biological-image analysis, FIJI 33 and the plugin for FIJI, Simple Neurite Tracer 24. The 3A10 (neurofilament antibody) developed by Jessell, Dodd, and Brenner-Morton was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Contributor Information
Rebecca K. Willits, Email: willits@uakron.edu.
Amy B. Harkins, Email: harkinsa@slu.edu.
References
- 1.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12:4381–90. [PubMed] [Google Scholar]
- 2.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–8. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell JH, Haycock JW. Next generation nerve guides: materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B Rev. 2012;18:116–28. doi: 10.1089/ten.TEB.2011.0498. [DOI] [PubMed] [Google Scholar]
- 5.Blewitt MJ, Willits RK. The effect of soluble peptide sequences on neurite extension on 2D collagen substrates and within 3D collagen gels. Ann Biomed Eng. 2007;35:2159–2167. doi: 10.1007/s10439-007-9389-4. [DOI] [PubMed] [Google Scholar]
- 6.Brown BR, Hughes ME, Russo C. Thermoelectricity in natural and synthetic hydrogels. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:031917. doi: 10.1103/PhysRevE.70.031917. [DOI] [PubMed] [Google Scholar]
- 7.Chang YJ, Hsu CM, Lin CH, Lu MS, Chen L. Electrical stimulation promotes nerve growth factor-induced neurite outgrowth and signaling. Biochim Biophys Acta. 2013;1830:4130–6. doi: 10.1016/j.bbagen.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 9.Cheng X, Gurkan UA, Dehen CJ, Tate MP, Hillhouse HW, Simpson GJ, Akkus O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29:3278–88. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Corredor RG, Goldberg JL. Electrical activity enhances neuronal survival and regeneration. J Neural Eng. 2009;6:055001. doi: 10.1088/1741-2560/6/5/055001. [DOI] [PubMed] [Google Scholar]
- 11.Davies AM, Thoenen H, Barde YA. The response of chick sensory neurons to brain-derived neurotrophic factor. J Neurosci. 1986;6:1897–904. doi: 10.1523/JNEUROSCI.06-07-01897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubey N, Letourneau PC, Tranquillo RT. Guided neurite elongation and schwann cell invasion into magnetically aligned collagen in simulated peripheral nerve regeneration. Exp Neurol. 1999;158:338–50. doi: 10.1006/exnr.1999.7095. [DOI] [PubMed] [Google Scholar]
- 13.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.English AW, Meador W, Carrasco DI. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005;21:2624–34. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- 15.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp Neurol. 2002;174:125–36. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 16.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205:347–59. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–50. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenebaum B, Sutton CH, Vadula MS, Battocletti JH, Swiontek T, DeKeyser J, Sisken BF. Effects of pulsed magnetic fields on neurite outgrowth from chick embryo dorsal root ganglia. Bioelectromagnetics. 1996;17:293–302. doi: 10.1002/(SICI)1521-186X(1996)17:4<293::AID-BEM5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Gross D, Loew LM, Webb WW. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986;50:339–48. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart A, Terenghi G, Wiberg M. Tissue Engineering for Peripheral Nerve Regeneration. In: Pallua N, Suscheck CV, editors. Tissue Engineering. Springer; Berlin Heidelberg: 2011. pp. 245–262. [Google Scholar]
- 21.Hory-Lee F, Russell M, Lindsay RM, Frank E. Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proc Natl Acad Sci U S A. 1993;90:2613–7. doi: 10.1073/pnas.90.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koppes AN, Seggio AM, Thompson DM. Neurite outgrowth is significantly increased by the simultaneous presentation of Schwann cells and moderate exogenous electric fields. J Neural Eng. 2011;8:046023. doi: 10.1088/1741-2560/8/4/046023. [DOI] [PubMed] [Google Scholar]
- 23.Kuntz RM, Saltzman WM. Neutrophil motility in extracellular matrix gels: mesh size and adhesion affect speed of migration. Biophys J. 1997;72:1472–80. doi: 10.1016/S0006-3495(97)78793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longair MH, Baker DA, Armstrong JD. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics. 2011;27:2453–4. doi: 10.1093/bioinformatics/btr390. [DOI] [PubMed] [Google Scholar]
- 25.Macias MY, Battocletti JH, Sutton CH, Pintar FA, Maiman DJ. Directed and enhanced neurite growth with pulsed magnetic field stimulation. Bioelectromagnetics. 2000;21:272–86. [PubMed] [Google Scholar]
- 26.Miller C, Jeftinija S, Mallapragada S. Micropatterned Schwann cell-seeded biodegradable polymer substrates significantly enhance neurite alignment and outgrowth. Tissue Eng. 2001;7:705–715. doi: 10.1089/107632701753337663. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen HT, Wei C, Chow JK, Nguy L, Nguyen HK, Schmidt CE. Electric field stimulation through a substrate influences Schwann cell and extracellular matrix structure. J Neural Eng. 2013;10:046011. doi: 10.1088/1741-2560/10/4/046011. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen HT, Wei C, Chow JK, Nguyen A, Coursen J, Sapp S, Luebben S, Chang E, Ross R, Schmidt CE. Electric field stimulation through a biodegradable polypyrrole-co-polycaprolactone substrate enhances neural cell growth. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuccitelli R. Physiological Electric Fields can Influence Cell Motility, Growth, and Polarity. In: Kenneth RM, editor. Advances in Molecular and Cell Biology. Elsevier; 1988. pp. 213–233. [Google Scholar]
- 30.Parkhurst MR, Saltzman WM. Quantification of human neutrophil motility in three-dimensional collagen gels. Effect of collagen concentration. Biophys J. 1992;61:306–15. doi: 10.1016/S0006-3495(92)81838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauer H, Rahimi G, Hescheler J, Wartenberg M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J Cell Biochem. 1999;75:710–23. doi: 10.1002/(sici)1097-4644(19991215)75:4<710::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Saygili E, Schauerte P, Kuppers F, Heck L, Weis J, Weber C, Schwinger RH, Hoffmann R, Schroder JW, Marx N, Rana OR. Electrical stimulation of sympathetic neurons induces autocrine/paracrine effects of NGF mediated by TrkA. J Mol Cell Cardiol. 2010;49:79–87. doi: 10.1016/j.yjmcc.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 35.Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1–12. doi: 10.1007/978-1-61779-536-7_1. [DOI] [PubMed] [Google Scholar]
- 36.Skaper SD, Varon S. Maintenance by nerve growth factor of the intracellular sodium environment in spinal sensory and sympathetic ganglionic cells. Brain Res. 1980;197:379–89. doi: 10.1016/0006-8993(80)91123-3. [DOI] [PubMed] [Google Scholar]
- 37.Swindle-Reilly KE, Papke JB, Kutosky HP, Throm A, Hammer JA, Harkins AB, Willits RK. The impact of laminin on 3D neurite extension in collagen gels. J Neural Eng. 2012;9:046007. doi: 10.1088/1741-2560/9/4/046007. [DOI] [PubMed] [Google Scholar]
- 38.Tep C, Lim TH, Ko PO, Getahun S, Ryu JC, Goettl VM, Massa SM, Basso M, Longo FM, Yoon SO. Oral administration of a small molecule targeted to block proNGF binding to p75 promotes myelin sparing and functional recovery after spinal cord injury. J Neurosci. 2013;33:397–410. doi: 10.1523/JNEUROSCI.0399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torbet J, Ronziere MC. Magnetic alignment of collagen during self-assembly. Biochem J. 1984;219:1057–9. doi: 10.1042/bj2191057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Udina E, Furey M, Busch S, Silver J, Gordon T, Fouad K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008;210:238–47. doi: 10.1016/j.expneurol.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed. 2004;15:1521–31. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- 42.Wood M, Willits RK. Short-duration, DC electrical stimulation increases chick embryo DRG neurite outgrowth. Bioelectromagnetics. 2006;27:328–31. doi: 10.1002/bem.20214. [DOI] [PubMed] [Google Scholar]
- 43.Wood MD, Willits RK. Applied electric field enhances DRG neurite growth: influence of stimulation media, surface coating and growth supplements. J Neural Eng. 2009;6:046003. doi: 10.1088/1741-2560/6/4/046003. [DOI] [PubMed] [Google Scholar]