Abstract
Importance
To understand how a model of Alzheimer disease pathophysiology based on β-amyloidosis and neurodegeneration predicts the regional anatomic expansion of hypometabolism and atrophy in persons with mild cognitive impairment (MCI).
Objective
To define the role of β-amyloidosis and neurodegeneration in the subsequent progression of topographic cortical structural and metabolic changes in MCI.
Design
Longitudinal, observational study with serial brain imaging.
Setting
Population-based cohort.
Participants
Ninety six MCI participants (all >70 years) with serial imaging biomarkers from the Mayo Clinic Study of Aging or Mayo Alzheimer Disease Research Center. Participants were characterized initially as having elevated or not elevated brain β-amyloidosis (“A+” or “A−“) based on 11C-Pittsburgh compound B positron emission tomography (PET). They were further characterized initially by the presence or absence of neurodegeneration (“N+” or “N−“), where presence of neurodegeneration was defined by abnormally low hippocampal volume or hypometabolism in an Alzheimer Disease (AD)-like pattern on 18fluoro-deoxyglucose (FDG) PET.
Main Outcome Measures
Regional FDG Standardized Uptake Value ratio (SUVR) and grey matter volumes in medial temporal, lateral temporal, lateral parietal and medial parietal regions.
Results
In the primary regions of interest, the A+N+ group had lower FDG SUVR and grey matter volumes at baseline, and showed large declines in FDG SUVR and grey matter volumes compared to the A−N+ and A−N−, but not the A+N− group. The A+N− group exhibited declines in FDG SUVR over time, which were not significantly different from the A−N+ or A−N− groups. The A−N+ group did not show declines in FDG SUVR or grey matter volume compared to A+N− or A−N− groups.
Conclusions and Relevance
Persons with MCI who were A+N+ demonstrated volumetric and metabolic worsening in temporal and parietal association areas, consistent with the expectation that the MCI stage in the Alzheimer pathway heralds incipient isocortical involvement. The A−N+ group, those with suspected non-Alzheimer pathophysiology, lacked a distinctive longitudinal volumetric or metabolic profile.
The clinical syndrome of mild cognitive impairment (MCI) when due to Alzheimer’s disease (AD) represents an inflection point at which the tempo of cognitive decline accelerates and expands1. Clinical acceleration is likely to be preceded or accompanied by neurodegenerative changes outside of the medial temporal lobe2, 3. The neurodegenerative expansion that occurs at the MCI stage is assumed to require the presence of β-amyloidosis but antemortem demonstrations are infrequent4, 5 and contradictory6. The many studies in MCI patients that examined region-specific declines with MR imaging7–9 or 18fluoro-deoxyglucose (FDG) positron emission tomography (PET)2, 10, 11 did not have amyloid imaging available. Our current model12, 13 posits that both β-amyloid and neurodegenerative biomarkers must be abnormal for higher rates of neurodegeneration to occur. We wished to test that hypothesis in persons with MCI.
We studied Mayo Clinic Study of Aging (MCSA) and the Mayo Alzheimer’s Disease Research Center (ADRC) participants who had undergone serial clinical evaluations as well as serial MR imaging, FDG PET imaging and 11C-Pittsburgh compound B (PiB) PET imaging. Our major aim was to determine the pattern of progression of regional volume loss and metabolic declines at the MCI stage as a function of β-amyloid and neurodegeneration biomarker status. We were interested both in those participants with elevated β-amyloid (those in the AD pathway) and those with non-elevated β-amyloid and neurodegeneration (the suspected non-Alzheimer pathophysiology (SNAP)).
Methods
Participants
We examined MCI participants in the MCSA or ADRC who had serial imaging biomarkers and who were ≥70 years of age at the baseline imaging visit. Participants had to be diagnosed as MCI at baseline but were not excluded if their diagnosis changed over the course of follow-up
Consensus clinical diagnoses were made using previously described methods in the MCSA14. A consensus diagnosis of MCI was made using these criteria: cognitive concern by a physician, patient, or nurse; impairment in 1 or more of the 4 cognitive domains; essentially normal functional activities; and not demented15 as previously described16–18. These criteria are identical to those proposed by the National Institute on Aging – Alzheimer’s Association workgroup19. We allowed any neuropsychologically-defined MCI subtype.
Standard protocol approvals, registrations, and patient consents
These studies were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards and written informed consent was obtained from all participants.
Imaging Methods
All subjects underwent MR, FDG PET and PiB PET following our previously described methodology20. MR and PET imaging were done within 6 months of a participant’s clinical visit. MR scanning was performed at 3 Tesla. Amyloid PET imaging was performed with PiB and consisted of four 5-minute dynamic frames acquired from 40–60 minutes after injection of 292 – 729 MBq 11C-PIB. PiB values were grey matter and white matter sharpened and partial volume corrected; they were normalized to the cerebellum. A global PiB standardized uptake value ratio (SUVR) was calculated from a group of regions including parietal, cingulate precuneus, prefrontal, orbito frontal, temporal, and anterior cingulate regions normalized to the cerebellum. All regions are summed over both hemispheres. FDG PET was obtained on the same day as the PIB scan and consisted of four 2-minute dynamic frames acquired from 30–38 minutes after injection of 366–399 MBq 18FDG. CT scans were obtained for attenuation correction. FDG values were non-sharpened and were not partial-volume corrected. Amyloid PET and FDG PET were analyzed with our in-house fully automated image processing pipeline20 where image voxel values are extracted from automatically labeled cortical regions of interest (ROIs).
Biomarker Characterization of Participants
Participants were characterized at baseline as having elevated or not elevated amyloid (A+ or A−) based on PiB SUVR > 1.40, and on having abnormal or normal neurodegenerative changes (N+ or N−) based on either hippocampal volume by MR or FDG hypometabolism in an AD signature meta-ROI. Hippocampal volume was measured with FreeSurfer (v5.3) and total intracranial volume (TIV) was measured using an in-house method. Each subject’s raw hippocampal volume was adjusted for TIV to create a TIV-adjusted hippocampal volume (HVa) by calculating the residual from a linear regression of hippocampal volume versus TIV among 133 cognitively normal subjects aged 30 to 5920). The cutpoint for hippocampal volume adjusted for SPM12 TIV (HVa) < −2.40 cm3. The FDG AD signature meta-ROI was defined as the average of uptake in defined voxels in angular gyrus, posterior cingulate gyrus, and left middle/inferior temporal gyrus normalized to the pons and vermis2. The cutpoint for abnormal FDG SUVR in the AD signature meta-ROI was < 1.3220. Cut-point values were derived from 75 persons with AD dementia from the Mayo ADRC or MCSA, and represented the 90th percentile for FDG and MRI measures and the 10th percentile for PiB SUVR21.
Outcome measures
Regional grey matter (GM) volume and FDG SUVR were the measures of change. They were computed for 15 cortical regions from an atlas22 modified in-house. Right and left hemisphere values for volumes were summed, and right and left hemisphere values for FDG ratios were averaged and weighted to the ROI size. Regional GM volumes were estimated using the TBM-Syn algorithm20. For the regional FDG SUVR used as outcome measures, values were normalized to the pons.
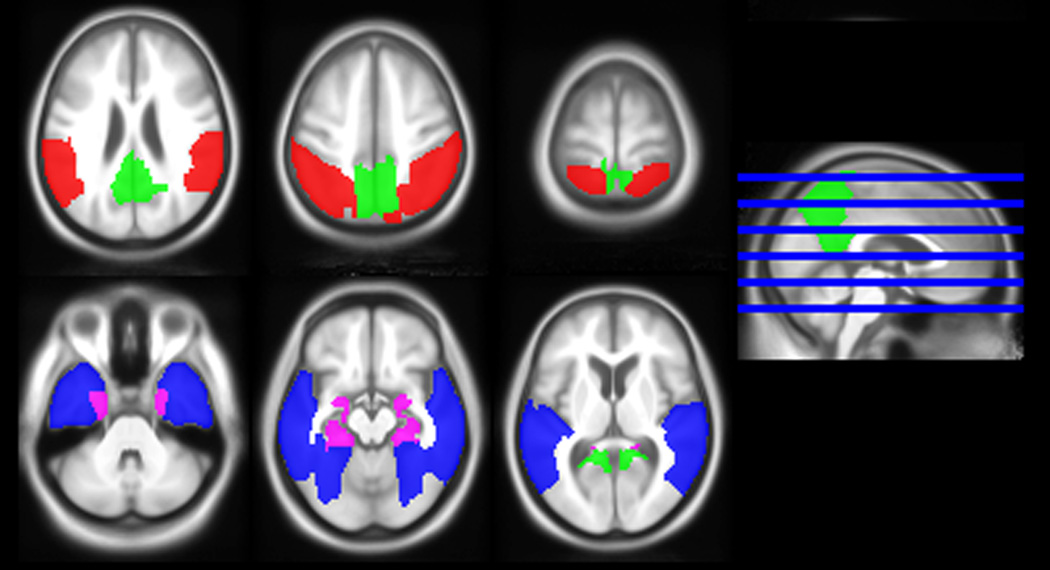
Volumetric and glucose metabolic annualized changes in four temporo-parietal ROIs were of primary interest: medial temporal, lateral temporal, medial parietal and lateral parietal. These were chosen based on prior work with MR imaging23. See Figure 1 for their locations. Eleven other regions were designated as secondary ROIs.
Figure 1.
Brain rendering of the four regions of interest: red = medial temporal; blue = lateral temporal; red = lateral parietal; and green = medial parietal.
Statistics
Our principal goal was to assess the role of elevated β-amyloidosis (A+) on progression of regional FDG SUVR and GM volume changes in the presence or absence of neurodegeneration (N+ or N−) defined at baseline. A secondary goal was to learn whether the A−N+ (suspected non-Alzheimer pathophysiology, SNAP) group differed from the other MCI groups.
Differences in baseline characteristics were assessed using either Pearson’s chi-squared test for 2×2 table using ‘N-1’ method or Welch’s two sample t-test. Linear mixed effects regression models were fit to assess group differences in baseline FDG SUVR and GM volumes, and to assess group differences in changes over time in FDG SUVR and GM volumes. One subject was excluded from all GM volume analyses due to having invalid volumetric estimates. Each model included main effects of time, baseline age, sex, biomarker group and interactions for age by time, and time by biomarker group and allowed for a random intercept and slope. These models allowed for group-wise differences at baseline and in rates of change and controlled for age-related baseline differences. All outcome measures were log-transformed to reduce skewness and to allow for interpretation of estimates on a percentage rather than absolute scale. We summarize baseline and annual percent change estimates using the usual 95% CIs and also using 84% CIs. We chose 84% CIs because at that level non-overlapping intervals correspond to a biomarker group difference that is significant at the p<0.05 level24. We did not correct for multiple comparisons. All analyses were performed using R Statistical Software (v3.0.1).
Results
The baseline characteristics of the biomarker-defined groups are shown in Table 1. The A+N+ and A+N− groups had a higher proportion of APOE e4 carriers and greater PiB SUVR levels compared to both A− groups, as expected. The A+N+ group had the lowest global and memory z-scores of the biomarker-defined groups. The A+N+ group also had the greatest decline in MMSE over time. Nearly half of participants in the N+ groups had both of the defining neurodegeneration features, hippocampal atrophy and FDG hypometabolism in an AD-like pattern, in 9/22 (41%) in the A−N+ group and 20/45 (44%) in the A+N+ group. The number of participants with only abnormal FDG was 9/22 (41%) in the A−N+ group and 14/45 (31%) in the A+N+ group and the number with only abnormal HVa was 4/22 (18%) in the A−N+ group and 11/45 (24%) in the A+N+ group. Participants were followed for an average of 2 years, and the duration of follow-up did not differ between the biomarker-defined groups.
Table 1. Participant characteristics.
Statistics shown are of the form mean (SD) unless otherwise specified.
| A+N+ (n = 45) |
A+N− (n = 17) |
A−N+ (n = 22) |
A−N− (n = 12) |
|
|---|---|---|---|---|
| Male, no. (%) | 29 (64%) | 11 (65%) | 18 (82%) | 10 (83%) |
| Age, yrs | 81 (5) | 79 (5) | 82 (5) | 79 (5) |
| Education, yrs | 14 (3) | 14 (4) | 15 (4) | 12 (3) |
| APOE e4 carrier, no. (%) | 23 (51%)a | 10 (59%) c | 2 (9%) | 0 |
| PIB SUVR | 2.11 (0.48)a | 1.95 (0.33)c | 1.31 (0.03) | 1.34 (0.06) |
| FDG SUVR in AD signature meta-ROI | 1.25 (0.14)b | 1.44 (0.10) | 1.27 (0.15)d | 1.44 (0.10) |
| Adjusted Hippocampal Volume | −2.81 (0.82)b | −1.75 (0.55) | −2.52 (0.67)d | −1.81 (0.47) |
| Total Intracranial volume (L) | 1.5 (0.1) | 1.5 (0.1) | 1.6 (0.2) | 1.5 (0.1) |
| Mini-Mental state exam | 25 (2.7) | 26 (2.0) | 26 (2.3) | 27 (1.3) |
| Annual change in MMSE† | −1.1 (0.20)i | −0.2 (0.32) | 0.2 (0.31) | −0.1 (0.39) |
| Global z-scores* | −0.74 (0.84)e | −0.29 (0.42) | −0.44 (0.91) | −0.44 (1.18) |
| Memory z-scores* | −1.23 (0.84)f | −0.74 (0.65) | −0.63 (0.93) | −0.68 (1.15) |
| Attention z-scores* | −0.23 (1.17) | 0.20 (0.92) | −0.46 (1.27) | −0.23 (1.10) |
| Language z-scores* | −0.75 (1.17)g | −0.16 (0.91) | −0.36 (0.69) | −0.27 (1.08) |
| Visual spatial z-scores* | −0.13 (1.00) | 0.05 (0.81) | 0.25 (1.25) | −0.34 (1.37) |
| Amnestic MCI, no. (%) | 41 (91%) | 16 (94%) | 19 (86%) | 9 (75%) |
| Mean number of Visits with imaging | 2 (0.7) | 2 (0.5)h | 2 (0.4) | 2 (0.3) |
| Those with 2 imaging visits | 33 (73%) | 10 (59%) | 17 (77%) | 11 (92%) |
| Those with ≥ 3 imaging visits | 12 (27%) | 7 (41%) | 5 (23%) | 1 (8%) |
| Range | 2 to 6 | 2 to 3 | 2 to 3 | 2 to 3 |
| Time in study, yrs | 2.1 (1.10) | 2.2 (0.99) | 1.9 (1.00) | 2.1 (1.03) |
| Range, yrs | 1 to 5 | 1 to 4 | 1 to 4 | 1 to 4 |
FDG = 18fluorodeoxyglucose positron emission tomography; PiB = Pittsburgh compound B positron emission tomography; SUVR = standardized uptake value ratio
z-scores derived from entire sample of 70+ year olds who were cognitively normal or who were diagnosed with MCI in the MCSA14
Estimates of mean change (standard error) in MMSE are from a linear mixed effects model. The mean change is estimated for an 80-year old participant. One participant was missing follow-up MMSE data and was not included in the model.
A+N+ differs from A−N+ and A−N−, p ≤ 0.001
A+N+ differs from A+N− and A−N−, p ≤ 0.001
A+N− differs from A−N+ and A−N−, p ≤ 0.001
A−N+ differs from A+N− and A−N−, p ≤ 0.001
A+N+ differs from A+N−, p=0.01
A+N+ differs from A+N− and A−N+, both p=0.02
A+N+ differs from A+N−, p=0.049
A+N− differs from A−N−, p=0.04
A+N+ differs from A+N−, A−N+, A−N−, p < 0.026
p-values are for descriptive purposes; none are corrected for multiple testing
Baseline
There were substantial baseline differences in regional FDG SUVR across the MCI groups that were largely a consequence of how group membership was defined. In the primary 4 temporal/parietal ROIs (Figure 2), the A+N+ and A−N+ groups had the lowest FDG SUVR, which significantly differed from either the A+N− or A− N− groups for all comparisons except for a borderline difference in the medial temporal region for A−N+ vs. A−N−. There were no significant differences in FDG SUVR between the A+N+ and A−N+ groups in these 4 regions. The A+N+ group had the smallest GM volumes in the medial temporal, lateral temporal, and lateral parietal, although not all differences were significant. See Figure 1 and it legend for p values for between-group comparisons.
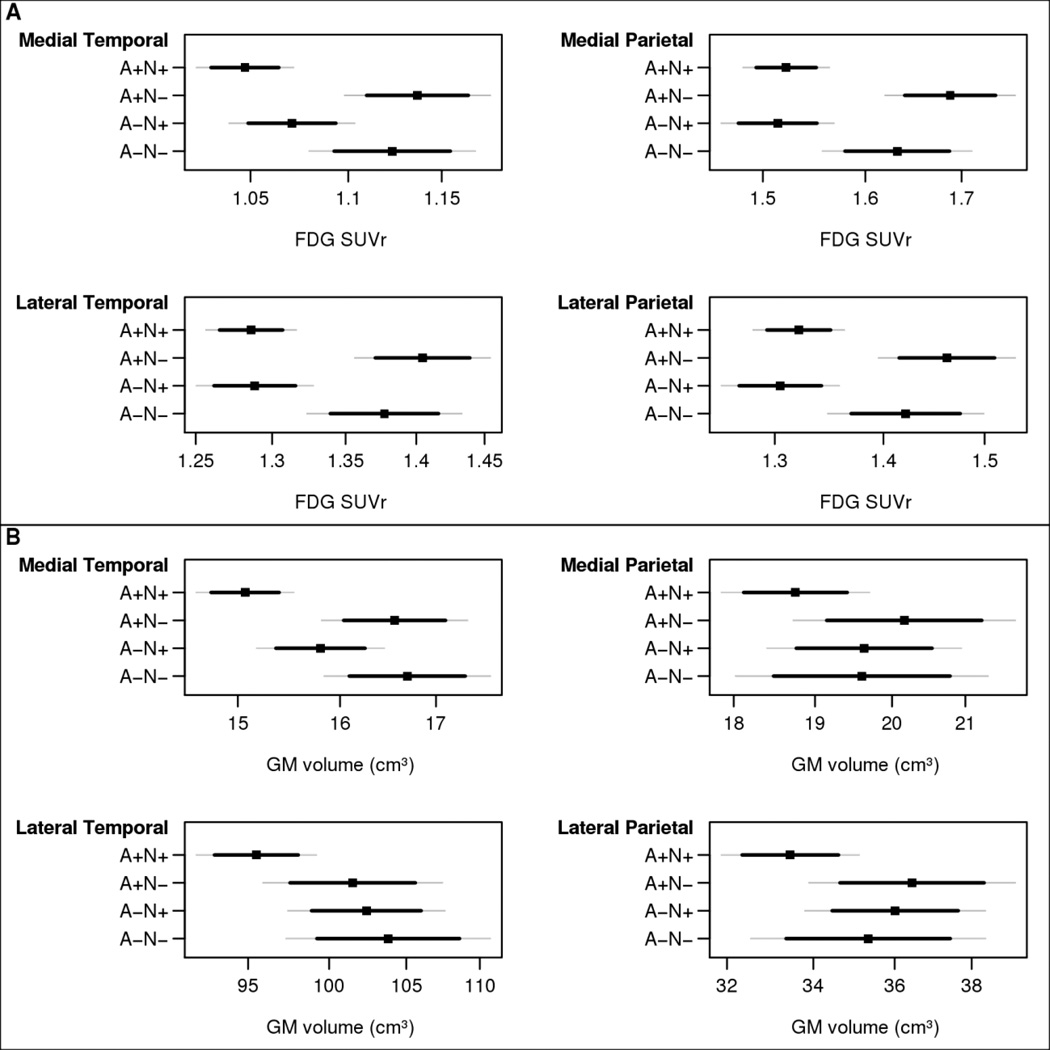
Figure 2.
Baseline FDG SUVR (A) and baseline grey matter volume (B) estimates with 95% confidence interval (CI) estimates (thin gray lines) and 84% CI estimates (thicker black lines) by biomarker group for primary ROIs: medial temporal, medial parietal, lateral temporal, and lateral parietal. The 84% CI allows for visual comparisons between groups where any amount of overlap indicates a lack of significance at the 0.05 level. Estimates are from a linear mixed model for a participant age 80 years. Statistical tests: FDG: for comparison of A+N+ to A+N− p<0.0001 for all 4 ROIs; for comparison of A+N+ to A−N− : medial temporal p=0.002, medial parietal p=0.01 , lateral temporal p=0.003; lateral parietal p=0.01. For comparison of A−N+ to A+N− group, p<0.01 for all 4 ROIs. For comparison of A−N+ to A−N− group p<0.01 for all 4 ROIs except medial temporal p=0.057. GM volume: for comparison of A+N+ to both A− N− and A+N− groups for medial temporal p<0.0001, for comparison to A+N+ to A−N+ group for medial temporal, p=0.054. All other medial temporal contrasts were not significant. Other 3 ROIs: all contrasts not significant.
Among the secondary ROIs (Figure e1), the A+N+ group had significantly lower FDG SUVR in all ROIs compared to the A+N− group, but not compared to the other two groups. The A−N+ group generally had lower FDG SUVR compared to A+N− but not different FDG SUVR compared to A−N−, though there were some exceptions. Across the secondary ROIs (Figure e2), regional GM volumes were generally not significantly different across the 4 biomarker-defined groups although a few regions in the A+N+ group compared to A+N− and A−N+ showed lower volumes.
Annual Change
The A+N+ group generally showed greater declines in both FDG SUVR and GM volume in the 4 primary temporal/parietal ROIs (Figure 3) compared to the A−N+ and A−N− groups, although not all of these differences were significant. The change in FDG SUVR and GM volume did not significantly differ between the A+N+ and A+N− groups for any of these 4 regions. Although the A+N− group exhibited point estimates that were lower than the values for the two A− groups, none of the differences exceeded the nominal significance level of 0.05. See Figure 2 and it legend for p values for between-group comparisons.
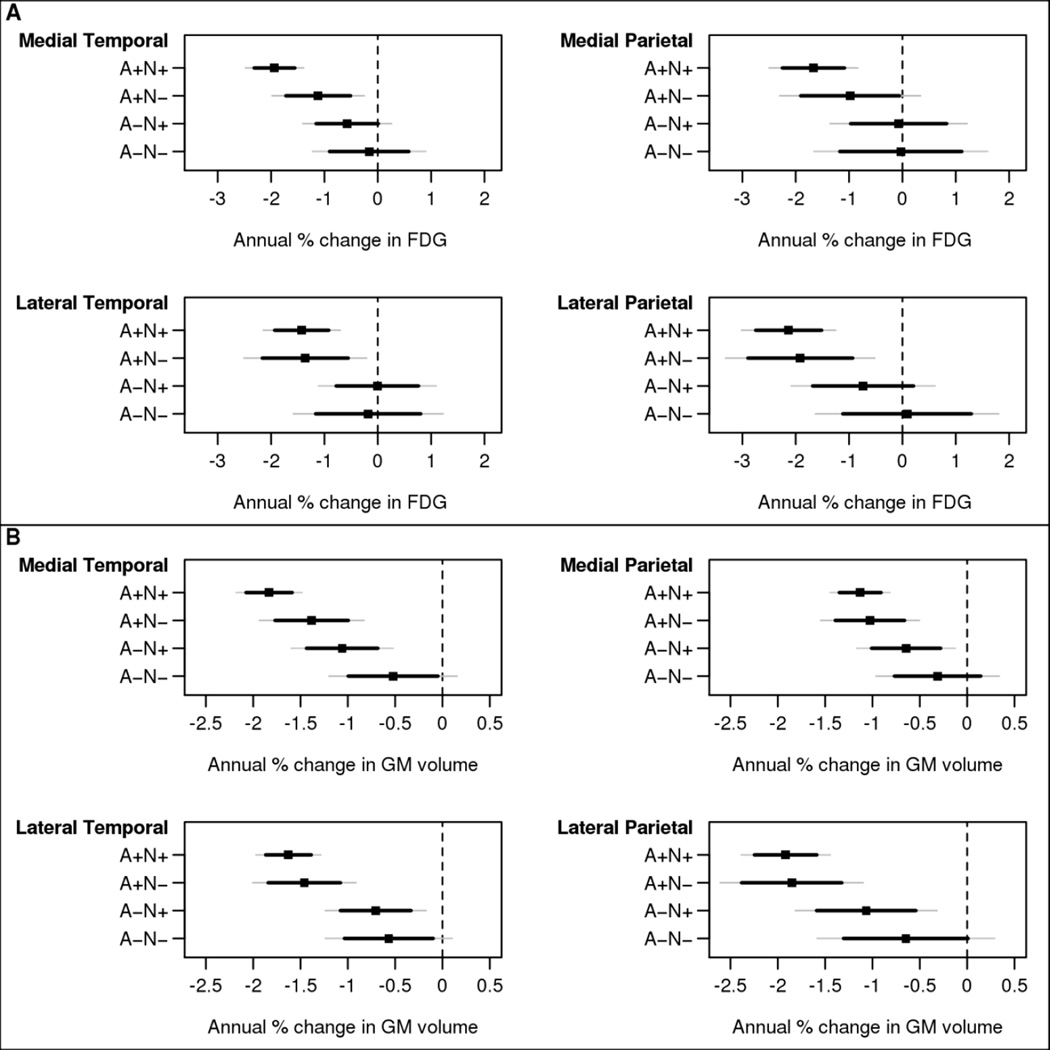
Figure 3.
Estimated annual percentage change for FDG SUVR (A) and grey matter volume (B) with 95% confidence interval (CI) (thin gray lines) and 84% CI (thicker black lines) by biomarker group for primary ROIs: medial temporal, medial parietal, lateral temporal and lateral parietal. The 84% CI allows for visual comparisons between groups where any amount of overlap indicates a lack of significance at the 0.05 level. Estimates are from a linear mixed model for a participant age 80 years. Statistical tests: FDG value by ROI for comparison of A+N+ versus A−N+: lateral temporal, p=0.03; medial temporal, p=0.007; medial parietal, p=0.04; lateral parietal, p=0.09. For comparison of A+N+ versus A−N−: lateral temporal, p=0.12; medial temporal, p=0.004; medial parietal, p=0.08; lateral parietal, p=0.02. All other contrasts not significant. GM volume: for comparison of A+N+ versus A−N+: lateral temporal, p=0.005; medial temporal, p=0.02; medial parietal, p=0.12; lateral parietal, p=0.06. For comparison of A+N+ versus A−N−: lateral temporal, p=0.006; medial temporal, p=<0.001; medial parietal, p=0.03; lateral parietal, p=0.02. All other contrasts not significant.
Across the secondary ROI’s (Figures e3 and e4), there were very few significant differences in either change in FDG SUVR or change in GM volume between the 4 groups. However, the A+N+ group was more likely than any of the other groups to show declines in FDG SUVR that were significantly different from zero. All groups showed declines in GM volume that were different from zero in most regions.
The findings are summarized in Figure 4. The A+N+ group demonstrated low glucose metabolism and GM volumes in the 4 primary temporo-parietal ROIs at baseline and large annual declines. In contrast the A+N− group exhibited higher metabolism and volumes at baseline but rates of decline that were not different from the A+N+. The A−N+ (SNAP) group had low glucose metabolism and smaller medial temporal GM volume at baseline, but experienced no regional metabolic or volumetric declines that distinguished them from the A+N− or A−N− groups.
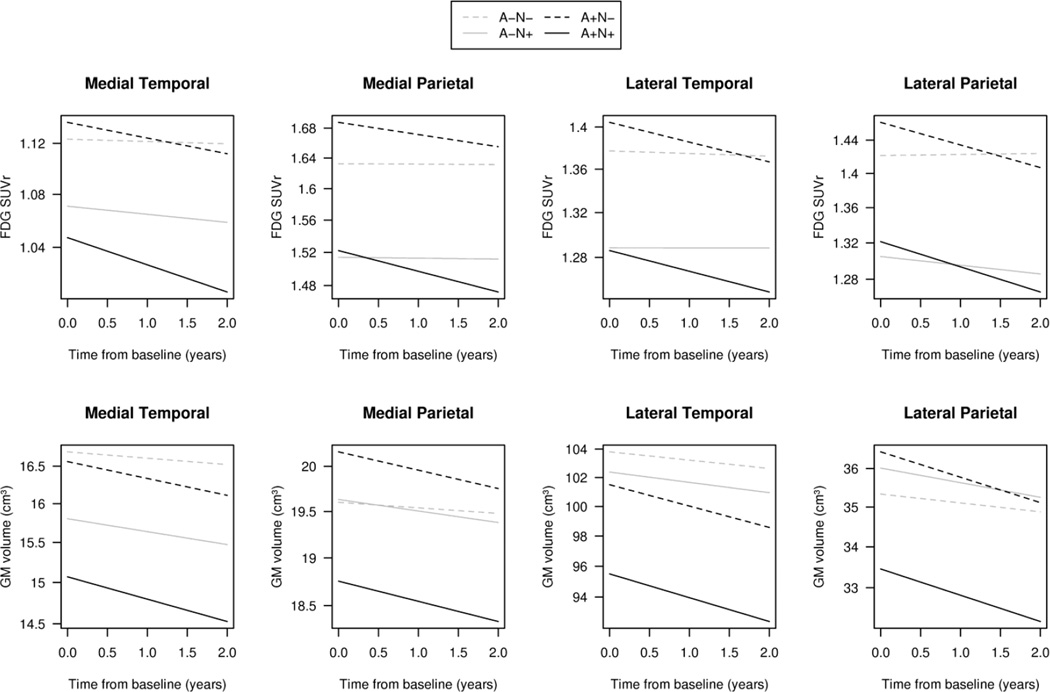
Figure 4.
Regression plots for the 4 primary ROIs showing trajectories of FDG SUVR (top panels) and grey matter (GM) (bottom panels) over time for the 4 biomarker defined groups for a hypothetical male 80 years of age. The x-axis is time from baseline. The time scale was limited to 2 years for illustrative purposes. See Figures 1 and 2 for confidence intervals of baseline values and slopes.
Discussion
Our principal finding was that MCI participants with elevated β-amyloidosis and neurodegeneration (“A+N+”) at baseline experienced worsening of FDG SUVR and cortical GM volume in the medial and lateral temporal and parietal ROIs compared to the MCI groups without elevated β-amyloid levels. Our findings support a model of AD pathophysiology12, 25 that requires the combined presence of brain β-amyloidosis and neurodegeneration, which in turn signifies a high likelihood of worsening of neurodegeneration in synaptically-connected extra-medial temporal regions26, 27. While some neurodegenerative changes arise independently of β-amyloid prior to MCI28, neurodegeneration at the MCI stage is facilitated when β-amyloid was elevated. The changes in the MCI A+N+ group were far larger than any seen in our prior study of cognitively normal individuals28.
The pattern of changes we observed in our A+N+ MCI patients who are on the AD pathway is what one would expect for “typical” AD progression on clinical-pathological grounds29, 30. While others have shown extratemporal changes in cognitively normal individuals31, analyses of ours using the same design as used here show that structural and metabolic abnormalities in persons who are A+N+ but cognitively normal are focused in the medial temporal lobe13. As overt cognitive impairment ensues, neurodegeneration spreads to the temporal and parietal isocortices, paralleling the progression of neurofibrillary tangle burden23, 32–34. The fact that we were able to demonstrate a clear pattern of structural and metabolic changes indicates a degree of homogeneity within the group possessing both elevated levels of β-amyloid and evidence of AD-like neurodegeneration. However, had we studied a younger group of MCI patients, in which the so-called “hippocampal sparing” subtype of AD is more common35, we might have found a different pattern of neurodegeneration related to β-amyloidosis.
The A+N− participants had larger metabolic declines than either of the A−N+ or A−N−groups, but none of the differences were significant. The percent of subjects who progressed to dementia at the last follow-up visit in the A+N− group was 12% (2 of 17), in contrast to the A+N+ group in which the percent of subjects who progressed was 21% (10 of 45). The pattern is similar but not identical to what we36 and others37–39 have previously reported. A more detailed analysis of the relationship of imaging findings to cognition is beyond the scope of the current report and requires longer periods of observation and more dementia “events” than we currently have available. The more favorable clinical outcomes in the A+N− group (who had less neurodegeneration at baseline) is consistent with the idea that a certain threshold of neurodegeneration must be exceeded for progression of cognitive impairment to occur. Over the course of the current study, the worsening of GM volumes and FDG SUVR meant that many in our A+N− group (8 of 17) would have been reclassified as A+N+ at the end of the observation period.
The A−N+ group (SNAP) had significantly lower FDG SUVR at baseline in most of cortical ROIs compared to the N− groups, but except for the medial temporal lobe, the A−N+ group did not have concomitant lower GM volumes elsewhere at baseline. While a few of the A−N+ subjects had neurodegeneration values at baseline that approached the cutpoints, the same was true for all of the biomarker groups. At the end of the current observation period, only one individual in the A−N+ group had transitioned to A+N+. Thus the majority of the A−N+ group was not on an amyloid-dependent pathway. Longitudinally, the A−N+ group experienced less worsening in atrophy or metabolism compared to the A+N+ group, and in no region did the A−N+ group experience significantly worse declines than the N− groups. These observations suggest that prevalent medial temporal atrophy was a major feature of MCI A−N+, in a manner not distinguishable from the A+N+ group. However, the amount of decline in medial temporal volume in the A−N+ group was much less than the A+N+ group. Primary age-related tauopathy40, cerebrovascular disease or hippocampal sclerosis41 could account for the more indolent medial temporal volume loss in the A−N+ group. The A−N+ group had widespread hypometabolism at the baseline, and this also worsened less compared to the A+N+ group. It is possible that multiple non-medial temporal tauopathy pathophysiologies (eg cerebrovascular or neurodegenerative) are driving the widespread hypometabolism. Such heterogeneity would tend to obscure any one distinct pattern of worsening. Visual inspection of the MR and FDG PET scans of the A−N+ group failed to reveal any cases in which an obvious etiological diagnosis (eg, frontotemporal degeneration) could be inferred from the scan. Biomarkers that are specific for non-AD degenerative processes are needed to address the progression of A−N+ cases. The proportion of A−N+ (SNAP) participants who progressed to dementia at the last follow-up was lower than in prior studies37–39 including our own36: 18% (4 of 22), about the same as the A+N− group (see above) but more than the A−N− group (8%, 1 of 12). As noted above, the clinical progression data should be treated with caution because of the small numbers who progressed to dementia at last follow-up and because these numbers do not adjust for differences in demographics among the groups. However, the indolent anatomic progression in the A−N+ group was mirrored in the low number of subjects who progressed to dementia at last follow-up.
Our observations support the claim that the presence of β-amyloid is required for anatomic and clinical progression, but that is not the same as claiming a causal role for β-amyloid at the MCI stage. Importantly, the increased risk of progression that elevated β-amyloid conferred does not clarify when in the sequence of events that the elevated β-amyloid actually mattered. As elevated β-amyloidosis has likely been present in these MCI patients for at least 15–20 years42, the point at which β-amyloid’s presence was causal could have been any time in that window. Furthermore, neurodegenerative changes in the medial temporal lobe (such as in middle-aged cognitively normal individuals) – which we observed in MCI participants without elevated β-amyloid imaging markers – are likely to be β-amyloid independent at least initially13, 32–34, 40, 43–45. It is hard to escape the conclusion that high levels of brain β-amyloidosis at some point play a facilitating role in progression of neurodegeneration. It is beyond the scope of this report to speculate on the mechanism of the interaction of β-amyloidosis and neurodegeneration at the molecular pathway level.
We relied on prior MR imaging8 and FDG PET studies of MCI patients who progressed to dementia2, 10, 11 to allow us to focus our attention on progression in temporal and parietal isocortical regions. However, the prior studies lacked amyloid imaging and used quite different approaches than ours to characterize participants at baseline. In persons with AD dementia, progression of hypometabolism into lateral temporo-parietal cortices occurs46, a pattern that our findings confirm. We extended the prior observations by showing that regional isocortical changes can be demonstrated at the MCI stage without preselecting those who progressed. We further clarified the role of elevated β-amyloidosis, after stratifying on baseline neurodegenerative status.
There are limitations to our analyses. We included all MCI neuropsychological subtypes, but we were not able to perform syndrome-specific analyses due to low numbers. Even so, the number of MCSA participants with MCI who have had serial imaging is small, particularly when subsets with specific imaging features are of interest. The number of participants available with serial imaging may have reduced our power to detect other smaller isocortical changes. In particular, the smaller sizes of A+N−, A−N+ and A−N− groups reduced our ability to detect differences in those groups. Our choice of neurodegeneration biomarkers was limited and unlikely to thoroughly cover the multiple processes that comprise AD neurodegenerative pathophysiology. Because each neurodegeneration biomarker is unique, ones other than hippocampal atrophy and FDG AD signature SUVR for defining baseline status might yield different conclusions.
Supplementary Material
Acknowledgments
Funding: This work was supported by NIH grants P50 AG16574, U01 AG06786 and R01 AG11378, the Elsie and Marvin Dekelboum Family Foundation and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation.
Abbreviations
- A−N−
amyloid not elevated and neurodegeneration absent
- A+N−
amyloid elevated and neurodegeneration absent
- A+N+
amyloid elevated and neurodegeneration present
- A−N+
amyloid not elevated and neurodegeneration present
- APOE
apolipoprotein E
- AD
Alzheimer’s disease
- ADRC
Alzheimer’s Disease Research Center
- FDG PET
18fluorodeoxyglucose positron emission tomography
- GM
grey matter
- HVa
adjusted hippocampal volume
- IQR
interquartile range
- MCSA
Mayo Clinic Study of Aging
- MR
magnetic resonance imaging
- MCI
mild cognitive impairment
- PiB PET
Pittsburgh compound B positron emission tomography
- ROI
region of interest
- SNAP
suspected non-Alzheimer pathophysiology
- SUVR
standardized uptake value ratio
- TIV
total intracranial volume
Figures for eAppendix
Figure e1. Baseline FDG SUVR estimates with 95% confidence interval (CI) (thin gray lines) and 84% CI (thicker black lines ) by biomarker group for secondary ROIs. The 84% CI allows for visual comparisons between groups where any amount of overlap indicates a lack of significance at the 0.05 level. Estimates are from a linear mixed model for a participant age 80 years.
FDG: for A+N+ versus A+N−, all ROI’s p<0.02 and for A+N+ versus A−N−, none significant. For A−N+ versus A+N−, most regions p<0.02 (except orbital frontal (p=0.08) and suppl motor area (p=0.048)) and for A−N+ versus A−N−, most ROIs not significant (except rolandic operculum (p=0.02), occipital (p=0.01) and primary visual (p=0.009)).
Figure e2. Baseline grey matter volume estimates with 95% confidence interval (CI) (thin gray lines) and 84% CI (thicker black lines) by biomarker group for secondary ROIs. The 84% CI allows for visual comparisons between groups where any amount of overlap indicates a lack of significance at the 0.05 level. Estimates are from a linear mixed model for a participant age 80 years.
GM volumes: for A+N+ versus A+N−, most ROI’s not significant (except precentral gyrus (p=0.03) and post central gyrus (p=0.02)). for A+N+ versus A−N−, all ROI’s not significant. For A−N+ versus A+N−, all ROI’s not significant, and for A−N+ versus A−N−, all ROI’s not significant
Figure e3. Estimated annual percentage change in FDG SUVR with 95% confidence interval (CI) (thin gray lines) and 84% CI (thicker black lines) by biomarker group for the secondary ROIs. The 84% CI allows for visual comparisons between groups where any amount of overlap indicates a lack of significance at the 0.05 level. Estimates are from a linear mixed model for a participant age 80 years.
No FDG SUVR changes were significant except for A+N+ versus A−N+ occipital and primary visual (p=0.03).
Figure e4. Estimated annual percentage change in grey matter volume with 95% confidence interval (CI) (thin gray lines) and 84% CI (thicker black lines) by biomarker group for the secondary ROIs. The 84% CI allows for visual comparisons between groups where any amount of overlap indicates a lack of significance at the 0.05 level. Estimates are from a linear mixed model for a participant age 80 years.
No GM volume loss differences were significant for A+N+ versus A−N+, and no differences were significant for A+N+ versus A−N− group, except orbital frontal (p=0.04) and postcentral gyrus (p=0.03).
Footnotes
Disclosures
Dr. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH.
Dr. Jack serves on scientific advisory board for Eli Lilly & Company; receives research support from the NIH/NIA, and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation; and holds stock in Johnson & Johnson.
Ms. Wiste reports no disclosures.
Mr. Weigand reports no disclosures.
Ms. Lundt reports no disclosures.
Dr. Vemuri receives research grants from the NIH/NIA.
Dr. Mielke receives research grants from the NIH/NIA, Alzheimer Drug Discovery Foundation, Lewy Body Dementia Association, and the Michael J Fox Foundation.
Dr. Machulda receives research support from the NIH/NIA & NIDCD.
Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI)
Dr. Kantarci receives research grants from the NIH/NIA.
Dr. Gunter reports no disclosures.
Mr. Senjem reports no disclosures.
Dr. Roberts reports no disclosures. She receives research grants from the NIH/NIA.
Dr. Boeve receives royalties from the publication of Behavioral Neurology of Dementia and receives research support from Cephalon, Inc., Allon Therapeutics, GE Healthcare, the NIH/NIA, and the Mangurian Foundation.
Ronald C. Petersen serves on data monitoring committees for Pfizer, Inc., Janssen Alzheimer Immunotherapy, and is a consultant for Roche, Inc., Merck, Inc. and Genentech, Inc`; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the National Institute of Health.
References
- 1.Wilson RS, Aggarwal NT, Barnes LL, et al. Cognitive decline in Incident Alzheimer's Disease in a community population. Neurology. 2010;74:951–955. doi: 10.1212/WNL.0b013e3181d64786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73(4):287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133(11):3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tosun D, Schuff N, Mathis CA, Jagust W, Weiner MW. Spatial patterns of brain amyloid-beta burden and atrophy rate associations in mild cognitive impairment. Brain. 2011;134(Pt 4):1077–1088. doi: 10.1093/brain/awr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chetelat G, Villemagne VL, Bourgeat P, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- 7.Henneman WJ, Sluimer JD, Barnes J, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72(11):999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70(7):512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard C, Helmer C, Dilharreguy B, et al. Time course of brain volume changes in the preclinical phase of Alzheimer's disease. Alzheimers Dement. 2014;10(2):143–151. e1. doi: 10.1016/j.jalz.2013.08.279. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Langbaum JB, Fleisher AS, et al. Twelve-month metabolic declines in probable Alzheimer's disease and amnestic mild cognitive impairment assessed using an empirically pre-defined statistical region-of-interest: findings from the Alzheimer's Disease Neuroimaging Initiative. Neuroimage. 2010;51(2):654–664. doi: 10.1016/j.neuroimage.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouquet M, Desgranges B, Landeau B, et al. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer's disease. Brain. 2009;132(Pt 8):2058–2067. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knopman DS, Jack CRJ, Wiste HJ, et al. Selective Worsening of Brain Injury Biomarker Abnormalities in Cognitively Normal Elderly with β-amyloidosis. JAMA Neurol. 2013;70:1030–1038. doi: 10.1001/jamaneurol.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts RO, Geda YE, Knopman D, et al. The Mayo Clinic Study of Aging: Design and Sampling, Participation, Baseline Measures and Sample Characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RO, Knopman DS, Mielke MM, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82(4):317–325. doi: 10.1212/WNL.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. Neurology. 2012;78(5):342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men than in women. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albert M, DeKosky ST, Dickson D, et al. The Diagnosis of Mild cognitive impairment due to Alzheimer's disease: Report of the National Institute on Aging and the Alzheimer's Association Workgroup. Alzheimer's & Dementia: Journal of the Alzheimer's Association. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR, Jr, Wiste HJ, Knopman DS, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82(18):1605–1612. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13(10):997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 23.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71(10):743–749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knol MJ, Pestman WR, Grobbee DE. The (mis)use of overlap of confidence intervals to assess effect modification. Eur J Epidemiol. 2011;26(4):253–254. doi: 10.1007/s10654-011-9563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopman DS. beta-Amyloidosis and neurodegeneration in Alzheimer disease: who's on first? Neurology. 2014;82(20):1756–1757. doi: 10.1212/WNL.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 26.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 27.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann Neurol. 2013;73:472–480. doi: 10.1002/ana.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 30.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1(1):103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson BC, Stoub TR, Shah RC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 2011;121(2):171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 33.Delacourte A, Sergeant N, Wattez A, et al. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp Gerontol. 2002;37(10–11):1291–1296. doi: 10.1016/s0531-5565(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 34.Duyckaerts C, Hauw JJ. Prevalence, incidence and duration of Braak's stages in the general population: can we know? Neurobiol Aging. 1997;18(4):362–369. doi: 10.1016/s0197-4580(97)00047-x. discussion 389-92. [DOI] [PubMed] [Google Scholar]
- 35.Murray ME, Graff-Radford NR, Ross OA, et al. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen RC, Aisen P, Boeve BF, et al. Mild Cognitive Impairment Due to Alzheimer’s Disease: Criteria in the Community. Ann Neurol. 2013;74:199–208. doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prestia A, Caroli A, van der Flier WM, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology. 2013;80(11):1048–1056. doi: 10.1212/WNL.0b013e3182872830. [DOI] [PubMed] [Google Scholar]
- 38.van Harten AC, Smits LL, Teunissen CE, et al. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology. 2013;81(16):1409–1416. doi: 10.1212/WNL.0b013e3182a8418b. [DOI] [PubMed] [Google Scholar]
- 39.Caroli A, Prestia A, Galluzzi S, et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): Prediction of progression. Neurology. 2015;84(5):508–515. doi: 10.1212/WNL.0000000000001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134(Pt 5):1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 43.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 44.Mungas D, Tractenberg R, Schneider JA, Crane PK, Bennett DA. A 2-process model for neuropathology of Alzheimer's disease. Neurobiol Aging. 2014;35(2):301–308. doi: 10.1016/j.neurobiolaging.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age, Sex, and APOE epsilon4 Effects on Memory, Brain Structure, and beta-Amyloid Across the Adult Life Span. JAMA Neurol. 2015 doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forster S, Grimmer T, Miederer I, et al. Regional expansion of hypometabolism in Alzheimer's disease follows amyloid deposition with temporal delay. Biol Psychiatry. 2012;71(9):792–797. doi: 10.1016/j.biopsych.2011.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.