Abstract
In new generation medical therapies for type 1 diabetes mellitus (DM), cell-based approaches using pancreatic islets have attracted significant attention worldwide. In particular, dispersed islet cells obtained from isolated pancreatic islets have been a valuable source in the cell biology and tissue engineering fields. Our experimental approach to the development of new islet-based DM therapies consisted of creating a monolithic islet cell sheet format using dispersed islet cells. In this experiment, we explored the potential of internationally transporting human islets from Alberta, Canada to Tokyo, Japan and obtaining viable dispersed islet cells. A total of 34 batches of isolated and purified human islets were transported using a commercial air courier service. Prior to shipping, the human islets had been in culture for 0–108 h at the University of Alberta. The transportation period from Alberta to Tokyo was 2–5 days. The transported human islet cells were enzymatically dispersed as single cells in Tokyo. The number of single islet cells decreased as the number of transportation days increased. In contrast, cell viability was maintained regardless of the number of transportation days. The preshipment culture time had no effect on the number or viability of single cells dispersed in Tokyo. When dispersed single islet cells were plated on laminin-5-coated temperature-responsive polymer-grafted culture dishes, the cells showed favorable attachment followed by extension as a monolithic format. The present study demonstrated that long-distance transported human islets are a viable cell source for experiments utilizing dispersed human islet cells.
Keywords: Islet transportation, Dispersed islet cells, Temperature-responsive polymer-grafted culture dish, Laminin-5
INTRODUCTION
As researchers continue to discover that human islets differ in several critical biological ways from nonhuman islets, continuing experimental research using human islets is anticipated (9). To meet this increasing demand for human islets, several attempts have been made to distribute isolated human islets nationally and internationally (8,9,12,19). To this end, the Clinical Islet Laboratory at the University of Alberta initiated its own human islet distribution program in 2007 to increase the opportunities for researchers to conduct these studies in remote locations (11). Transported human islets have been proven to be valuable and have contributed to the continued progress of islet-based basic research (16,17,27,29).
Cell-based therapy using pancreatic islets has been established as a promising new therapy for patients with type 1 diabetes mellitus (DM). To enhance the success of islet-based therapies, the establishment of new approaches incorporating novel technologies that prolong transplanted islet cell longevity is vital. Accordingly, the use of dispersed single islet cells has been described as a viable approach to creating functional islet tissues (14,25,26). In particular, our laboratory has successfully fabricated a contiguous cell sheet format (islet cell sheet) in a culture condition using dispersed islet cells (25,26). This technology is based on culturing dispersed islet cells on a temperature-responsive polymer-grafted culture dish followed by temperature-dependent, nonchemical cell harvesting (32). Transplanting the islet cell sheets into a subcutaneous space resulted in the creation of neoislet tissues. The engineered neoislet tissues were found to provide therapeutic value by normalizing blood glucose levels in mice with DM (24). To use islet cell sheets as a clinical treatment for DM, human islet cell sheets made of dispersed islet cells must first be created. To date, investigations of the quality and values of dispersed single islet cells obtained over long distances have not been addressed.
In this study, we internationally transported 34 batches of human islet cells from the University of Alberta, Canada to Tokyo Women’s Medical University, Japan via a commercial air courier service. We obtained dispersed islet cells from the transported human islets and investigated the effects of the transportation period and the preshipment culture time on the cell quantity and quality. We also assessed the biological viability of the obtained dispersed islet cells by culturing the cells on culture surfaces specifically designed by our group for islet cell culturing (26).
MATERIALS AND METHODS
Human Islets
Thirty-four human islet preparations from deceased donors that failed to meet defined release criteria for clinical transplantation were used in this study. Written consent for research was provided by donor relatives in all 34 cases. The study further obtained ethical approval from the Human Research Ethics Board of the University of Alberta and Tokyo Women’s Medical University. Islets were cultured in Connaught Medical Research Laboratories (CMRL) 1066 medium (Mediatech, Herndon, VA, USA) at 22°C until they were transported. Mean age of the 34 donors was 49 ± 12 (range 16–70) years, and 14 were male and 20 were female. The viability (%) of islets before shipping was measured by using SYTO Green (Life Technologies, Carlsbad, CA, USA) and ethidium bromide (Sigma, St. Louis, MO, USA) dyes.
Transportation
Human islets resuspended in CMRL 1066 medium in a 50-ml conical tube (BD Biosciences, San Jose, CA, USA) were shipped from Edmonton to Tokyo by a commercial air courier. The cargo space in the aircraft was set at 15–17°C.
Dispersed Islet in Tokyo
Transported islets were subsequently cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and 5.5 mM glucose (Otsuka Pharmaceutical, Tokyo, Japan) overnight. On the following day, islets were incubated with 0.125% trypsin–EDTA (Life Technologies) at 37°C for 3 min and dispersed by a 20-time pipetting for 30 s, followed by trypsin inactivation by addition of 10% FBS. Finally, the suspension of dispersed single islet cells was obtained by filtering through a 70-µm cell strainer (BD Biosciences).
Assessments of the Dispersed Single Islet Cells in Tokyo
The number and the viability (%) of single dispersed islet cells were determined by counting with trypan blue (Life Technologies) exclusion test. The effects of transportation time from Canada to Tokyo and preshipment culture time at Canada on the number and viability of cells were investigated. Transport time was split into two groups: 2 days (n = 26) and greater than 2 days (n = 8). In the latter experiment, islet batches transported with the shortest transportation days (2 days) were analyzed. Cell recovery was expressed by the actual number of dispersed islet cells per volume of islet (packed cell volume) before shipment (×107 cells/ml).
Islet Cell Culturing on Temperature-Responsive Culture Dishes
Culture surfaces specifically designed for dispersed islet cell culturing were prepared by coating rat laminin-5 in phosphate-buffered saline (PBS) (0.21 mg/cm2; Chemicon International, Temecula, CA, USA) on temperature-responsive polymer [poly(N-isopropylacrylamide); PIPAAm]-grafted culture dishes (UpCell, CellSeed, Tokyo, Japan) as described previously (25,26). PBS was used as noncoated control. Dispersed human islet cells resuspended with RPMI 1640 medium containing 10% FBS were plated at a density of 1.3 × 105 cells/cm2 and cultured at 37°C. Twenty-four hours later, the culture dishes were washed to remove the nonattached cells and replenished with RPMI 1640 containing 10% FBS.
Measurement of Cell Attachment and Confluency Rates
To quantify the cell attachment rate of dispersed human islet cells to noncoated and laminin-5-coated PIPAAm-grafted dishes, the number of viable cells was assessed using a water-soluble tetrazolium salt (WST) method with a Cell Counting Kit-8 (Dojindo Laboratory, Kumamoto, Japan) on day 1 (24 h after cell seeding) after the unattached cells were washed away (n = 4 per each group). On day 3 (72 h after the cell seeding), we calculated the islet cell confluency, which was expressed as the percentage of the total surface of attached cells per culture surface area (n = 4 for each group).
Insulin Secretion Assay
On day 3, the insulin secretion assay was conducted as described previously. In brief, the culture medium was changed with fresh RPMI medium containing 3.3 mmol/L glucose and cultured for 180 min. After this incubation step, the medium was then replaced with RPMI medium containing 3.3 mmol/L glucose for 60 min and then replaced with RPMI with 20 mmol/L glucose for 60 min. For the final step, the medium was replaced with RPMI medium containing 3.3 mmol/L glucose for 60 min. At the end of each time point, the culture medium was collected and kept frozen at −20°C until analysis. The amount of secreted insulin in the culture medium (n = 3 per time point) was measured using an Ultra Sensitive Human Insulin ELISA Kit (Morinaga Institute of Biological Science, Kanagawa, Japan). The data allowed an insulin stimulation index (SI) to be calculated using the following equation: SI = (insulin content in the 20 mmol/L glucose medium)/(insulin content in the initial 3.3 mmol/L glucose medium).
Statistical Analyses
All values are expressed as mean ± standard deviation (SD). Student’s t test was used to compare data between two groups. The significance of differences among three or more groups was tested by one-way ANOVA, followed by Scheffe’s least significant difference post hoc analysis (PASW Statistics 18 software, IBM Japan, Tokyo, Japan). The probability less than 0.05 was considered to be statistically significant.
RESULTS
Quality and Quantity of Dispersed Islet Cells in Tokyo
The viability of islets at Canada before shipping was 81.3 ± 8.1%. After transportation, the number and viability of dispersed islet cells were 6.4 ± 4.7 × 107 per batch and 75.6 ± 12.8%, respectively.
Effect of Transportation Time on the Quantity and Quality of Dispersed Islet Cells in Tokyo
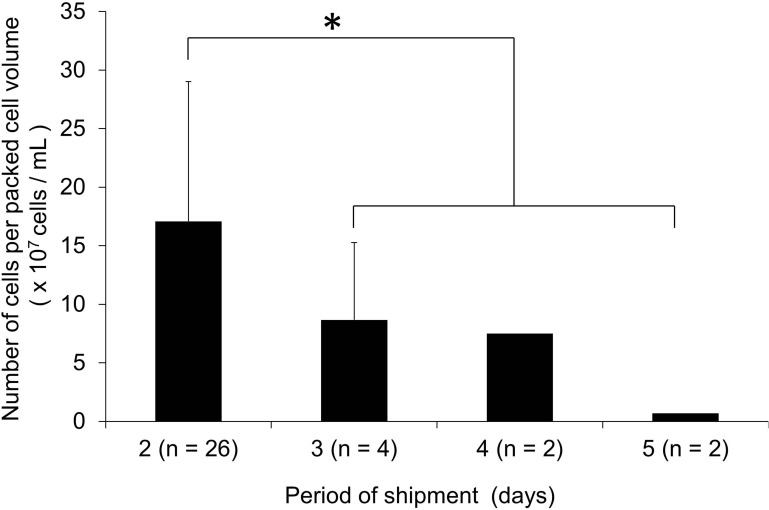
The transportation time from Alberta to Tokyo was between 2 and 5 days. The number of dispersed islet cells obtained in Tokyo decreased with increasing transportation time (Fig. 1). The number of cells in the 2-day group (n = 26) showed significantly higher values compared with that of more than 2-day group (n = 8). The cell viability of 2-day and more than 2-day groups was 77.2 ± 12.2% and 72.8 ± 14.2%, respectively (p = 0.40).
Figure 1.
Effect of transportation time on the number of dispersed islet cells obtained from transported islets in Tokyo. The transported human islets were cultured overnight, followed by digestion for obtaining single dispersed islet cells. The transportation time was 2 days (n = 26), 3 days (n = 4), 4 days (n = 2), or 5 days (n = 2). Cell recovery was expressed by the obtained number of dispersed islet cells per volume of islet (packed cell volume) before shipment (×107 cells/ml). *p < 0.05 between 2-day group and a combined “more than 2-day” group (n = 8) by Student’s t test.
Effect of Preshipment Culture Time on the Quantity and Quality of Dispersed Islet Cells in Tokyo
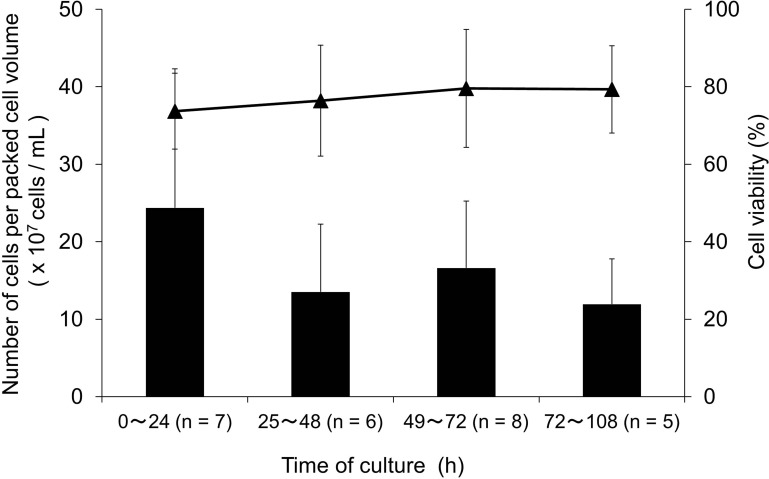
Transported islets in the 2-day transportation were assessed among groups stratified by preshipment culture time (<25 h, 25–48 h, 49–72 h, and >72 h). No significant differences among groups were observed in both quantity and quality of the dispersed islet cells (Fig. 2).
Figure 2.
Effect of preshipment culture time at the University of Alberta on the number of dispersed islet cells obtained from transported islets in Tokyo. Dispersed islet cell number and their cell viability obtained in Tokyo in the 2-day transportation group (n = 26) were determined according to the culture time conducted at the University of Alberta before the shipping. The preshipment culture time was less than 25 h (n = 7), 25–48 h (n = 6), 49–72 h (n = 8), or longer than 72 h (n = 5). Cell recovery was expressed by the obtained number of dispersed islet cells per volume of islet (packed cell volume) before shipment (×107 cells/ml). No statistical intergroup differences were found in both cell recovery and viability by one-way ANOVA with multiple comparisons. The bars indicate cell recovery, and the closed triangles (▴) indicate cell viability.
Dispersed Islet Cell Culture
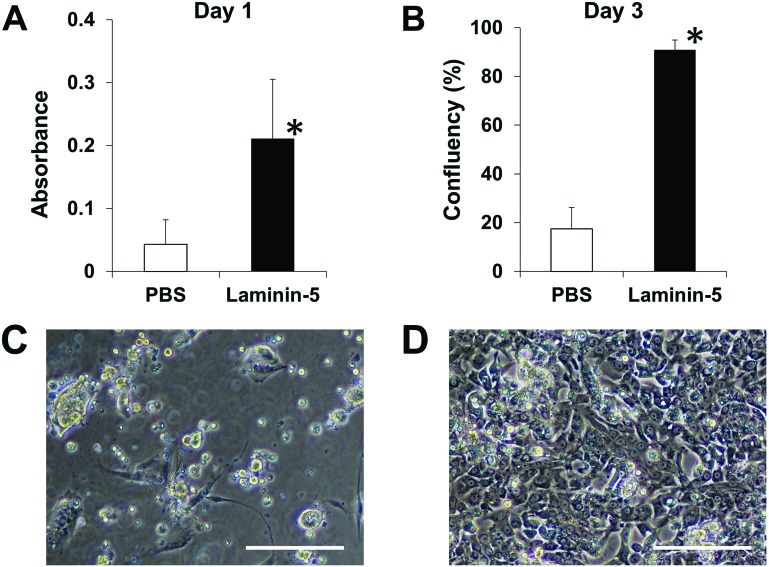
To investigate whether dispersed single islets obtained from transported human islets could be used for monolayer culturing, cells were plated on laminin-5-coated PIPAAm dishes. The cells showed significantly higher attachment ratio at day 1 on the coated versus noncoated dishes (Fig. 3A). The cells on the laminin-5-coated PIPAAm dishes subsequently showed stable attachment and cellular extension and reached near confluency on day 3 (Fig. 3B–D). The biological functionality of the cultured human islet cells was determined by their ability to increase the secretion of insulin in response to increasing doses of glucose into the medium. Five of five different lots of human dispersed islet cells showed a positive stimulation index (SI > 1) when the cells were cultured on laminin-5-coated PIPAAm dishes. In contrast, only one of five cell lots showed a positive SI when the cells were cultured on noncoated PIPAAm surfaces. SIs were 1.7 ± 0.6 and 1.0 ± 1.1, in the laminin-5-coated and noncoated groups, respectively (n = 5 for each group).
Figure 3.
Attachment and confluency rates of dispersed human islet cells on laminin-5-coated temperature-responsive culture dishes. Dispersed rat islet cells were plated at a density of 1.3 × 105 cells/cm2 on noncoated and rat laminin-5-coated PIPAAm-grafted dishes. (A) Cell attachment status was assessed on day 1 by counting attached cells using water-soluble tetrazolium salt assay (n = 4). (B) The confluency of islet cells on noncoated and laminin-5-coated PIPAAm-grafted dishes on day 3. *p < 0.05 versus control (PBS). Representative images of cultured islet cells on noncoated (C) and laminin-5-coated (D) PIPAAm-grafted dishes on day 3. Scale bars: 100 µm.
DISCUSSION AND CONCLUSION
The present study explored 34 international long-distance transportations of human islets for the use of dispersed islet cells. The recovery rate of dispersed islet cells was clearly higher in the shorter transportation time group, whereas cell viability was not associated with transportation time. The preshipment culture time had no negative impact on cell quantity or quality. The dispersed single islet cells favorably attached to the laminin-5-coated PIPAAm dishes and retained their intrinsic ability to secrete insulin in a glucose-dependent manner.
To provide the higher chance of islet transplantation to the patients, Langer et al. (15) initiated international islet shipping to allow patients in other countries to receive islet cell transplantation. To maximize the success of international islet distribution, several islet shipping procedure refinements have been made, including the use of a gas permeable bag (2,7) and encapsulating the islets (30). In our study, the shipments were conducted in a simpler system in which the islets were resuspended in regular culture medium and packed in 50-ml conical tubes. Although the number of dispersed islet cells decreased as transportation time increased (≥3 days), 2-day transportation resulted in a sufficient number of cells. Furthermore, cell viability was maintained regardless of transportation time. These data demonstrated that dispersed islet cells obtained from long-distance transported human islets were valuable for use in basic medical research even at longer transportation distances.
Several studies had addressed the effect of preshipment culture on transported islet quality (13). Ichii et al. (6) reported that preshipment islet culturing minimally affects islet viability but that culturing results lower yield. Culturing before transplantation is known to be related with islet loss (13). A previous investigation by Kin et al. (13) provided evidence that islet culture period (median, 20 h; 16–30 h) is associated with the increase of morphology score and islet viability without affecting islet purity. The present study showed that preshipment culture period minimally affected cell number and viability assessed after the transportation.
In our islet transportation process, human islets resuspended in 50-ml conical tubes were shipped from Alberta to Tokyo by a commercial air courier. This packing offered simple and easy carrying. Noguchi et al. (20) reported that low culture temperature prevented islet damage. Medications such as glucagon-like receptor inhibitor that can be used to prevent islet apoptosis have recently been reported (22). In the future, transportation temperature or culture medium may be modified to obtain better cell viability.
The value of dispersed islet cells has been examined in different experimental conditions, including an assessment platform for islet cell biological functions and cellular signaling (4,5,10), cell proliferation analyses (23), and reforming cellular aggregates (pseudoislets) or hybrid islet tissue devices (14,31). One of our approaches to the creation of functional islet tissues consists of bioengineering monolayer islet cell sheets (21,25,26). An important feature of this approach is the preparation of culture surfaces specifically defined for islet cell culturing; that is, rat laminin-5-coated PIPAAm surfaces. Laminin-5 is known to contribute to β-cell proliferation, β-cell apoptosis protection, and insulin secretion (1,3,25). The present study showed that rat laminin-5 resulted in significant cellular attachment of dispersed human islet cells followed by biological functionality expression in terms of glucose-stimulated insulin secretion. Regardless, the present study established that biologically functional dispersed human islet cells could be obtained from isolated islet batches that had been transported over long distances.
The present study underscores the value of transported human islets as a cell source for research using dispersed islet cells. Since ductal and exocrine cells exist in islet batches isolated for clinical transplantation purposes (28), the investigation of cell composition in dispersed islet cells from transported human islets is important for maximizing the investigational potential of using cells dispersed from isolated islet batches. Regardless, this study highlighted the value of human islets transported over long distances by a commercial air courier service to supply dispersed islet cells. Further studies utilizing dispersed islet cells are highly anticipated.
ACKNOWLEDGMENTS
The authors would like to thank the Clinical Islet Laboratory staff at the University of Alberta/Alberta Health Services for the islet isolation and coordination of shipping; Dr. T. Masuda, Dr. S. Mukobata (CellSeed Inc.), and Ms. K. Kanegae (Tokyo Women’s Medical University) for technical assistance; and Dr. N. Ueno (Tokyo Women’s Medical University) for his editorial assistance in the preparation of this manuscript. This work was supported in part by Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems (K. O., T. O.), Global Center of Excellence Program (K. O.), and Grant-in-Aid for Scientific Research (MEXT KAKENHI No. 21300180 to K. O.) from the Japanese Ministry of Education, Culture, Sports, Science and Technology. Teruo Okano is an investor in CellSeed Inc. and an investor/developer who is designated on the patent for temperature-responsive culture surfaces. All other authors declare no conflict of interest.
REFERENCES
- 1. Armanet M.; Wojtusciszyn A.; Morel P.; Parnaud G.; Rousselle P.; Sinigaglia C.; Berney T.; Bosco D. Regulated laminin-332 expression in human islets of Langerhans. FASEB J. 23(12):4046–4055; 2009. [DOI] [PubMed] [Google Scholar]
- 2. Bodo M.; Muzi G.; Bellucci C.; Lilli C.; Calvitti M.; Lumare A.; Dell’Omo M.; Gambelunghe A.; Baroni T.; Murgia N. Comparative in vitro studies on the fibrogenic effects of two samples of silica on epithelial bronchial cells. J. Biol. Regul. Homeost. Agents 21(3–4):97–104; 2007. [PubMed] [Google Scholar]
- 3. Bosco D.; Meda P.; Halban P. A.; Rouiller D. G. Importance of cell–matrix interaction in rat islet β cell secretion in vitro role of α6β1 integrin. Diabetes 49(2):233–243; 2000. [DOI] [PubMed] [Google Scholar]
- 4. Bosco D.; Rouiller D. G.; Halban P. A. Differential expression of E-cadherin at the surface of rat beta-cells as a marker of functional heterogeneity. J. Endocrinol. 194(1):21–29; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Hammar E.; Parnaud G.; Bosco D.; Perriraz N.; Maedler K.; Donath M.; Rouiller D. G.; Halban P. A. Extracellular matrix protects pancreatic beta-cells against apoptosis: Role of short- and long-term signaling pathways. Diabetes 53(8):2034–2041; 2004. [DOI] [PubMed] [Google Scholar]
- 6. Ichii H.; Sakuma Y.; Pileggi A.; Fraker C.; Alvarez A.; Montelongo J.; Szust J.; Khan A.; Inverardi L.; Naziruddin B.; Levy M. F.; Klintmalm G. B.; Goss J. A.; Alejandro R.; Ricordi C. Shipment of human islets for transplantation. Am. J. Transplant. 7(4):1010–1020; 2007. [DOI] [PubMed] [Google Scholar]
- 7. Ikemoto T.; Matsumoto S.; Itoh T.; Noguchi H.; Tamura Y.; Jackson A. M.; Shimoda M.; Naziruddin B.; Onaca N.; Yasunami Y.; Levy M. F. Assessment of islet quality following international shipping of more than 10,000 km. Cell Transplant. 19(6):731–741; 2010. [DOI] [PubMed] [Google Scholar]
- 8. Integrated Islet Distribution Program: Available at:http://iidp.coh.org/Default.aspx, Accessed February 1, 2013.
- 9. Kaddis J. S.; Hanson M. S.; Cravens J.; Qian D.; Olack B.; Antler M.; Papas K. K.; Iglesias I.; Barbaro B.; Fernandez L.; Powers A. C.; Niland J. C. Standardized transportation of human islet: An islet cell resource center study of more than 2000 shipments. Cell Transplant 22(7):1101–1111; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katsuta H.; Akashi T.; Katsuta R.; Nagaya M.; Kim D.; Arinobu Y.; Hara M.; Bonner-Weir S.; Sharma A. J.; Akashi K.; Weir G. C. Single pancreatic beta cells co-express multiple islet hormone genes in mice. Diabetologia 53(1):128–138; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kin T.; O’Gorman D.; Schroeder A.; Onderka C.; Richer B.; Rosichuk S.; Zhai X.; Shapiro A. M. J. Human islet distribution program for basic research at a single center. Transplant. Proc. 43(9):3195–3197; 2011. [DOI] [PubMed] [Google Scholar]
- 12. Kin T.; O’Gorman D.; Zhai W.; Schroeder A.; Onderka C.; Richer B.; Rosichuk S.; Shapiro A. M. J. Human islet distribution activity for basic research: Annual report 2011 Alberta islet distribute program. Available at:https://sites.google.com/a/ualberta.ca/alberta-islet-distribution-program/home/annual-report-2011, Accessed February 1, 2013. [DOI] [PubMed]
- 13. Kin T.; Senior P.; O’Gorman D.; Richer B.; Salam A.; Shapiro A. M. J. Risk factors for islet loss during culture prior to transplantation. Transplantation 21(11):1029–1035; 2008. [DOI] [PubMed] [Google Scholar]
- 14. Kodama S.; Kojima K.; Furuta S.; Chambers M.; Paz A. C.; Vacanti C. A. Engineering functional islets from cultured cells. Tissue Eng. Part A 15(11):3321–3329; 2009. [DOI] [PubMed] [Google Scholar]
- 15. Langer R. M.; Máthé Z.; Doros A.; Máthé Z. S.; Weszelits V.; Filó A.; Bucher P.; Morel P.; Berney T.; Járay J. Successful islet after kidney transplantations in a distance over 1000 kilometers: Preliminary results of the Budapest–Geneva collaboration. Transplant. Proc. 36(10):3113–3115; 2004. [DOI] [PubMed] [Google Scholar]
- 16. Lee S. H.; Hao E.; Levine F.; Itkin-Ansari P. Id3 upregulates BrdU incorporation associated with a DNA damage response, not replication, in human pancreatic β-cells. Islets 3(6):358–366; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee S. H.; Itkin-Ansar P.; Levine F. CENP-A, a protein required for chromosome segregation in mitosis, declines with age in islet but not exocrine cells. Aging 2(11):785–790; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mandrup-Poulsen T.; Billestrup N.; Halban P. A. Proliferation of sorted human and rat beta cells. Diabetologia 51(1):91–100; 2008. [DOI] [PubMed] [Google Scholar]
- 19. National Islet Cell Resource Center Consortium: Available at: http://www.citisletstudy.org/index.html, Accessed February 1, 2013.
- 20. Noguchi H.; Naziruddin B.; Jackson A.; Shimoda M.; Ikemoto T.; Fujita Y.; Chujo D.; Takita M.; Kobayashi N.; Onaca N.; Levy M. F.; Matsumoto S. Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation. Transplantation 89(1):47–54; 2010. [DOI] [PubMed] [Google Scholar]
- 21. Ohashi K.; Mukobata S.; Utoh R.; Yamashita S.; Masuda T.; Sakai H.; Okano T. Production of islet cell sheets using cryopreserved islet cells. Transplant. Proc. 43(9):3188–3191; 2011. [DOI] [PubMed] [Google Scholar]
- 22. Park Y. J.; Ao Z.; Kieffer T. J.; Chen H.; Safikhan N.; Thompson D. M.; Meloche M.; Warnock G. L.; Marzban L. The glucagon-like peptide-1 receptor agonist exenatide restores impaired pro-islet amyloid polypeptide processing in cultured human islets: Implications in type 2 diabetes and islet transplantation. Diabetologia 56(3):508–519; 2013. [DOI] [PubMed] [Google Scholar]
- 23. Parnaud G.; Bosco D.; Berney T.; Pattou F.; Kerr-Conte J.; Donath M. Y.; Bruun C.; Mandrup-Poulsen T.; Billestrup N.; Halban P. A. Proliferation of sorted human and rat beta cells. Diabetologia 51(1):91–100; 2008. [DOI] [PubMed] [Google Scholar]
- 24. Parnaud G.; Hammar E.; Rouiller D. G.; Armanet M.; Halban P. A.; Bosco D. Blockade of beta1 integrin–laminin-5 interaction affects spreading and insulin secretion of rat beta-cells attached on extracellular matrix. Diabetes 55(5):1413–1420; 2006. [DOI] [PubMed] [Google Scholar]
- 25. Saito T.; Ohashi K.; Utoh R.; Ise K.; Gotoh M.; Yamato M.; Okano T. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation 92(11):1231–1236; 2011. [DOI] [PubMed] [Google Scholar]
- 26. Shimizu H.; Ohashi K.; Utoh R.; Ise K.; Gotoh M.; Yamato M.; Okano T. Bioengineering of a functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials 30(30):5943–5949; 2009. [DOI] [PubMed] [Google Scholar]
- 27. Smukler S. R.; Arntfield M. E.; Razavi R.; Bikopoulos G.; Karpowicz P.; Seaberg R.; Dai F.; Lee S.; Ahrens R.; Fraser P. E.; Wheeler M. B.; van der Kooy D. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8(3):281–293; 2011. [DOI] [PubMed] [Google Scholar]
- 28. Street C. N.; Lakey J. R.; Shapiro A. M. J.; Imes S.; Rajotte R. V.; Ryan E. A.; Lyon J. G.; Kin T.; Avila J.; Tsujimura T.; Korbutt G. S. Islet graft assessment in the Edmonton Protocol: Implications for predicting long-term clinical outcome. Diabetes 53(12):3107–3114; 2004. [DOI] [PubMed] [Google Scholar]
- 29. Takane K. K.; Kleinberger J. W.; Salim F. G.; Fiaschi-Taesch N. M.; Stewart A. F. Regulated and reversible induction of adult human β-cell replication. Diabetes 61(2):418–424; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vaithilingam V.; Barbaro B.; Oberholzer J.; Tuch B. E. Functional capacity of human islets after long-distance shipment and encapsulation. Pancreas 40(2):247–252; 2011. [DOI] [PubMed] [Google Scholar]
- 31. Williams S. J.; Wang Q.; Macgregor R. R.; Siahaan T. J.; Stehno-Bittel L.; Berkland C. S. Adhesion of pancreatic beta cells to biopolymer films. Biopolymers 91(8):676–685; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang J.; Yamato M.; Sekine H.; Sekiya S.; Tsuda Y.; Ohashi K.; Shimizu T.; Okano T. Tissue engineering using laminar cellular assemblies. Adv. Mater. 21(32–33):3404–3409; 2009. [DOI] [PubMed] [Google Scholar]