Abstract
Nonalcoholic steatohepatitis (NASH) has become a major concern in clinical hepatology. To elucidate the disease mechanisms and to develop a treatment, the advent of an appropriate experimental model is crucial. Pregnant Sprague–Dawley rats were fed a high-fat diet from gestational day 16. Two days after birth, the neonates were injected subcutaneously with streptozotocin (STZ) (180, 200, or 256 mg/kg). The mothers were fed a high-fat diet during the nursing period. After being weaned (4 weeks of age), the juvenile rats were fed the same high-fat diet. The survival rates at the time of weaning were 25.6% (180 mg/kg STZ), 22.8% (200 mg/kg STZ), and 19.4% (256 mg/kg STZ). The mean body weight of NASH rats was approximately 20% less than that of normal rats. Serum levels of glucose, alanine aminotransferase, and hyaluronic acid increased in NASH rats. Histologically, typical features of steatohepatitis such as ballooning, inflammatory cell infiltration, and perivenular and pericellular fibrosis were observed. In an indocyanine green loading test, the blood half-life was significantly longer in NASH rats (5.04 ± 2.14 vs. 2.72 ± 0.72 min; p < 0.05), which was suggestive of an impaired hepatobiliary transportation function. Concomitantly, biliary ICG concentrations in NASH rats stabilized in a delayed fashion compared with normal rats. In addition, the amount of bile excreted in NASH rats was significantly lower than that in normal rats (4.32 ± 0.83 vs. 7.66 ± 1.05 mg/min; p < 0.01). The rat NASH model presented here mimics the clinical features of the disease and will be a helpful tool for medical and bioscience research.
Keywords: Nonalcoholic steatohepatitis (NASH), Streptozotocin (STZ), High-fat diet, Rat
INTRODUCTION
Nonalcoholic steatohepatitis (NASH) is a relatively new disease concept and is thought to be highly associated with recent lifestyle changes, especially dietary patterns. The first report on NASH was published in 1980 (28). NASH is considered a severe form of nonalcoholic fatty liver disease (NAFLD), which has also become a common liver abnormality showing steatosis, steatohepatitis, and cirrhosis not related to alcohol abuse (15,17,21,25). Progression to NASH is thought to occur due to the following reasons—the “two-hit” hypothesis (6): (a) steatosis caused by insulin resistance and obesity leads to sensitization and (b) inflammation, oxidative stress, lipid peroxidation, and proinflammatory cytokines initiate steatohepatitis. NASH is currently defined as steatohepatitis resulting in ballooning hepatocyte degeneration, inflammatory infiltration of polymorphonuclear leukocytes, and perivenular or pericellular fibrosis (12,15,28). To elucidate the disease mechanisms and to develop a treatment, an appropriate experimental animal model that possesses the typical disease characteristics must first be established.
A number of experimental animal NASH models have been proposed (10,13). In addition to the use of genetically mutated animals, high-fat and methionine- and choline-deficient diet treatments are currently used (3,7,16,30). However, a reliable model of progressive steatohepatitis with severe fibrosis has not yet been realized.
Streptozotocin (STZ) is a well-known inducer of experimental diabetes. While its mechanism consists of damaging pancreatic islets, it also induces insulin-resistant type 2 diabetes in a dose-dependent fashion (4,11). Some researchers have successfully induced type 2 diabetes using a high-fat diet (2,18,22,23,29). Type 2 diabetes is a potent risk factor of NASH, so we investigated the potency of the combination of STZ and a high-fat diet treatment for inducing NASH. The purpose of this study was to verify the appropriateness of a rat NASH model by comparing its characteristics to human NASH pathology.
MATERIALS AND METHODS
Ethical Considerations
All experimental procedures were approved by the institutional animal ethics committee (Reference No. 2010-005).
Materials
Pregnant Sprague–Dawley (SD) rats (gestation day 16; specific pathogen free) were purchased from Sankyo Labo Service Co., Inc. (Tokyo, Japan). The high-fat diet, in which 60% of total calories were derived from fat, was purchased from Research Diets Inc. (D12492, New Brunswick, NJ, USA). STZ (S0130-500MG) was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA), and indocyanine green (ICG) was purchased from Daiichi Sankyo Co. Ltd. (Tokyo, Japan).
Production of NASH Rats
Pregnant rats were given a high-fat diet and water ad libitum during the gestation and nursing periods. Two days after birth, the neonates were injected subcutaneously with 180, 200, or 256 mg/kg of STZ. The rats were weaned 4 weeks after birth and then fed the high-fat diet.
Estimation of Disease State
Survival rate, body weight, blood glucose, alanine aminotransaminase (ALT), and hyaluronic acid were evaluated. Blood samples (approximately 150 µl) were taken from the tail vein and centrifuged (1,000 × g for 5 min) to obtain sera. Glucose and ALT levels were measured using an automated analyzer (DRI-CHEM 3500i; FUJIFILM, Tokyo, Japan). Hyaluronic acid levels were measured using an ELISA kit (Mitsubishi Chemical Medience Corporation, Tokyo, Japan).
Functional Evaluation of Hepatobiliary Transport
NASH and normal SD rats (12–16 weeks old) were anesthetized with 2.0% isoflurane (Mylan Inc., Canonsburg, PA, USA), placed in a supine position, and subjected to a medial abdominal incision. Heart rate was monitored using electrocardiography (M1166A, Model 66S; Agilent Technologies, Inc., Santa Clara, CA, USA) and maintained at 180–220/min by regulating anesthesia. A total of 250 µg/kg of ICG was injected via the abdominal vena cava, and 400 µl sample of blood was collected from the vein at 1, 2.5, 5, 10, and 15 min after the injection.
Rat bile was collected using a cannula (SR-FF2419 24 G cannula; Terumo, Tokyo, Japan) inserted into the bile duct. Bile was collected in aliquots at fixed intervals, that is, 2.5, 5, 7.5, 10, 12.5, 15, 20, 25, 30, 40, 50, and 60 min after ICG injection, and then weighed.
ICG concentration in the sera and bile was determined spectroscopically at 805 nm (DU 640; Beckman Coulter Inc., Brea, CA, USA). The amount of ICG was calculated using an absorption coefficient of 204,000 cm−1 × (mol/L)−1 (14).
Histological Examination
The rat livers were fixed with 20% neutralized formalin (068-01727, Wako Pure Chemical Industries, Ltd., Osaka, Japan) and prepared as histological sections using Masson trichrome staining (HT-15, Sigma-Aldrich Co.).
Statistics
Statistical significance was determined by one-way analysis of variance and Fisher’s protected least significant difference test. Values of p < 0.05 were considered significant.
RESULTS
Survival Rate and Body Weight Change
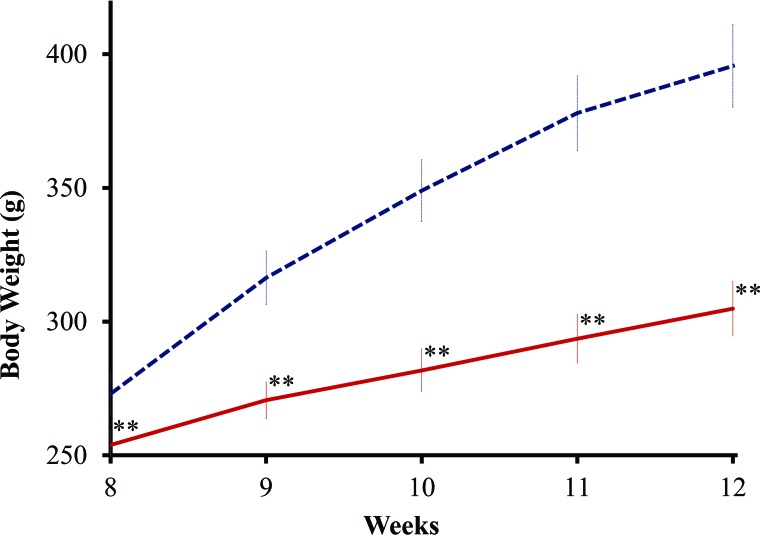
After the injection of STZ, some neonates died during the suckling period. The cause of death was thought to be type 1 diabetes because the neonates showed polyuria after the injection of STZ. The survival rate at the time of weaning was 25.6–19.4% correlating reversely with the STZ dose (Table 1). The male and female ratio of the survivors was not disproportionate (data not shown). After weaning, all rats survived and matured. Although the NASH rats were fed a high-fat diet, their body weight gain was approximately 20% less than that of normal rats from 8 to 12 weeks of age (Fig. 1).
Table 1.
Survival Rate of the Nonalcoholic Steatohepatitis (NASH) Rats at the Time of Weaning (4 Weeks Old) After Different Doses of Streptozotocin Injection
| Streptozotocin Dose* | |||
|---|---|---|---|
| 180 mg/kg | 200 mg/kg | 256 mg/kg | |
| Survival rate | 25.7% | 22.4% | 19.4% |
| Alive/dead† | 44/127 | 57/197 | 7/29 |
| Alive‡ | |||
| Male/female | 20/24 | 31/26 | 3/4 |
Injected subcutaneously 2 days after birth.
Number of alive and dead rats during the suckling period.
Gender of alive rats was determined at the time of weaning.
Figure 1.
Body weight changes. Body weight changes in nonalcoholic steatohepatitis (NASH) (solid red line, n = 8, male) and normal (dashed blue line, n = 8, male) rats from 8 to 12 weeks of age. Each point was expressed as mean ± SD, and statistical significance is shown by asterisks (p < 0.01). NASH rats were injected with 200 mg/kg streptozotocin 2 days after birth and fed a high-fat diet as described in the text.
Blood Biochemistry Test
Blood glucose levels were significantly higher in NASH rats than in normal rats (382 ± 117 mg/dl vs. 163 ± 22 mg/dl) (Table 2). Similarly, ALT and hyaluronic acid levels were significantly higher in NASH rats. There was mild elevation of ALT and hyaluronic acid levels in male NASH rats compared to females (Table 2). Therefore, we focused on the male NASH rats thereafter.
Table 2.
Blood Biochemistry Test of 12-Week-Old Nonalcoholic Steatohepatitis (NASH) Rats
| Group (n) | Glucose (mg/dl) | Alanine Aminotransferase (IU/L) | Hyaluronic Acid (mg/dl) |
|---|---|---|---|
| Control (12)* | 164 ± 21 | 36.2 ± 7.8 | 19.6 ± 6.2 |
| NASH (15) | 382 ± 117† § | 61.7 ± 32.4‡ | 71.5 ± 45.7§ |
| Male (8) | 349 ± 138§ | 71.0 ± 32.9‡ | 92.0 ± 53.9§ |
| Female (7) | 419 ± 83§ | 51.1 ± 30.6 | 48.1 ± 16.8§ |
Rats were injected with 200 mg/kg streptozotocin 2 days after birth and fed a high-fat diet.
Twelve-week-old normal Sprague–Dawley rats (eight males and four females). There was no statistically significant difference between males and females.
Statistical significance compared to controls using Fisher’s protected least significant difference test.
p < 0.05.
p < 0.01.
Histopathology Findings
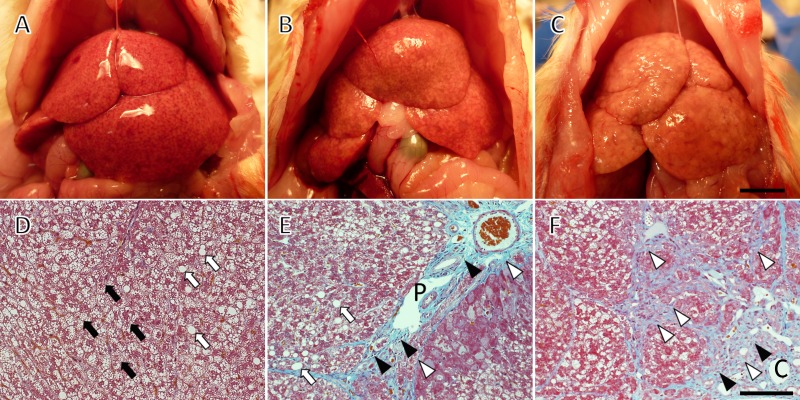
In macroscopic observation, the NASH rat livers exhibited the typical fatty liver feature of a pale yellow appearance (Fig. 2A–C). A netlike pattern on the surface indicated the existence of fibrosis and pseudolobule development. Although all of the livers were from the same group of rats treated with 200 mg/kg STZ, the progression of pathological changes varied from mild to severe and cirrhotic. Most livers showed medium changes as typified in Figure 2B. Microscopic sections were prepared from such livers with medium degrees of change. Ballooning degeneration was observed in parenchymal hepatocytes (Fig. 2D), as was macrovesicular steatosis (Fig. 2D, E). Inflammatory cell infiltration was detected mainly in the portal region (Fig. 2E). Severe perivenular and pericellular fibrosis were sharply stained light blue by the Masson trichrome (Fig. 2E, F), supporting the pseudolobule appearance in macroscopic observation.
Figure 2.
Macroscopic (A–C) and microscopic (D–F) observation of nonalcoholic steatohepatitis (NASH) rat livers. All rats were treated with a neonatal streptozotocin injection (200 mg/kg) and a high-fat diet, and livers were collected at 8 weeks of age. There was variance in the progression of pathological change: (A) mild; (B) medium; (C) severe and cirrhotic. Histology was taken from medium to severe specimens. Ballooning degeneration (black arrows, D), inflammatory cell infiltration (black arrowheads, E), macrovesicular steatosis (white arrows, D), and perivenular and pericellular fibrosis (white arrowheads, E and F) are evident. The sections were stained with Masson trichrome. C, central vein; P, portal vein. Scale bars: 1 cm (C) and 25 µm (F).
Hepatobiliary Transport Activity
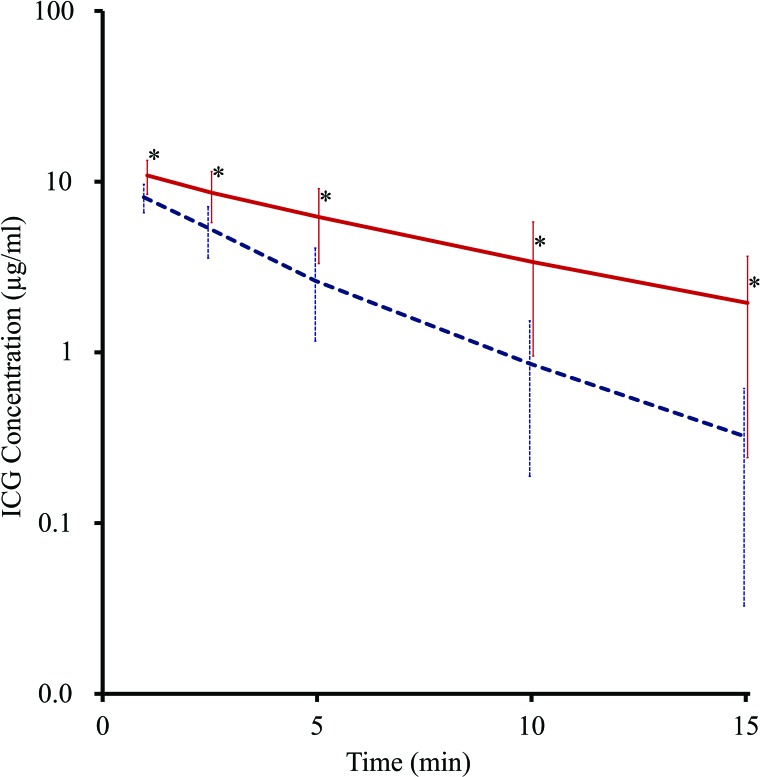
The clearance of blood ICG was significantly slower in NASH rats than in normal rats (Fig. 3). The half-life of ICG in NASH rats was significantly longer than that in normal rats (5.04 ± 2.14 min, n = 6 vs. 2.72 ± 0.72 min, n = 6; p < 0.05). The plasma disappearance rate of ICG was also calculated from the data as being 13.8%/min in NASH rats and 25.5%/min in normal rats, indicating impaired hepatobiliary transport.
Figure 3.
Time course change of indocyanine green (ICG) concentration. Time course change of ICG concentration of nonalcoholic steatohepatitis (NASH) (solid red line, n = 6) and normal (dashed blue line, n = 6) rats. Bars indicate SD, and asterisks indicate statistical significance (p < 0.05). The half-life of ICG was calculated from the slope, and that of the NASH rats was significantly longer than that of normal rats (5.04 ± 2.1 min vs. 2.72 ± 0.7 min; p < 0.05).
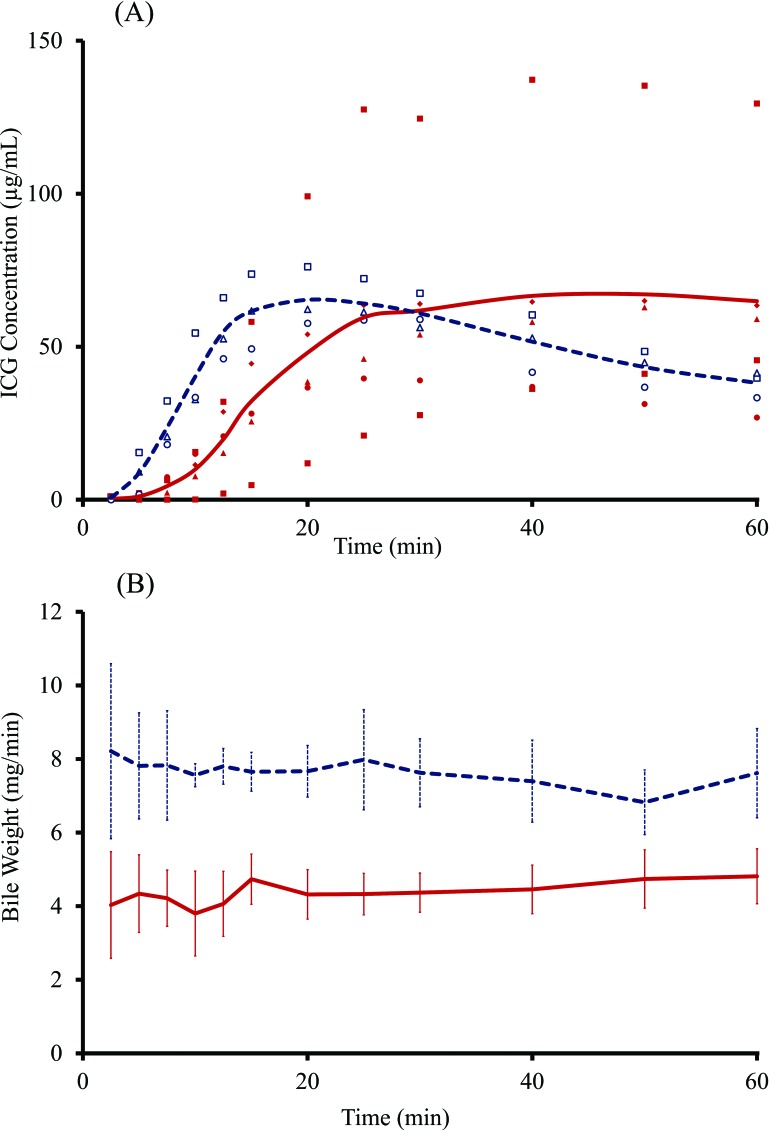
The biliary ICG concentration in the NASH rats plateaued in a delayed fashion compared to that in the normal rats (Fig. 4A). The amount of bile secreted per minute was significantly lower in NASH rats than that in normal rats throughout the collection period (Fig. 4B). The mean ± SD of all points was 4.32 ± 0.83 and 8.09 ± 0.98 mg/min in NASH and normal rats, respectively. Thus, the bile-producing activity was cut in half by the presence of NASH.
Figure 4.
Time course change of indocyanine green (ICG) concentration in the bile (A) and the amount of bile secreted (B) in nonalcoholic steatohepatitis (NASH) and normal rats. In (A), closed squares, circles, triangles, and diamonds denote ICG concentration of NASH rats (n = 5), while open squares, circles, and triangles denote ICG concentration of normal rats (n = 3). The solid red line and the dashed blue line indicate mean ICG concentration of NASH and normal rats, respectively. The mean ICG concentration of the NASH group increased relatively slowly. In (B), the solid red line and the dashed blue line indicate the mean amount of bile secreted per minute, respectively, while the bars indicate SD (NASH rats, n = 5; normal rats, n = 3). The mean amount of bile secreted in the NASH group was significantly lower than that in the normal group (4.32 ± 0.83 mg/min vs. 7.66 ± 1.05 mg/min; p < 0.01).
DISCUSSION
The NASH model described here implemented almost every feature of the clinical state observed in humans. The significantly increased glucose level was indicative of underlying diabetes, although it was not clear whether insulin resistance was induced. Levels of ALT, a marker of hepatocellular damage, and hyaluronic acid, an indicator of fibrosis, increased in concert with the histological observations (Fig. 2). The ALT elevation was not as marked as that seen in other experimental hepatic failure models such as those involving hepatotoxin treatment or hepatectomy, probably because the cirrhotic damage proceeded gradually in the present model. In contrast, the progression of fibrosis was confirmed by both the hyaluronic acid elevations and the histology. We previously observed elevated malondialdehyde levels in the blood and liver tissues suggestive of the progression of lipid peroxidation due to oxidative stress (9).
Interestingly, a difference between the sexes was observed in the development of NASH in the present study. As shown in Table 2, NASH severity was more pronounced in males than in females. Clinically, the prevalence of NAFLD and NASH in males has been reported, and the mechanism is thought to be associated with differences in the expression of estrogen-dependent genes (1,8,20). Sex-related differences in the sensitivity to insulin have also been reported (24). Our NASH model reflects the clinical situation in terms of sex differences.
We attempted to perform stable bile collection in this study. In a preliminary experiment, the measured amount of bile dispersed every trial. Keeping the heart rate at 180–220/min using electrocardiography provided reproducible results. This beat rate is slightly lower than the normal range of awake rats (260–400/min). Under this condition, however, both normal and NASH rats breathed regularly, and their blood color remained intact during the 60-min sampling period. In the literature, positive end-expiratory pressure affected hepatic blood flow in humans (27), while cardiac output and hepatic blood flow decreased during isoflurane anesthesia in dogs (26).
The ascending pattern of ICG in the bile was delayed in NASH rats. The time to plateau was approximately 30 min in NASH rats and 12 min in normal rats. By comparison, the plateau time was reported to be 120 min in two patients undergoing cholecystectomy (5). The delayed transportation of circulating ICG to the bile as well as the decreased bile secretion confirmed the kinetic attenuation of hepatic function.
In conclusion, our NASH rat model induced by the combination of neonatal STZ injection and a high-fat diet mimics almost every feature of human NASH. This model also follows the present “two-hit” hypothesis of NASH etiology and will be a good model to elucidate its mechanism.
ACKNOWLEDGMENTS
The authors thank Dr. Noboru Machida for his heartfelt encouragement. This work was supported by a grant-in-aid from the National Center for Child Health and Development. The authors declare no conflicts of interest.
REFERENCES
- 1. Anezaki Y.; Ohshima S.; Ishii H.; Kinoshita N.; Dohmen T.; Kataoka E.; Sato W.; Iizuka M.; Goto T.; Sasaki J.; Sasaki T.; Suzuki A.; Ohnishi H.; Horie Y. Sex difference in the liver of hepatocyte-specific Pten-deficient mice: A model of nonalcoholic steatohepatitis. Hepatol. Res. 39:609–618; 2009. [DOI] [PubMed] [Google Scholar]
- 2. Arulmozhi D. K.; Veeranjaneyulu A.; Bodhankar S. L. Neonatal streptozotocin-induced rat model of Type 2 diabetes mellitus: A glance. Indian J. Pharmacol. 36:217–221; 2004. [Google Scholar]
- 3. Baumgardner J. N.; Shankar K.; Hennings L.; Badger T. M.; Ronis M. J. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 294:G27–G38; 2008. [DOI] [PubMed] [Google Scholar]
- 4. Bonner-Weir S.; Trent D. F.; Honey R. N.; Weir G. C. Responses of neonatal rat islets to streptozotocin: Limited B-cell regeneration and hyperglycemia. Diabetes 30:64–69; 1981. [DOI] [PubMed] [Google Scholar]
- 5. Cherrick G. R.; Stein S. W.; Leevy C. M.; Davidson C. S. Indocyanine green: Observations on its physical properties, plasma decay, and hepatic extraction. J. Clin. Invest. 39:592–600; 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Day C. P.; James O. F. Steatohepatitis: A tale of two “hits”? Gastroenterology 114:842–845; 1998. [DOI] [PubMed] [Google Scholar]
- 7. Deng Q. G.; She H.; Cheng J. H.; French S. W.; Koop D. R.; Xiong S.; Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology 42:905–914; 2005. [DOI] [PubMed] [Google Scholar]
- 8. Eguchi Y.; Hyogo H.; Ono M.; Mizuta T.; Ono N.; Fujimoto K.; Chayama K.; Saibara T.; JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J. Gastroenterol. 47:586–595; 2012. [DOI] [PubMed] [Google Scholar]
- 9. Enosawa S.; Dozen M.; Tada Y.; Hirasawa K. Electron therapy attenuated elevated alanine aminotransferase and oxidative stress values in type 2 diabetes-induced non-alcoholic steatohepatitis of rats. Cell Medicine (submitted to the same issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan J. G.; Qiao L. Commonly used animal models of non-alcoholic steatohepatitis. Hepatobiliary Pancreat. Dis. Int. 8:233–240; 2009. [PubMed] [Google Scholar]
- 11. Katsilambros N.; Rahman Y. A.; Hinz M.; Fussgänger R.; Schröder K. E.; Straub K.; Pfeiffer E. F. Action of streptozotocin on insulin and glucagon responses of rat islets. Horm. Metab. Res. 2:268–270; 1970. [DOI] [PubMed] [Google Scholar]
- 12. Lall C. G.; Aisen A. M.; Bansal N; Sandrasegaran K. Nonalcoholic fatty liver disease. AJR Am. J. Roentgenol. 190:993–1002; 2008. [DOI] [PubMed] [Google Scholar]
- 13. Larter C. Z.; Yeh M. M. Animal models of NASH: Getting both pathology and metabolic context right. J. Gastroenterol. Hepatol. 23:1635–1648; 2008. [DOI] [PubMed] [Google Scholar]
- 14. Lee H.; Mason J. C.; Achilefu S. Heptamethine cyanine dyes with a robust C-C bond at the central position of the chromophore. J. Org. Chem. 71:7862–7865; 2006. [DOI] [PubMed] [Google Scholar]
- 15. McCullough A. J. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 1:S17–S29; 2006. [DOI] [PubMed] [Google Scholar]
- 16. Mu Y. P.; Ogawa T.; Kawada N. Reversibility of fibrosis, inflammation, and endoplasmic reticulum stress in the liver of rats fed a methionine-choline-deficient diet. Lab. Invest. 90:245–562; 2010. [DOI] [PubMed] [Google Scholar]
- 17. Pascale A.; Pais R.; Ratziu V. An overview of nonalcoholic steatohepatitis: Past, present and future directions. J. Gastrointest. Liver Dis. 19:415–423; 2010. [PubMed] [Google Scholar]
- 18. Sahin K.; Onderci M.; Tuzcu M.; Ustundag B.; Cikim G.; Ozercan I. H.; Sriramoju V.; Juturu V.; Komorowski J. R. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: The fat-fed, streptozotocin-treated rat. Metabolism 56:1233–1240; 2007. [DOI] [PubMed] [Google Scholar]
- 19. Sakka S. G.; VanHout N. Relation between indocyanine green (ICG) plasma disappearance rate and ICG blood clearance in critically ill patients. Intens. Care Med. 32:766–769; 2006. [DOI] [PubMed] [Google Scholar]
- 20. Sanyal A. J.; American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 123:1705–1725; 2002. [DOI] [PubMed] [Google Scholar]
- 21. Shimada M.; Takase S. Clinical features of NASH. Nihon Rinsho 64:1114–1118; 2006. [PubMed] [Google Scholar]
- 22. Srinivasan K.; Viswanad B.; Asrat L.; Kaul C. L.; Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 52:313–320; 2005. [DOI] [PubMed] [Google Scholar]
- 23. Takada J.; Machado M. A.; Peres S. B.; Brito L. C.; Borges-Silva C. N.; Costa C. E.; Fonseca-Alaniz M. H.; Andreotti S.; Lima F. B. Neonatal streptozotocin-induced diabetes mellitus: A model of insulin resistance associated with loss of adipose mass. Metabolism 56:977–984; 2007. [DOI] [PubMed] [Google Scholar]
- 24. Vital P.; Larrieta E.; Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J. Endocrinol. 190:425–432; 2006. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe S.; Yaginuma R.; Ikejima K.; Miyazaki A. Liver diseases and metabolic syndrome. J. Gastroenterol. 43:509–518; 2008. [DOI] [PubMed] [Google Scholar]
- 26. Weiss M.; Krejcie T. C.; Avram M. J. A physiologically based model of hepatic ICG clearance: Interplay between sinusoidal uptake and biliary excretion. Eur. J. Pharm. Sci. 44:359–365; 2011. [DOI] [PubMed] [Google Scholar]
- 27. Winsö O.; Biber B.; Gustavsson B.; Holm C.; Milsom I.; Niemand D. Portal blood flow in man during graded positive end-expiratory pressure ventilation. Intens. Care Med. 12:80–85; 1986. [DOI] [PubMed] [Google Scholar]
- 28. Yeh M. M.; Brunt E. M. Pathology of nonalcoholic fatty liver disease. Am. J. Clin. Pathol. 128:837–847; 2007. [DOI] [PubMed] [Google Scholar]
- 29. Zhang M.; Lv X. Y.; Li J.; Xu Z. G.; Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008:704045; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zou Y.; Li J.; Lu C.; Wang J.; Ge J.; Huang Y.; Zhang L.; Wang Y. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 79:1100–1107; 2006. [DOI] [PubMed] [Google Scholar]