Abstract
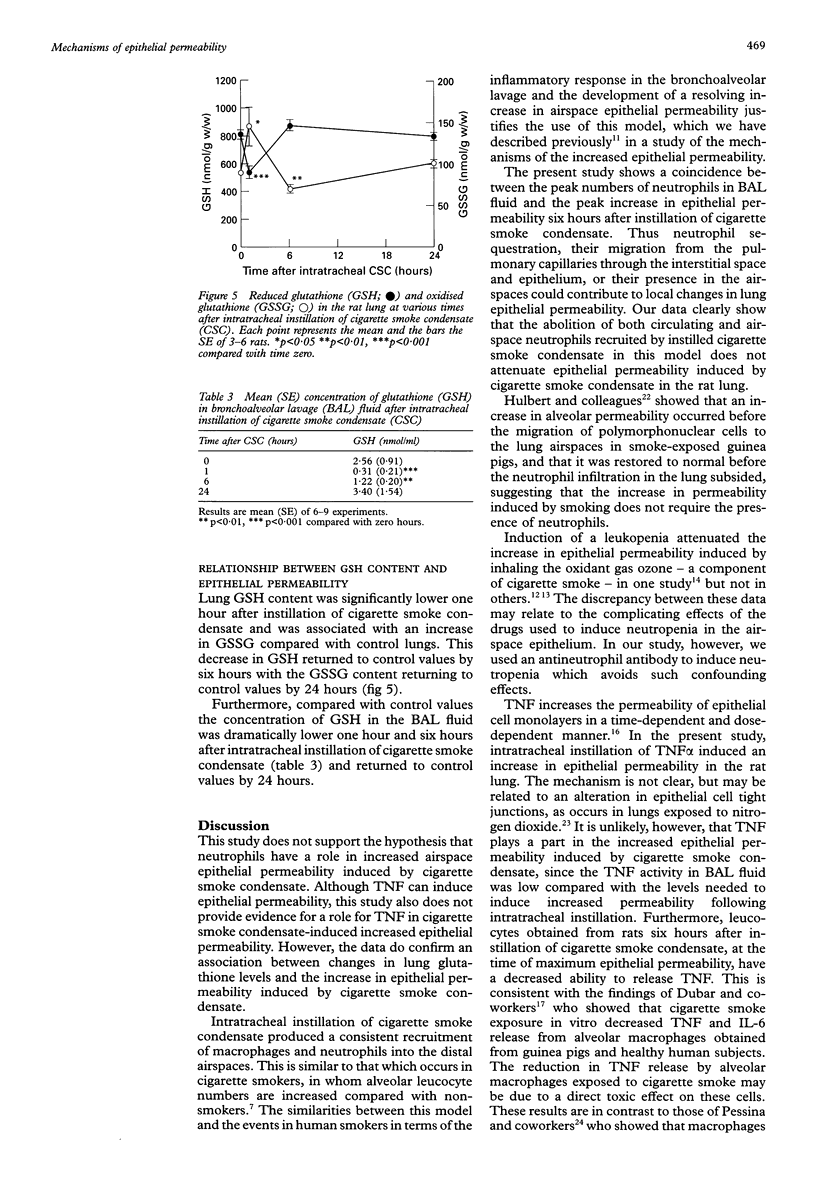
BACKGROUND: Increased epithelial permeability of the airspaces occurs commonly in the lungs of cigarette smokers. It is likely to be important in augmenting the inflammatory response in the airspaces and hence may have a role in the pathogenesis of emphysema. It has previously been shown that intratracheal instillation of cigarette smoke condensate induces increased epithelial permeability in vivo in rats and in vitro in epithelial cell monolayers, associated with a disturbance in the lung antioxidant, glutathione (GSH). The aim of this study was to assess the role of neutrophils, GSH, and tumour necrosis factor (TNF) in the increased epithelial permeability following intratracheal instillation of cigarette smoke condensate. METHODS: Epithelial permeability of the airspaces was measured in rat lungs as the passage of intratracheally instilled 125-iodine labelled bovine serum albumin (BSA) into the blood. The permeability of a monolayer of human type II alveolar epithelial cells to 125I-BSA was also measured. RESULTS: Cigarette smoke condensate produced a 59.7% increase in epithelial permeability over control values peaking six hours after instillation and returning to control values by 24 hours. Depletion of neutrophils and, to a lesser extent, macrophages by an intraperitoneal injection of antineutrophil antibody did not influence the increased epithelial permeability induced by cigarette smoke condensate. Although instillation of human recombinant TNF alpha produced an increase in epithelial permeability in the rat lung from 0.62 (0.61)% to 1.27 (0.08)%, only a trivial amount of TNF alpha was detected in bronchoalveolar lavage (BAL) fluid in vivo or in culture medium from BAL leucocytes obtained from animals treated with cigarette smoke condensate (94.9 (28.8) units/ml). Furthermore, antiTNF antibody did not abolish the increased epithelial permeability produced by cigarette smoke condensate. The role of GSH was assessed by measuring the changes in both the reduced (GSH) and oxidised form (GSSG) in lung tissue and in BAL fluid. One hour after instillation of cigarette smoke condensate there was a marked fall in the GSH content in the lung (from 809.8 (31.8) to 501.7 (40.5) nmol/g) in association with increased GSSG levels (from 89.8 (2.7) to 148.7 (48.8) nmol/g). This was followed by a return of GSH levels to control values, with a concomitant decrease in GSSG levels six hours after instillation. GSH levels in BAL fluid fell dramatically following cigarette smoke condensate (from 2.56 (0.30) to 0.31 (0.21) nmol/ml) and this fall was sustained up to six hours after instillation of cigarette smoke condensate. CONCLUSIONS: These studies suggest that neutrophils and TNF do not have a major role in the increased epithelial permeability induced by cigarette smoke condensate. However, the data support a role for the depletion of the antioxidant glutathione in the increased epithelial permeability caused by cigarette smoke condensate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhalla D. K., Daniels D. S., Luu N. T. Attenuation of ozone-induced airway permeability in rats by pretreatment with cyclophosphamide, FPL 55712, and indomethacin. Am J Respir Cell Mol Biol. 1992 Jul;7(1):73–80. doi: 10.1165/ajrcmb/7.1.73. [DOI] [PubMed] [Google Scholar]
- Burns A. R., Hosford S. P., Dunn L. A., Walker D. C., Hogg J. C. Respiratory epithelial permeability after cigarette smoke exposure in guinea pigs. J Appl Physiol (1985) 1989 May;66(5):2109–2116. doi: 10.1152/jappl.1989.66.5.2109. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987 Jul;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Church D. F., Pryor W. A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985 Dec;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell O., Hamid Q., Corrigan C. J., Barkans J., Meng Q., Collins P. D., Kay A. B. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992 Nov;77(3):330–337. [PMC free article] [PubMed] [Google Scholar]
- Dubar V., Gosset P., Aerts C., Voisin C., Wallaert B., Tonnel A. B. In vitro acute effects of tobacco smoke on tumor necrosis factor alpha and interleukin-6 production by alveolar macrophages. Exp Lung Res. 1993 May-Jun;19(3):345–359. doi: 10.3109/01902149309064351. [DOI] [PubMed] [Google Scholar]
- Dusser D. J., Minty B. D., Collignon M. A., Hinge D., Barritault L. G., Huchon G. J. Regional respiratory clearance of aerosolized 99mTc-DTPA: posture and smoking effects. J Appl Physiol (1985) 1986 Jun;60(6):2000–2006. doi: 10.1152/jappl.1986.60.6.2000. [DOI] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Gordon R. E., Solano D., Kleinerman J. Tight junction alterations of respiratory epithelium following long-term NO2 exposure and recovery. Exp Lung Res. 1986;11(3):179–193. doi: 10.3109/01902148609064295. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Heffner J. E., Repine J. E. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989 Aug;140(2):531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Hogg J. C. The effect of smoking on airway permeability. Chest. 1983 Jan;83(1):1–2. doi: 10.1378/chest.83.1.1. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Niewoehner D. E. Pathogenesis of emphysema. Chest. 1983 Apr;83(4):679–685. doi: 10.1378/chest.83.4.679. [DOI] [PubMed] [Google Scholar]
- Hulbert W. C., Walker D. C., Jackson A., Hogg J. C. Airway permeability to horseradish peroxidase in guinea pigs: the repair phase after injury by cigarette smoke. Am Rev Respir Dis. 1981 Mar;123(3):320–326. doi: 10.1164/arrd.1981.123.3.320. [DOI] [PubMed] [Google Scholar]
- Jones J. G., Minty B. D., Lawler P., Hulands G., Crawley J. C., Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980 Jan 12;1(8159):66–68. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Kleeberger S. R., Hudak B. B. Acute ozone-induced change in airway permeability: role of infiltrating leukocytes. J Appl Physiol (1985) 1992 Feb;72(2):670–676. doi: 10.1152/jappl.1992.72.2.670. [DOI] [PubMed] [Google Scholar]
- Lannan S., Donaldson K., Brown D., MacNee W. Effect of cigarette smoke and its condensates on alveolar epithelial cell injury in vitro. Am J Physiol. 1994 Jan;266(1 Pt 1):L92–100. doi: 10.1152/ajplung.1994.266.1.L92. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Donaldson K., Rahman I., MacNee W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. Am J Respir Crit Care Med. 1994 Jun;149(6):1518–1525. doi: 10.1164/ajrccm.149.6.8004308. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Van Epps D. E. In vivo neutrophil emigration in response to interleukin-1 and tumor necrosis factor-alpha. J Leukoc Biol. 1989 Jan;45(1):62–68. doi: 10.1002/jlb.45.1.62. [DOI] [PubMed] [Google Scholar]
- McCusker K., Hoidal J. Selective increase of antioxidant enzyme activity in the alveolar macrophages from cigarette smokers and smoke-exposed hamsters. Am Rev Respir Dis. 1990 Mar;141(3):678–682. doi: 10.1164/ajrccm/141.3.678. [DOI] [PubMed] [Google Scholar]
- Smith L. J., Anderson J. Oxygen-induced lung damage. Relationship to lung mitochondrial glutathione levels. Am Rev Respir Dis. 1992 Dec;146(6):1452–1457. doi: 10.1164/ajrccm/146.6.1452. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Lukacs N. W., Standiford T. J., Kunkel S. L. Cytokines. 2. Cytokines and lung inflammation: mechanisms of neutrophil recruitment to the lung. Thorax. 1993 Jul;48(7):765–769. doi: 10.1136/thx.48.7.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Voisin C., Aerts C., Fournier E., Firlik M. Acute effects of tobacco smoke on alveolar macrophages cultured in gas phase. Eur J Respir Dis Suppl. 1985;139:76–81. [PubMed] [Google Scholar]



