Abstract
The molecular mechanisms underlying the ability of axons to regenerate after injury remain poorly understood. Here we show that in Caenorhabditis elegans, axotomy induces ectopic expression of serotonin (5-HT) in axotomized non-serotonergic neurons via HIF-1, a hypoxia-inducible transcription factor, and that 5-HT subsequently promotes axon regeneration by autocrine signalling through the SER-7 5-HT receptor. Furthermore, we identify the rhgf-1 and rga-5 genes, encoding homologues of RhoGEF and RhoGAP, respectively, as regulators of axon regeneration. We demonstrate that one pathway initiated by SER-7 acts upstream of the C. elegans RhoA homolog RHO-1 in neuron regeneration, which functions via G12α and RHGF-1. In this pathway, RHO-1 inhibits diacylglycerol kinase, resulting in an increase in diacylglycerol. SER-7 also promotes axon regeneration by activating the cyclic AMP (cAMP) signalling pathway. Thus, HIF-1-mediated activation of 5-HT signalling promotes axon regeneration by activating both the RhoA and cAMP pathways.
 The molecular mechanism of axon regeneration after injury is poorly understood. Here the authors show that in C. elegans, the HIF-1 transcription factor induces ectopic synthesis of serotonin in severed ‘non-serotonergic' neurons and that serotonin activates downstream signaling pathways leading to axon regeneration.
The molecular mechanism of axon regeneration after injury is poorly understood. Here the authors show that in C. elegans, the HIF-1 transcription factor induces ectopic synthesis of serotonin in severed ‘non-serotonergic' neurons and that serotonin activates downstream signaling pathways leading to axon regeneration.
An axon's ability to regenerate after injury depends on both its intrinsic growth capacity and the external environment. Most invertebrate neurons, as well as neurons in the mammalian peripheral nervous system are able to regenerate. Although intrinsic regeneration signals can influence regenerative success, the variables determining a neuron's intrinsic regrowth capacity remain poorly defined at the molecular level. The nematode Caenorhabditis elegans has recently emerged as a tractable genetic model for studying neuron regenerative processes1,2. Two recent screens have identified candidate genes that may be involved in axon regeneration: Chen et al.3 screened a collection of mutants of C. elegans, and Nix et al.4 screened using an RNA interference (RNAi) library. The candidate genes identified include those encoding both growth-promoting and growth-inhibiting factors. Several of these genes have been implicated previously in regeneration and others define new and conserved pathways of interest.
In the present study, we investigate the roles of the tph-1 and rga-5 genes in the regulation of axon regeneration. The tph-1 and rga-5 genes were identified as candidate genes in a screen of mutants and by an RNAi screen, respectively3,4. The tph-1 gene encodes an enzyme involved in 5-HT biosynthesis5, while the rga-5 gene encodes a homolog of the mammalian p190 Rho GTPase-activating protein. We show that the hypoxia-inducible transcription factor (HIF-1) induces expression of the tph-1 gene in axotomized neurons, which are not serotonergic. 5-HT subsequently promotes axon regeneration by signalling through the serotonin receptor SER-7. We further demonstrate that the RhoA signalling pathway is important for neuronal regeneration via its ability to inhibit diacylglycerol (DAG) kinase, thus increasing DAG concentrations. These two effects are linked, as C. elegans RhoA is activated by SER-7 and by G12α through RhoGEF encoded by the rhgf-1 gene. SER-7 is also required for axon regeneration via its ability to activate the cyclic AMP (cAMP) signalling pathway. Thus, HIF-1-mediated activation of 5-HT synthesis and consequent autocrine signalling promotes the neuron regenerative response by activating two separate signalling pathways.
Results
5-HT is required for axon regeneration
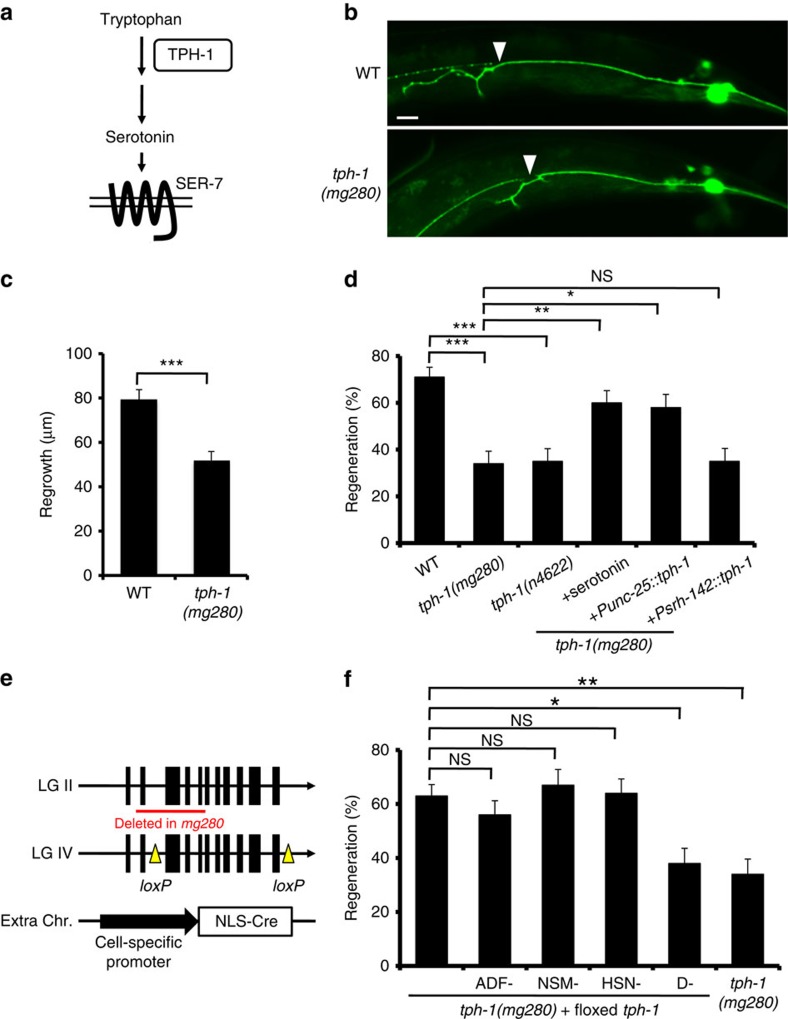
It has been suggested that axon injury may represent a type of cellular stress. In response to specific types of stress, neurotransmitters are deployed to effect the appropriate physiological and behavioural modifications6,7,8. In C. elegans, 5-HT modulates a variety of stress responses9,10. However, little is known about how specific types of stress induce 5-HT-mediated signal transduction in the nervous system. Interestingly, Chen et al.3 recently carried out a systematic screen of mutants looking for defects in axon regeneration and identified tph-1 among several genes that positively regulate axon regeneration in a glutaminergic touch sensory neuron3. The tph-1 gene encodes tryptophan hydroxylase5, which is required for 5-HT biosynthesis (Fig. 1a). We confirmed that a loss-of-function mutation (mg280) of tph-1 caused a defect in axon regeneration in touch sensory posterior lateral microtubule (PLM) neurons (Fig. 1b,c and Supplementary Table 1). Furthermore, the mg280 and n4622 mutations of tph-1 were also defective in axon regeneration in GABA-releasing D-type motor neurons (Fig. 1d and Supplementary Table 2). Exogenous 5-HT treatment restored axon regeneration in tph-1 mutants. These results suggest that 5-HT is generally required during axon regeneration.
Figure 1. Serotonin activates axon regeneration.
(a) The synthesis of serotonin from tryptophan catalyzed by TPH-1. (b) Representative PLM sensory neurons in wild type and tph-1 mutant animals 24 h after laser surgery. Arrowheads indicate cut sites. Scale bar=20 μm. (c) Regeneration of PLM sensory neurons. Lengths of PLM regrowth at 24 h after laser surgery are shown. Error bars indicate s.e.m. ***P<0.001 (n≥30; unpaired t-test, two-tailed and unequal variances). (d,f) Regeneration of D-type motor neurons. Percentages of axons that initiated regeneration 24 h after laser surgery are shown. Error bars indicate 95% CI. ***P<0.001; **P<0.01; *P<0.05; NS, not significant (Fisher's exact test, two-tailed, n≥50). (e) Cell-specific deletion of tph-1 using a Cre-loxP recombination system. CI, confidence interval.
We next searched for the 5-HT-producing neurons essential for axon regeneration. To match serotonin levels to the normal range for each neuron, we used a system recently developed by Flavell et al.11 A single floxed copy of the tph-1 gene, including the promoter, exons and introns, was inserted into a defined location on chromosome IV (Fig. 1e). This inserted gene was able to rescue the tph-1 defect in axon regeneration of D-type motor neurons (Fig. 1f and Supplementary Table 2). This was combined with cell-specific transgenes expressing Cre recombinase in amphid sensory neuron, dual cilia, designation F. (ADF), neurosecretory motor neuron (NSM) or hermaphrodite specific neuron (HSN) neurons, which are known to be 5-HT-producing neurons5, or in D-type neurons. This system allowed the excision of the inserted tph-1 gene and elimination of 5-HT production in these individual neurons. We found that the tph-1 defect was still rescued when Cre was expressed in ADF, NSM or HSN neurons (Fig. 1f and Supplementary Table 2). In contrast, the tph-1 defect was not rescued when Cre was expressed in D-type motor neurons. Furthermore, expression of tph-1 in ADF neurons by the srh-142 promoter was unable to rescue the tph-1 defect (Fig. 1d and Supplementary Table 2). These results suggest that axon regeneration in D-type motor neurons requires 5-HT production by D neurons. To confirm this, we ectopically expressed tph-1 in D-type motor neurons in a tph-1(mg280) background using the unc-25 promoter. We found that expression of tph-1 in D neurons did indeed suppress the defect in axon regeneration of tph-1 mutants (Fig. 1d and Supplementary Table 2). These results demonstrate that TPH-1 acts to promote regeneration of the damaged neuron in a cell-autonomous manner.
HIF-1 upregulates 5-HT expression in injured neurons
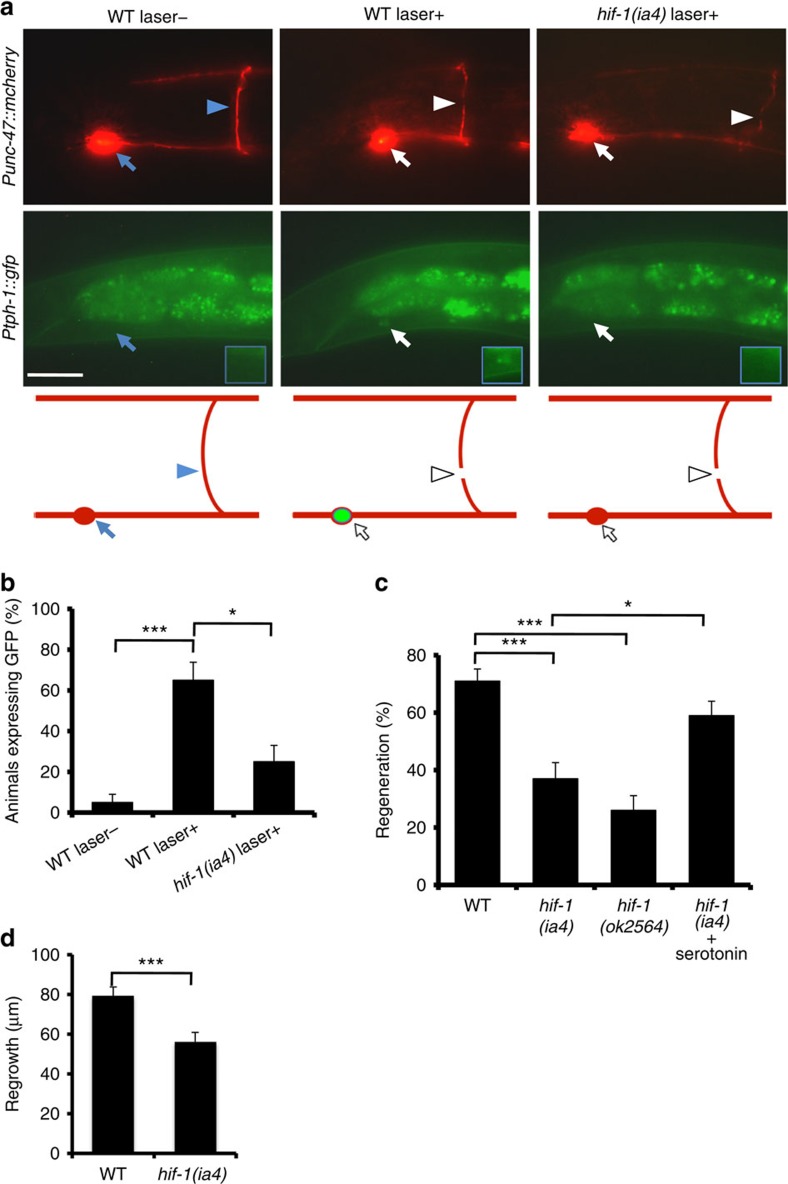
The identification of D-type neurons as the 5-HT-producing neurons essential for axon regeneration was unexpected, as D neurons are known to be GABAergic and the expression of 5-HT synthesis enzymes in these neurons has not been found5,12,13,14. We therefore addressed the possibility that axon severing might induce transcription of the tph-1 gene in D-type neurons. To test this, we monitored expression of the Ptph-1::gfp transgene, which consists of the tph-1 promoter driving the fluorescent protein green fluorescent protein (GFP). We found that expression of tph-1 was altered in D-type neurons by axon injury. In control animals, Ptph-1::gfp was robustly expressed in the head of animals in the NSM and ADF neurons (Supplementary Fig. 1), and was not expressed in D neurons. However, after laser surgery of D-type neurons, Ptph-1::gfp expression was strongly upregulated in these neurons (Fig. 2a,b). This expression became visible at 30 min after surgery, and returned to undetectable levels by 24 h. Thus, axon injury induces the transient expression of Ptph-1::gfp in severed D-type neurons. 5-HT immunofluorescence staining of animals treated with laser surgery also revealed induction of 5-HT expression, compared with control animals (Supplementary Fig. 2). Expression of Punc-47::mcherry, which reflects the expression of vesicular GABA transporter, was constant over this time period (Fig. 2a). Therefore, the severed GABAergic neurons still keep the GABAergic characteristics, and do not transdifferentiate into serotonergic, non-GABAergic neurons. Furthermore, axon severing also induced 5-HT and Ptph-1::gfp expression in PLM neurons (Supplementary Figs 2 and 3), which are glutamatergic, non-serotonergic touch sensory neurons15. Our results demonstrate that axotomized ‘non-serotonergic' neurons temporarily gain the ability to synthesize serotonin in response to axon injury.
Figure 2. HIF-1 positively regulates axon regeneration.
(a) Induction of Ptph-1::gfp expression in D-type motor neurons by laser surgery. Expression of fluorescent proteins in D-type motor neurons 30 min after laser surgery is shown. A schematic representation of D-type motor neurons is shown on the bottom. Arrowheads and arrows indicate axons and cell bodies of D-type neurons with (white) or without (blue) laser surgery, respectively. D-type neurons are visualized by mCherry under control of the unc-47 promoter. Cell bodies of D-type neurons are magnified (blue boxes). Scale bar=20 μm. (b) Percentages of animals expressing tph-1. Induction of Ptph-1::gfp expression in D-type motor neurons with (+) or without (−) laser surgery was assayed as described in Methods section. Twenty neurons were examined for each condition. Error bars indicate 95% CI. ***P<0.001; *P<0.05 (Fisher's exact test, two-tailed, n=20). (c) Regeneration of D-type motor neurons. Percentages of axons that initiated regeneration 24 h after laser surgery are shown. Error bars indicate 95% CI. ***P<0.001; *P<0.05 (Fisher's exact test, two-tailed, n≥50). (d) Regeneration of PLM sensory motor neurons. Lengths of PLM regrowth at 24 h after laser surgery are shown. Error bars indicate s.e.m. ***P<0.001 (n≥30; unpaired t-test, two-tailed, unequal variances). CI, confidence interval.
What determines expression of the ectopic tph-1 gene in response to axon injury? Interestingly, the promoter region of the tph-1 gene contains hypoxia response elements, which are binding sites for HIF-1 (ref. 16). The HIF-1 protein is a transcription factor containing a bHLH-PAS domain that mediates transcriptional responses to hypoxic stress17. Indeed, it has been demonstrated that HIF-1 binds to the tph-1 promoter via hypoxia response elements to drive serotonin production under hypoxic conditions in C. elegans16. We therefore examined whether HIF-1 is involved in tph-1 expression in response to axon injury. The hif-1(ia4) mutation had no effect on expression of Ptph-1::gfp in the head of animals (Supplementary Fig. 1) but decreased its induction in response to laser surgery in D-type neurons (Fig. 2a,b), indicating that induction is dependent on HIF-1 function. In addition, expression of Ptph-1::gfp in PLM neurons following laser surgery decreased in hif-1 mutants (Supplementary Fig. 3). Thus, HIF-1 is required for induction of 5-HT expression in injured neurons in response to axon injury.
These results raised the possibility that hypoxic stress might enhance axon regeneration. In normoxic conditions, HIF-1 is degraded through the E3 ubiquitin ligase VHL-1 (von Hippel–Lindau tumour suppressor protein)-dependent degradation pathway18. Degradation is initiated through hydroxylation of a specific proline residue in HIF-1 by the oxygen-dependent prolyl 4-hydroxylase EGL-9 (ref. 18). VHL-1 recognizes the hydroxylated proline and targets HIF-1 for ubiquitin-mediated proteasomal degradation19. Hypoxic conditions inhibit the hydroxylation of HIF-1, stabilizing the protein and allowing it to transcriptionally regulate expression of its target genes19,20. Thus, stabilization of HIF-1 through removal of the HIF-1 degradatory pathway phenocopies the effects of hypoxia seen in neurons. We examined the effect of HIF-1 stabilization on tph-1 expression in D-type motor neurons. For this purpose, we used vhl-1(ok161) mutants and a mutated form of HIF-1, HIF-1(P621A), in which the proline in the conserved LXXLAP motif required for degradation of HIF-1 has been altered to an alanine18,21. As observed previously16, the vhl-1 mutation or expression of HIF-1(P621A) caused constitutive expression of tph-1 in glutamatergic sensory amphid sensory neuron, single cilia, designation G. (ASG) neurons under normoxic conditions (Supplementary Figs 1 and 4). Normally, tph-1 is only weakly expressed under normoxic conditions in these neurons. However, we found that the vhl-1 mutation or expression of HIF-1(P621A) failed to induce Ptph-1::gfp expression constitutively in D neurons (Supplementary Fig. 4). These results suggest that the mechanism that activates HIF-1 in response to axon injury is different from the HIF-1-stabilizing mechanism that is involved in the response to hypoxia.
Next, we examined whether HIF-1 is required for 5-HT-driven axon regeneration. We found that the frequency of axon regeneration in D neurons was reduced significantly, in hif-1(ia4) and hif-1(ok2564) mutants, and was restored by exogenous 5-HT treatment (Fig. 2c and Supplementary Table 2). This is consistent with the possibility that HIF-1 regulates axon regeneration by driving 5-HT production. Furthermore, the hif-1(ia4) mutation was also defective in axon regeneration in PLM neurons following laser surgery (Fig. 2d and Supplementary Table 1). These results suggest that HIF-1 is generally required during axon regeneration.
SER-7 activates axon regeneration via GPA-12 and RHGF-1
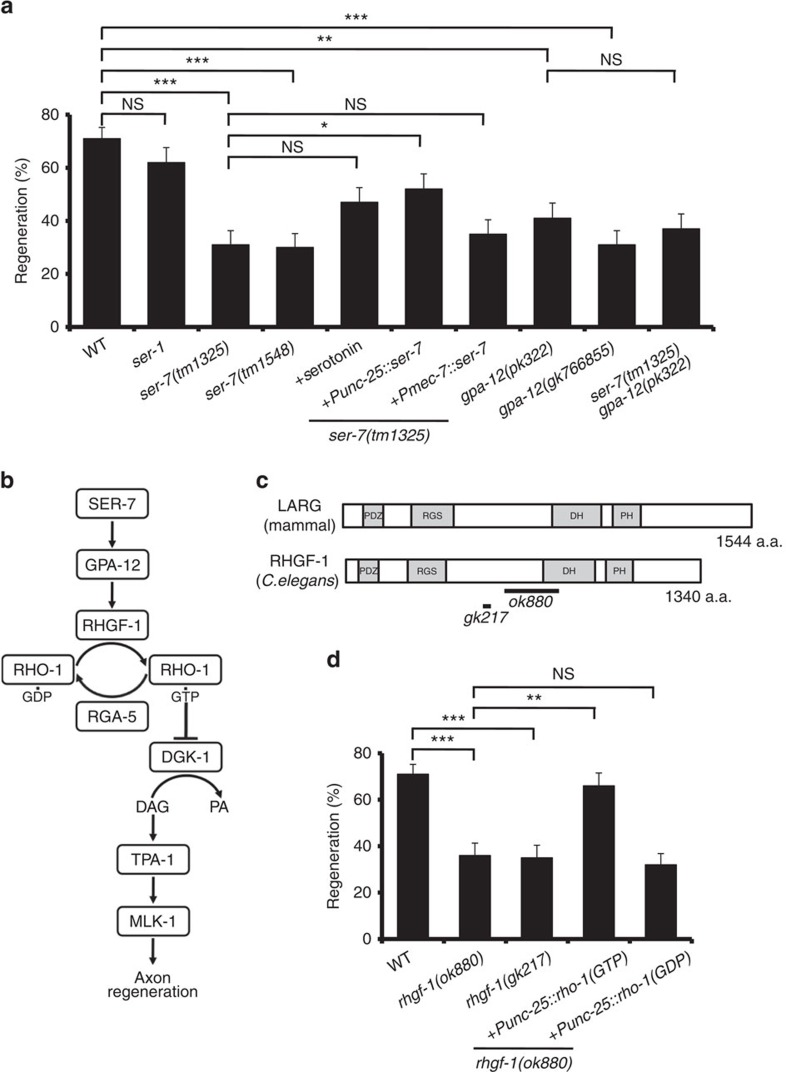
The biological effects of 5-HT are mediated through its interactions with G protein-coupled receptors22. Since SER-7 is one of the 5-HT receptors, we examined whether SER-7 participates in axon regeneration. We found that two mutations (tm1325 and tm1548) in the ser-7 gene caused defects in axon regeneration (Fig. 3a and Supplementary Table 2). In contrast to ser-7, the ok345 null mutation of ser-1 encoding another serotonin receptor had no effect on axon regeneration. Thus, SER-7 plays a specific role in axon regeneration. To test whether SER-7 can act in a cell-autonomous manner, we expressed ser-7 from the unc-25 or mec-7 promoters in ser-7(tm1325) mutants. The ser-7 defect in axon regeneration was rescued by expression of ser-7 in D-type motor neurons by the unc-25 promoter but not by expression in sensory neurons by the mec-7 promoter (Fig. 3a and Supplementary Table 2). These results demonstrate that SER-7 acts to promote regeneration in a cell-autonomous manner in the damaged neuron. Exogenous 5-HT treatment was unable to efficiently suppress the ser-7 defect (Fig. 3a and Supplementary Table 2).
Figure 3. 5-HT-SER-7 signalling activates axon regeneration via GPA-12 and RHGF-1.
(a,d) Percentages of axons that initiated regeneration 24 h after laser surgery. Error bars indicate 95% CI. ***P<0.001; **P<0.01; *P<0.05; NS, not significant (Fisher's exact test, two-tailed, n≥50). (b) SER-7 signalling pathway. SER-7 activates the GPA-12-RHGF-1-RHO-1 pathway. Activation of RHO-1 inhibits DGK-1, leading to a stabilization of DAG levels. (c) Structure of RHGF-1. Schematic diagrams of RHGF-1 and its human counterpart are shown. Domains are shown as follows: a PDZ domain, a regulator of G protein signalling domain (RGS), a Dbl homology domain (DH) and a Pleckstrin homology domain (PH). The bold lines underneath indicate the extent of the deleted regions in the rhgf-1(ok880) and rhgf-1(gk217) mutants.
What functions downstream of SER-7 to regulate axon regeneration? SER-7 is coupled to GPA-12, a C. elegans homologue of the α subunit of the heterotrimeric G12 protein (Fig. 3b)23. We found that the gpa-12(pk322) and gpa-12(gk766855) mutations significantly reduced axon regeneration after axon injury (Fig. 3a and Supplementary Table 2). To further address whether ser-7 and gpa-12 function in the same pathway, we constructed ser-7(tm1325); gpa-12(pk322) double mutants. We found that the gpa-12 mutation did not enhance the axon regeneration defect of ser-7 mutants (Fig. 3a and Supplementary Table 2), suggesting that SER-7 and GPA-12 function in the same pathway.
In mammals, the RhoGEF LARG protein specifically interacts with G12α via its regulator of G protein signalling (RGS) domain24. C. elegans RHGF-1, a homolog of mammalian LARG (Fig. 3c), binds to GPA-12 and acts as a regulator of neurotransmitter release at a subset of cholinergic motor synapses25,26. These observations raised the possibility that RHGF-1 may act downstream of GPA-12 in the axon regeneration pathway (Fig. 3b). Indeed, we found that the rhgf-1(ok880) and rhgf-1(gk217) mutants were defective in axon regeneration (Fig. 3d and Supplementary Table 2). Taken together, these results suggest that SER-7 activates axon regeneration through GPA-12 and RHGF-1.
RHO-1 activates axon regeneration by inhibiting DGK-1
The activity of Rho proteins is modulated by their cycling between the active GTP- and inactive GDP-bound states. Rho activity is further regulated by other proteins, including GAPs, which enhance GTP hydrolysis and the consequent inactivation of Rho, and guanine nucleotide exchange factors (GEFs), which promote the binding of GTP and, thus, activation of Rho27. RHO-1 also cycles between the GTP-bound active and the GDP-bound inactive forms. To confirm that RHGF-1 acts as a GEF for RHO-1 and to test if it thereby controls axon regeneration, we expressed a mutant RHO-1 that is locked in the GTP-bound state, RHO-1(G14V), in D-type motor neurons using the unc-25 promoter. We found that expression of RHO-1(G14V) was able to suppress the rhgf-1 defect in axon regeneration, whereas a GDP-bound RHO-1(T19N) expressed from the unc-25 promoter was not (Fig. 3d and Supplementary Table 2).
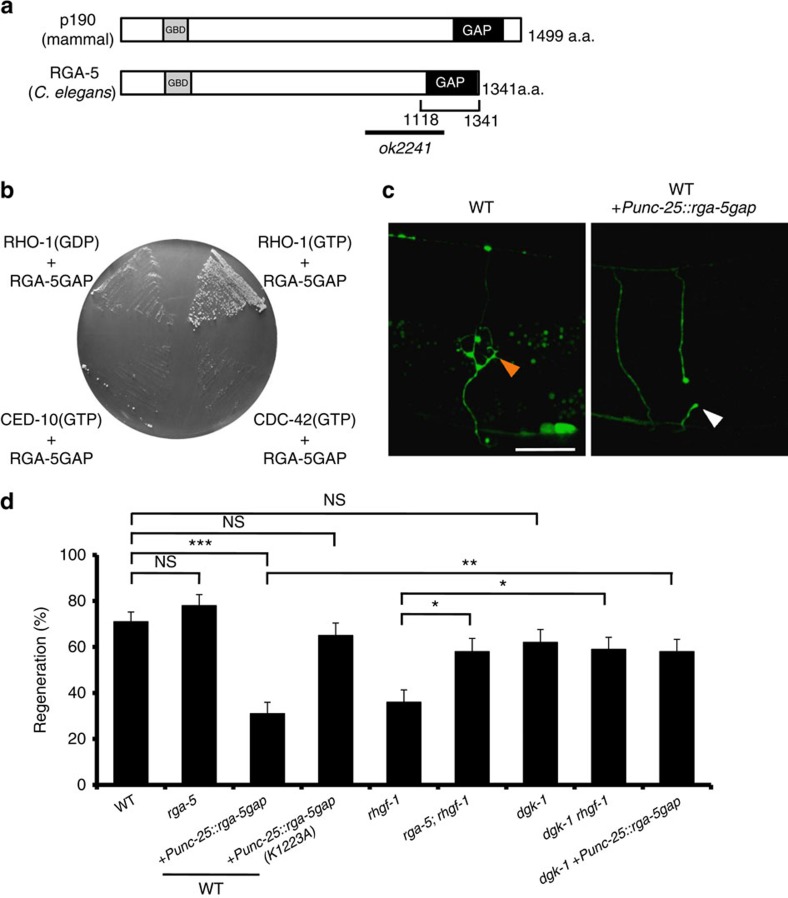
What functions as a RhoGAP in the regulation of axon regeneration? One of the genes identified in our previous RNAi screen in β-spectrin mutant animals (unc-70 ; ref. 28) was rga-5, the product of which belongs to the Rho GTPase-activating protein (GAP) family4. RGA-5 has an N-terminal GTP binding domain (GBD) and a C-terminal GAP domain. RGA-5 displays strong homology across its GBD and GAP domains to the mammalian p190 RhoGAP (Fig. 4a and Supplementary Fig. 5). Since the GAP domain in p190 RhoGAP is specific for the Rho family of GTPases, particularly RhoA (ref. 29), we expected that RGA-5 would specifically act on RHO-1. To test this prediction, we performed yeast two-hybrid binding assays to test for interactions between RGA-5 and two RHO-1 variants that are locked in the GTP- and GDP-bound states (G14V and T19N, respectively). We found that the RGA-5 GAP domain (1118–1341 amino acids) specifically interacted with the GTP bound, but not GDP-bound version of RHO-1 (Fig. 4b), consistent with the property of GAP proteins to associate with the GTP-bound version of GTPases. The RGA-5 GAP domain failed to bind to GTP-bound CDC-42 or CED-10, two other Rho/Rac family members, demonstrating that the RGA-5-RHO-1 interaction is specific.
Figure 4. Effect of RhoGAP on axon regeneration.
(a) Structure of RGA-5. Schematic diagrams of RGA-5 and its human counterpart are shown. Domains are shown as follows: a GTP binding domain (GBD) and a Rho GAP domain (GAP). The bold line underneath indicates the extent of the deleted region in the rga-5(ok2241) mutant. (b) A two-hybrid assay for the interaction of RGA-5 with RHO-1. The reporter strain PJ69-4A was co-transformed with expression vectors encoding GAL4 DBD-RHO-1 (T19N) (GDP), GAL4 DBD-RHO-1 (G14V) (GTP), GAL4 DBD-CED-10 (G12V) (GTP), GAL4 DBD-CDC-42 (G12V) (GTP) and GAL4 AD-RGA-5 (GAP domain) as indicated. (c) Representative D-type motor neurons in wild-type animals and wild-type animals overexpressing RGA-5 GAP domain 24 h after laser surgery. In wild-type animals, severed axons have regenerated growth cones (orange arrowhead). In wild-type animals overexpressing the RGA-5 GAP domain, the proximal end of axon failed to regenerate (white arrowhead). Scale bar=20 μm. (d) Percentages of axons that initiated regeneration 24 h after laser surgery. Error bars indicate 95% CI. ***P<0.001; **P<0.01; *P<0.05; NS, not significant (Fisher's exact test, two-tailed, n≥50). CI, confidence interval.
Next, we determined the function of RGA-5 in axon regeneration. First, we examined the rga-5(ok2241) mutation, which deletes the GAP domain in RGA-5 (Fig. 4a). After laser surgery, axons in rga-5(ok2241) mutant animals regenerated normally. However, overexpression of the RGA-5 GAP domain reduced regeneration (Fig. 4c,d and Supplementary Table 2). It has been confirmed that the GAP domain of p190 RhoGAP possesses GAP activity and that the conserved Lys-1311 (Lys-1223 in RGA-5) residue is essential for this activity (Supplementary Fig. 5b)29,30. Overexpression of the mutant GAP(K1223A), in which the Lys-1223 in the RGA-5 GAP domain was mutated to alanine, did not cause reduced regeneration (Fig. 4d and Supplementary Table 2). These results suggest that the RGA-5 GAP domain stimulates the conversion of RHO-1 to the GDP-bound inactive form, resulting in the inhibition of axon regeneration. In this manner, RGA-5 acts as a potent inhibitor of axon regeneration. We further examined possible genetic interactions between rga-5 and rhgf-1, and found that the rga-5(ok2241) mutation was able to suppress the rhgf-1(ok880) defect (Fig. 4d and Supplementary Table 2). This can be explained because RHO-1 can still be weakly activated in the absence of its GEF, thus concomitant loss of GAP affects the phenotype.
How does RHO-1 regulate axon regeneration? Mammalian RhoA has been shown to bind and inhibit the DAG kinase DGKθ (refs 31, 32). DAG kinases remove DAG by phosphorylating the membrane-bound secondary messenger DAG, converting it to phosphatidic acid33,34, which implicates RhoA in regulating DAG levels31. In addition, C. elegans RHO-1 is able to bind to DGK-1, the C. elegans ortholog of DGKθ (ref. 35). Thus, DGK-1 is a possible target of negative regulation by RHO-1 (Fig. 3b). If RHO-1 modulates axon regeneration through the inhibition of DGK-1, then we would predict that a dgk-1 loss-of-function mutation should suppress the regeneration defect by inactivation of RHO-1. As expected, we found that the dgk-1(ok1462) null mutation suppressed the defect caused by the rhgf-1(ok880) deletion mutation or overexpression of the RGA-5 GAP domain (Fig. 4d and Supplementary Table 2). Our results are most consistent with a signal transduction cascade involving activation of RHO-1, inhibition of DGK-1, and a consequent increase in DAG (Fig. 3b).
SER-7 promotes axon regeneration via the cAMP pathway
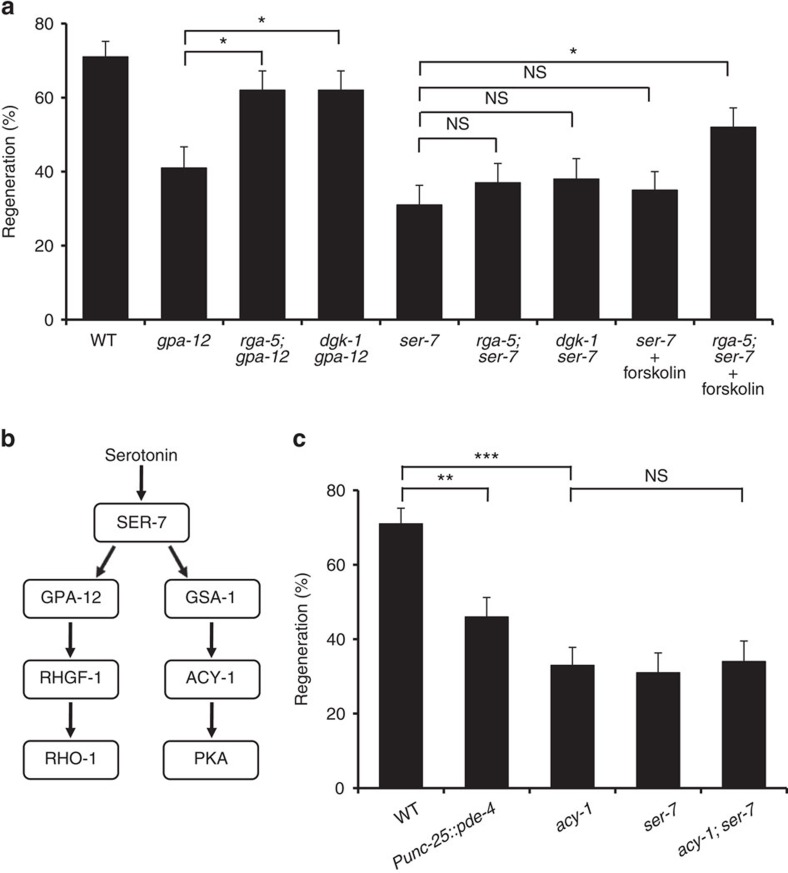
If the SER-7-GPA-12 pathway acts upstream of RHO-1 to promote axon regeneration, an rga-5 or dgk-1 mutation should suppress the defect in axon regeneration seen in ser-7 and gpa-12 mutants. As expected, the rga-5(ok2241) and dgk-1(ok1462) mutations were each able to suppress the gpa-12 defect (Fig. 5a and Supplementary Table 2). However, in contrast to the gpa-12 mutation, the ser-7 defect was not suppressed by the rga-5(ok2241) or dgk-1(ok1462) mutation (Fig. 5a and Supplementary Table 2). These results suggest that another regulatory pathway exists downstream of SER-7, in addition to the GPA-12-RHO-1 pathway, that is required for axon regeneration.
Figure 5. SER-7 promotes axon regeneration via the cAMP pathway.
(a,c) Percentages of axons that initiated regeneration 24 h after laser surgery. Error bars indicate 95% CI. ***P<0.001; **P<0.01; *P<0.05; NS, not significant (Fisher's exact test, two-tailed, n≥50). (b) SER-7 signalling pathways. SER-7 activates both GPA-12-RHGF-1-RHO-1 and GSA-1-ACY-1-PKA pathways.
Since the SER-7 5-HT receptor has also been reported to be coupled to GSA-1 Gsα (Fig. 5b)23,36, we hypothesized that an additional pathway of SER-7-mediated axon regeneration could involve the GSA-1 signalling pathway. Since Gsα is coupled to adenylyl cyclase, Gsα signalling can be monitored by intracellular cAMP levels. As observed previously37, axon regeneration was inhibited by overexpression of pde-4, which encodes a cAMP-phosphodiesterase and reduces intracellular cAMP levels (Fig. 5c and Supplementary Table 2). Furthermore, a loss-of-function mutation of acy-1, which encodes adenylyl cyclase, was defective in axon regeneration (Fig. 5c and Supplementary Table 2). These results suggest that activation of GSA-1 signalling is necessary to activate axon regeneration. To confirm that ser-7 and acy-1 function in the same pathway, we constructed ser-7(tm1325); acy-1(nu329) double mutants. The ser-7 mutation did not enhance the axon regeneration defect of acy-1 mutants (Fig. 5c and Supplementary Table 2), supporting the possibility that SER-7 functions in the GSA-1 signalling pathway to activate axon regeneration.
Since SER-7 activates both GPA-12 and GSA-1 signalling pathways (Fig. 5b)23, we speculated that activation of both pathways are required for axon regeneration. If so, activation of the GSA-1 signalling pathway should suppress the ser-7 defect in axon regeneration in a manner dependent on the activation of the GPA-12 signalling pathway. Treatment of animals with forskolin is expected to cause an increase in cAMP levels by activating adenylyl cyclase37,38. We found that forskolin treatment failed to suppress the single mutant ser-7(tm1325) defect in axon regeneration (Fig. 5a and Supplementary Table 2). However, forskolin treatment was able to suppress the axon regeneration defect in ser-7; rga-5 double mutants, in which the GPA-12 signalling pathway is activated (Fig. 5a and Supplementary Table 2). These results suggest that SER-7 activates GPA-12 and GSA-1, which act in concert to activate axon regeneration.
Discussion
The neurotransmitter 5-HT plays multiple roles in the enteric, peripheral and central nervous system, and its most prominent biological function is as a messenger transmitting signals from pre- to post-synaptic neurons. However, 5-HT has been reported to play other roles, such as the regulation of neurite growth39,40, suggesting that 5-HT also plays a prominent role in regulating the neuronal cytoarchitecture41,42. In C. elegans, 5-HT modulates cognitive behaviours such as chemosensation, learning and memory in addition to a variety of stress responses10,13,16. Here our results demonstrate that C. elegans 5-HT is synthesized in axotomized ‘non-serotonergic' neurons and is required for axon regeneration. This effect is likely due to a direct action of 5-HT on injured neurons through the SER-7 5-HT receptor, which activates axon regeneration in a cell-autonomous manner.
Neurons are known to be able to produce distinct sets of neurotransmitters43, and some studies have shown that certain stimuli can induce neurotransmitter switching44,45. In mammals, leukaemia inhibitory factor and ciliary neurotrophic factor have been shown to induce the differentiation of developing peripheral sympathetic neurons, which normally release noradrenaline, into cholinergic neurons46,47. Another study revealed that the balance of the two hippocampal granule cell neurotransmitters, glutamate and GABA, changes during post-embryonic development, as well as with seizures and long-term potentiation48. Recently, Dulcis et al.49 reported that the neurotransmitters produced by hypothalamic neurons switch between dopamine and somatostatin in response to photoperiod stimuli. Although these studies were largely carried out using populations of neuronal cells that could include different types of cells such as reserve pool neurons, they indicate that neurotransmitter production varies dynamically in neurons. Our findings in C. elegans demonstrate that a single, differentiated GABAergic D-type motor neuron or glutamatergic PLM sensory neuron that is never serotonergic under standard laboratory conditions can be induced to produce 5-HT as a result of axotomy in a manner dependent on HIF-1. This represents a novel case in which a switch in neurotransmitter expression can be regulated. Hypoxia also stimulates expression of 5-HT via HIF-1 in glutamatergic ASG neurons16,50. However, stabilization of HIF-1, which phenocopies the hypoxia-induced neuronal effects, is sufficient to induce tph-1 expression in ASG neurons, but not in D or PLM neurons. Therefore, HIF-1-dependent tph-1 expression in severed neurons is regulated by a mechanism different from the HIF-1-stabilizing mechanism.
In mammals, the expression and stability of HIF-1, as well as the expression of its target genes, are all increased after spinal cord injury51,52. Thus, activation of HIF-1 by axotomy may be conserved among animals. Furthermore, the HIF-induced upregulation of 5-HT in specific regions of the vertebrate brain53 suggests that the mechanisms coupling axon regeneration and 5-HT signalling to produce an alteration in neuronal responses may be conserved among animals. Therefore, it would be interesting to know whether axotomy of vertebrate neurons also stimulates expression of 5-HT or other neurotransmitters that influence regeneration. The inability of axons to regenerate in the adult mammalian central nervous system is a major problem in a number of diseases, and understanding the multi-functional roles of 5-HT signalling on neurite growth may lead to the development of novel neuroregenerative therapies by targeting serotonin-regulated signalling pathways.
The biological effects of 5-HT are mediated through interactions with G protein-coupled receptors22. Here we have shown that the activated SER-7 5-HT receptor promotes axon regeneration in injured neurons by activating G12α and Gsα signalling pathways in a cell-autonomous manner. How do G12α and Gsα signalling cascades activate axon regeneration? Several studies suggested that DAG and cAMP act downstream of the G12α and Gsα cascades, respectively54,55,56,57. In mammals, G12α activates an RGS-containing RhoGEF, which in turn activates RhoA. Several lines of evidence suggest that this pathway also acts within C. elegans motor neurons26. The axon regeneration pathway is regulated by both of these components acting in the G12α pathway, as either a mutation in rhgf-1 encoding the RGS-containing RhoGEF or inactivation of RHO-1 by RhoGAP RGA-5 inhibits axon regeneration. In addition, RHO-1 activation is able to suppress the defect in axon regeneration caused by the gpa-12 mutation. Thus, the genetic data support a model in which the G12α-RhoGEF-RhoA signalling pathway is conserved in the control of axon regeneration in C. elegans. Consistent with this, GPA-12 and RHGF-1 are both expressed in motor neurons and physically interact25. In C. elegans, activated RHO-1 causes an increase in DAG by directly binding to and inhibiting DGK-1 (ref. 35). High levels of DAG activate the PKC homolog TPA-1, leading to activation of the JNK pathway by phosphorylation of MLK-1 MAPKKK58. These results raise the possibility that the G12α pathway activates axon regeneration by inducing an increase in DAG, resulting in the activation of the JNK pathway.
The Gsα cascade involves the activation of adenylyl cyclase by Gsα, and the consequent increase in cAMP production leads to the activation of PKA. Our recent results suggest that in response to axon injury, PKA upregulates transcription of the svh-2 gene, which encodes a growth factor receptor functioning in the JNK pathway59,60. Thus, one effector of the Gsα pathway that stimulates axon regeneration is a transcription factor that activates transcription of the svh-2 gene, resulting in activation of the JNK pathway. However, the Gsα pathway likely has additional targets. Taken together, these results suggest that the G12α and Gsα pathways converge to activate the JNK pathway in axon regeneration.
Methods
C. elegans strains
C. elegans strains used in this study are listed in Supplementary Table 3. All strains were maintained on nematode growth medium (NGM) plates and fed with bacteria of the OP50 strain, as described previously61.
Plasmids
Punc-25::tph-1 was made by inserting the tph-1 cDNA isolated from a cDNA library by PCR into the pSC325 vector. Psrh-142::tph-1 was made by replacing an NLS-Cre cDNA fragment of Psrh-142::nls-cre plasmid11 (Gift from Dr Cori Bargmann) with a tph-1 cDNA fragment. Punc-25::nls-cre was generated by inserting NLS-Cre cDNA (Addgene) into the pSC325 vector. Punc-47::mcherry was generated by replacing the gfp DNA of the pOKU64 vector (Punc-47::gfp, a gift from Dr Ikue Mori) with the mCherry cDNA (DNA2.0). Punc-25::ser-7 and Pmec-7::ser-7 were made by inserting the ser-7 cDNA isolated from a cDNA library by PCR into the pSC325 and pPD52.102 vectors, respectively. Punc-25::rho-1 was generated by inserting the rho-1 cDNA, provided from Dr Kozo Kaibuchi (Nagoya University, JAPAN), into the pSC325 vector. Punc-25::rho-1 (G14V) and Punc-25::rho-1 (T19N) were made by oligonucleotide-directed PCR using Punc-25::rho-1 as a template and the mutations were verified by DNA sequencing. The Punc-25::rga-5gap plasmid contains the GAP domain of rga-5 cDNA isolated from a cDNA library by PCR using oligonucleotides 5′-gtcagcATGAAGAACAAAGTGCAACTCATGATC-3′ and 5′-ccatGGCCCGATAATTATCCACGTCATAG-3′, inserted into the pSC325 vector. The Punc-25::rga-5gap(K1223A) was made by oligonucleotide-directed PCR using Punc-25::rga-5gap as a template and the mutation was verified by DNA sequencing. Punc-25::pde-4 was generated by inserting the pde-4 cDNA isolated from a cDNA library into the pSC325 vector. The GAL4 DBD-RHO-1 (G14V), GAL4 DBD-RHO-1 (T19N), GAL4 DBD-CED-10 (G12V) and GAL4 DBD-CDC-42 (G12V) plasmids are also generous gifts from Dr Kozo Kaibuchi. The GAL4 AD-RGA-5 (GAP domain) plasmid was constructed by inserting the rga-5gap cDNA into the pACTII vector. The Pmyo-2::dsred-monomer plasmid has been described previously59.
Transgenic animals
Transgenic animals were obtained as described in ref. 62. Punc-25::rga-5gap (50 ng μl−1), Punc-25::rga-5gap(K1223A) (50 ng μl−1), Punc-25::rho-1 (G14V) (5 ng μl−1), Punc-25::rho-1 (T19N) (5 ng μl−1), Punc-25::ser-7 (50 ng μl−1), Pmec-7::ser-7 (5 ng μl−1), Punc-25::pde-4 (50 ng μl−1), Punc-25::tph-1 (50 ng μl−1), Psrh-142::tph-1 (50 ng μl−1), Punc-25::nls-cre (50 ng μl−1) and Punc-47::mcherry (100 ng μl−1) plasmids were used to generate kmEx1301 [Punc-25::rga-5gap+Pmyo-2::dsred-monomer], kmEx1312 [Punc-25::rga-5gap(K1223A)+Pmyo-2::dsred-monomer], kmEx1302 [Punc-25::rho-1 (G14V)+Pmyo-2::dsred-monomer], kmEx1303 [Punc-25::rho-1 (T19N)+Pmyo-2::dsred-monomer], kmEx1304 [Punc-25::ser-7+Pmyo-2::dsred-monomer], kmEx1305 [Pmec-7::ser-7+Pmyo-2::dsred-monomer], kmEx1306 [Punc-25::pde-4+Pmyo-2::dsred-monomer], kmEx1307 [Punc-25::tph-1+Pmyo-2::dsred-monomer], kmEx1311 [Psrh-142::tph-1+Pmyo-2::dsred-monomer], kmEx1308 [Punc-25::nls-cre+Pmyo-2::dsred-monomer] and kmEx1309 [Punc-47::mcherry+Pmyo-2::dsred-monomer], respectively. The kyEx4057 [Pceh-2::nls-cre], kyEx4077 [Psrh-142::nls-cre], kyEx4107 [Pegl-6::nls-cre] and kySi56 [floxed tph-1 genomic rescue] were generous gifts from Dr Cori Bargmann11. The otIs197, zdIs13, wpIs36 and wdEx848 transgenes63,64,65 were obtained from CGC.
Yeast two-hybrid assay
GAL4 DBD-RHO-1(G14V, T19N), GAL4 DBD-CED-10 (G12V), GAL4 DBD-CDC-42 (G12V) and GAL4 AD-RGA-5 (GAP domain) plasmids were co-transformed into the Saccharomyces cerevisiae reporter strain PJ69-4A and allowed to grow on SC-Leu-Trp plates. Transformants grown on these plates were then streaked out onto SC-Leu-Trp-His plates and incubated at 30 °C for 4 days.
Axotomy
All animals were subjected to axotomy at the young adult stage at 20 °C. Young adult hermaphrodites were immobilized with 20 mM sodium azide in M9 buffer on 2% agarose pad under a cover slip. D-type motor neurons expressing GFP were imaged with a fluorescence microscope. Selected commissural axons, mainly posterior D-type neurons, were cut using a 440-nm MicroPoint ablation Laser System from Photonic Instruments. After surgery, animals were allowed to recover on an agar plate and remounted for fluorescent imaging ∼24 h after surgery. For measurements of D neurons, imaged commissures that exhibited growth cones or small branches on the proximal fragment were counted as ‘regenerated'. The proximal fragments that showed no change after 24 h were counted as ‘no regeneration'. A minimum of 50 individuals with 1–3 axotomized commissures were observed for most experiments. For PLM touch neurons, extended process lengths were measured from maximum transparency projections of a single z-stack, by using the Keyence BZ-X700 analysis software.
Drug experiments
5-HT and forskolin were obtained from Wako and Focus Biomolecules, respectively. For 5-HT treatment, worms were grown from egg to adult, up until axotomy, on NGM plates containing 6.5 mM 5-HT and OP50 Escherichia coli. After axotomy, the worms were plated on NGM plates containing 5-HT and OP50 E. coli for 24 h. For forskolin treatment, the mixed stages of worms were incubated with M9 buffer containing 100 μm forskolin and heat-killed OP50 for 12 h at 20 °C, and the worms at young adult stage after the treatment were used.
Immunofluorescence
5-HT immunofluorescence was essentially performed as described previously5. Briefly, the worms axotomized either D neurons or PLM neurons were fixed by 4% formaldehyde in PBS buffer at 4 °C for overnight, washed three times by PBS and then treated by PBS containing 5% β-mercaptoethanol and 1% Triton X-100 for 24 h. Then, the worms were washed by PBS and digested in 100 mM Tris-HCl (pH7.5), 1 mM CaCl2 and 5 mg ml−1 collagenase (Wako, 034–10533). After digestion, a rat anti-serotonin antibody (Chemicon, Cat. No. MAB352), 100X diluted with Can Get Signal Immunostain (TOYOBO), was used as the first antibody solution. A chicken anti-rat Alexa 594 (Molecular probes), 200X diluted with Can Get Signal Immunostain (TOYOBO), was used as the second antibody solution.
Microscopy
Standard fluorescence images of transgenic worms were observed and photographed, using either a Zeiss Plan-APOCHROMAT × 100 objective of a Zeiss Axioplan II fluorescence microscope with a Hamamatsu 3CCD camera, or a × 100 objective of a Keyence BZ-X700 all-in-one fluorescence microscope. Confocal fluorescence images were taken on an Olympus FV500 and FV1000 confocal laser scanning microscopes with × 60 and × 100 objectives.
Quantification of GFP expression
Expression of GFP fluorescence was quantified using the ImageJ program (NIH). The cell bodies of severed D or PLM neurons were outlined with closed polygons and the fluorescence intensities within these areas were determined (Is). Then Is was divided by the area to derive intensity per area (Isa). To determine the background intensity of each cell, the same polygon was placed in an area neighbouring the cell body, fluorescence was measured (Ib), and this was divided by area (Iba). The relative signal intensity per area (Ira) was calculated as Isa−Iba. Cells having Ira>7 (for D neuron) or Ira>4 (for PLM neuron) were categorized as ‘expressed', respectively.
Statistical analysis
Statistical analyses were carried out as described previously58. Briefly, confidence intervals (95%) were calculated by the modified Wald method and two-tailed P values were calculated using Fisher's exact test (http://www.graphpad.com/quickcalcs/). The Welch's t -test was performed by using a t-test calculator (http://www.graphpad.com/quickcalcs/ttest1/).
Additional information
How to cite this article: Alam, T. et al. Axotomy-induced HIF-serotonin signalling axis promotes axon regeneration in C. elegans. Nat. Commun. 7:10388 doi: 10.1038/ncomms10388 (2016).
Supplementary Material
Supplementary Figures 1-5 and Supplementary Tables 1-3.
Acknowledgments
We thank Dr Cori Bargmann, Dr Kozo Kaibuchi, Dr Ikue Mori, Caenorhabditis Genetic Center (CGC), National Bio-Resource Project and C. elegans Knockout Consortium for materials. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD10440). This work was supported by grants from the Ministry of Education, Culture and Science of Japan (to K.M. and N.H.), as well as from the Naito Foundation (to N.H.).
Footnotes
Author contributions T.A., H.M., N.H. and K.M. designed the experiments and analyzed data. T.A., H.M., C.L. and S.P. performed genetic and biological experiments. P.N. and M.B. identified rga-5 and shared the information. T.A. and H.M. performed all of the other experiments. N.H. and K.M. wrote the manuscript.
References
- Yanik M. F. et al. Neurosurgery: functional regeneration after laser axotomy. Nature 432, 822 (2004). [DOI] [PubMed] [Google Scholar]
- Hammarlund M. & Jin Y. Axon regeneration in C. elegans. Curr. Opin. Neurobiol. 27, 199–207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. et al. Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron 71, 1043–1057 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix P. et al. Axon regeneration genes identified by RNAi screening in C. elegans. J. Neurosci. 34, 629–645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze J. Y., Victor M., Loer C., Shi Y. & Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403, 560–564 (2000). [DOI] [PubMed] [Google Scholar]
- de Kloet E. R., Joels M. & Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475 (2005). [DOI] [PubMed] [Google Scholar]
- McEwen B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007). [DOI] [PubMed] [Google Scholar]
- Joels M. & Baram T. Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 10, 459–466 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. J., Chronis D. S., Karow D. S., Marletta M. A. & Bargmann C. I. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 4, e274 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Moussaif M., Kuan C. J., Gargus J. J. & Sze J. Y. Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab. 4, 429–440 (2006). [DOI] [PubMed] [Google Scholar]
- Flavell S. W. et al. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154, 1023–1035 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand J. B. & Nonet M. L. Synaptic transmission in C. elegans II eds Riddle D. L.et al. 611–643Cold spring Harbor Laboratory Press (1997). [PubMed] [Google Scholar]
- Chase D. L. & Koelle M. R. Biogenic amine neurotransmitters in C. elegans. The C. elegans research community, WormBook, doi/10.1895/wormbook.1.132.1, http://www.wormbook.org (2007). [DOI] [PMC free article] [PubMed]
- Jorgensen E. M. GABA. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.14.1, http://www.wormbook.org (2005).
- Lee R. Y. N., Sawin E. R., Chalfie M., Horvitz H. R. & Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J. Neurosci. 19, 159–167 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock R. & Hobert O. Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nat. Neurosci. 13, 610–614 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Guo R. & Powell-Coffman J. A. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl Acad. Sci. USA 98, 7916–7921 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001). [DOI] [PubMed] [Google Scholar]
- Bishop T. et al. Genetic analysis of pathways regulated by the von Hippel-Lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol. 2, e289 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Nettleton D., Jiang M., Kim S. K. & Powell-Coffman J. A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 280, 20580–20588 (2005). [DOI] [PubMed] [Google Scholar]
- Pocock R. & Hobert O. Oxygen levels affect axon guidance and neuronal migration in Caenorhabditis elegans. Nat. Neurosci. 11, 894–900 (2008). [DOI] [PubMed] [Google Scholar]
- Hoyer D., Hannon J. P. & Martin G. R. Molecular, pharmacylogical and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 71, 533–554 (2002). [DOI] [PubMed] [Google Scholar]
- Song B. & Avery L. Serotonin activates overall feeding by activating two separate neural pathways in Caenorhabditis elegans. J. Neurosci. 32, 1920–1931 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Nakamura S., Mano H. & Kozasa T. Gα 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc. Natl Acad. Sci. USA 100, 733–738 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau D. M. et al. Identification and molecular characterization of the Gα12-Rho guanine nucleotide exchange factor pathway in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 100, 14748–14753 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley E., McMullan R. & Nurrish S. J. The Gα12-RGS RhoGEF-RhoA signalling pathway regulates neuromodulator release in C. elegans. EMBO J. 25, 5884–5895 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Rehmann H. & Wittingghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (2007). [DOI] [PubMed] [Google Scholar]
- Hammarlund M., Nix P., Hauth L., Jorgensen E. M. & Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science 323, 802–806 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J. et al. Rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo. EMBO J. 12, 5151–5160 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. M. et al. p190RhoGAP can act to inhibit PDGF-induced gliomas in mice: a putative tumor suppressor encoded on human chromosome 19q13.3. Genes Dev. 17, 476–487 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssa B., de Widt J., Kranenburg O., Moolenaar W. H. & van Blitterswijk W. J. Diacylglycerol kinase θ binds to and is negatively regulated by active RhoA. J. Biol. Chem. 274, 6820–6822 (1999). [DOI] [PubMed] [Google Scholar]
- Los A. P., Vinke F. P., de Widt J., Divecha N. & van Blitterswijk W. J. Structure-activity relationship of diacylglycerol kinase θ. Biochim. Biophys. Acta. 1636, 169–174 (2004). [DOI] [PubMed] [Google Scholar]
- van Blitterswijk W. J. & Houssa B. Properties and functions of diacylglycerol kinase. Cell Signal. 12, 595–605 (2000). [DOI] [PubMed] [Google Scholar]
- Luo B., Prescott S. M. & Topham M. K. Diacylglycerol kinase ζ regulates phosphatidylinositol 4-phosphate 5-kinase I α by a novel mechanism. Cell Signal. 16, 891–897 (2004). [DOI] [PubMed] [Google Scholar]
- McMullan R., Hiley E., Morrison P. & Nurrish S. J. Rho is a presynaptic activator of neuromodulator release at pre-existing synapses in C. elegans. Gene Dev 20, 65–76 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson R. J., Geng J., Gray A. D. & Komuniecki R. W. SER-7b, a constitutively active Gαs coupled 5-HT7-like receptor expressed in the Caenorhabditis elegans M4 pharyngeal motorneuron. J. Neurochem. 87, 22–29 (2003). [DOI] [PubMed] [Google Scholar]
- Ghosh-Roy A., Wu Z., Goncharov A., Jin Y. & Chisholm A. D. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J. Neurosci. 30, 3175–3183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W. & Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl Acad. Sci. USA 78, 3363–3367 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakhtenberg E. F. & Goldberg J. L. The role of serotonin in axon and dendrite growth. Int. Rev. Neurobiol. 106, 105–126 (2012). [DOI] [PubMed] [Google Scholar]
- Rojas P. S., Neira D., Muñoz M., Lavandero S. & Fielder J. L. Serotonin (5-HT) regulates neurite outgrowth through 5-HT1A and 5-HT7 receptors in cultured hippocampal neurons. J. Neurosci. Res. 92, 1000–1009 (2014). [DOI] [PubMed] [Google Scholar]
- Hydon P. G., McCobb D. P. & Kater S. B. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science 226, 561–564 (1984). [DOI] [PubMed] [Google Scholar]
- Kvachnina E. et al. 5-HT7 receptor is coupled to Gα subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J. Neurosci. 25, 7821–7830 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr. Opin. Phamacol 4, 47–52 (2004). [DOI] [PubMed] [Google Scholar]
- Potter D. D., Landis S. C., Matsumoto S. G. & Furshpan E. J. Synaptic functions in rat sympathetic neurons in microcultures. II. Adrenergic/cholinergic dual status and plasticity. J. Neurosci. 6, 1080–1098 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C. Neurotransmitter switching? No surprise. Neuron 86, 1131–1144 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S., Sendtner M. & Rohrer H. Ciliary neurotrophic factor induces cholinergic differentiation of rat sympathetic neurons in culture. J. Cell Biol. 108, 1807–1816 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T. et al. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science 246, 1412–1416 (1989). [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 28, 297–303 (2005). [DOI] [PubMed] [Google Scholar]
- Dulcis D., Jamshidi P., Leutgeb S. & Spitzer N. C. Neurotransmitter switching in the adult brain regulates behavior. Science 340, 449–453 (2013). [DOI] [PubMed] [Google Scholar]
- Serrano-Saiz E. et al. Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell 155, 659–673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y., He M. & Mao B. Sequential changes of hypoxia-inducible factor 1 α in experimental spinal cord injury and its significance. Clin. J. Traumatol 5, 103–106 (2002). [PubMed] [Google Scholar]
- Xiaowei H., Ninghui Z., Wei X., Yiping T. & Linfeng X. The experimental study of hypoxia-inducible factor-1 α and its target genes in spinal cord injury. Spinal Cord 44, 35–43 (2006). [DOI] [PubMed] [Google Scholar]
- Poncet L., Denoroy L., Daimaz Y. & Pequignot J. M. Activity of tryptophan hydroxylase and content of indolamines in discrete brain regions after a long-term hypoxic exposure in the rat. Brain Res. 765, 122–128 (1997). [DOI] [PubMed] [Google Scholar]
- Berger A. J., Hart A. C. & Kaplan J. M. Gαs-induced neurodegeneration in Caenorhabditis elegans. J. Neurosci. 18, 2871–2880 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korwagen H. C., van der Linden A. M. & Plasterk R. H. A. G protein hyperactivation of the Caenorhabditis elegans adenylyl cyclase SGS-1 induces neuronal degeneration. EMBO J. 17, 5059–5065 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden A. M., Moorman C., Cuppen E., Korwagen H. C. & Plasterk R. H. A. Hyperactivation of the G12-mediated signaling pathway in Caenorhabditis elegans induces a developmental growth arrest via protein kinase C. Curr. Biol. 13, 516–521 (2003). [DOI] [PubMed] [Google Scholar]
- Schade M. A., Reynolds N. K., Dollins C. M. & Miller K. G. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the Gαs pathway and define a third major branch of the synaptic signaling network. Genetics 169, 631–649 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuhov S. I. et al. Endocannabinoid-Goα signalling inhibits axon regeneration in Caenorhabditis elegans by antagonizing Gqα-PKC-JNK signalling. Nat. Commun. 3, e1136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. et al. The growth factor SVH-1 regulates axon regeneration in C. elegans via the JNK MAPK cascade. Nat. Neurosci. 15, 551–557 (2012). [DOI] [PubMed] [Google Scholar]
- Li C., Hisamoto N. & Matsumoto K. Axon regeneration is regulated by Ets C/EBP transcription complexes generated by activation of the cAMP/Ca2+ signaling pathways. PLoS Genet. 11, e1005603 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D. & Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. G. & Chiu C. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development 130, 3781–3794 (2003). [DOI] [PubMed] [Google Scholar]
- Firnhaber C. & Hammarlund M. Neuron-specific RNAi in C. elegans and its use in a screen for essential genes required for GABA neuron function. PLoS Genet. 9, e1003921 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J. et al. Sensory neuron fates are distinguished by a transcriptional switch that regulates dendrite branch stabilization. Neuron 79, 266–280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-5 and Supplementary Tables 1-3.