Abstract
Background:
Deranged body fat and muscle mass are aftermaths of uncontrolled diabetes. Anthropometric methods like body mass index (BMI) do not give qualitative inferences like total body fat (TBF), visceral fat (VF) or subcutaneous fat (SF) that can be given by bio-electrical impedance analysis (BIA). We studied body composition of type 2 diabetics in comparison to controls matched by age-sex, weight and BMI separately.
Methods:
Seventy-eight under-treatment type 2 diabetics of either sex with known glycemic and lipidemic control and equal number of controls with three patterns of matching were taken from our city. We derived parameters of body composition in both groups by Omron Karada Scan (Model HBF-510, China), using the principle of tetra poplar BIA and compared them for statistical significance.
Results:
We found significantly more SF (30.47% ± 7.73%), VF (11.94% ± 4.97%) and TBF (33.96% ± 6.07%) and significantly lesser skeletal muscle mass (23.39% ± 4.49%) in type 2 diabetics as compared to controls, persisting even after matching with weight or BMI. On assessing qualitatively, the risk of high VF, high TBF, low skeletal muscle mass was significantly high in type 2 diabetics, which were 10.41, 3.01, 9.21 respectively for comparable BMI and 6.78, 3.51, 11.93 respectively for comparable weight.
Conclusions:
BIA reveals that type 2 diabetics have more ectopic fat on the expense of skeletal muscle that persists even after matching by weight or BMI, both quantitatively and qualitatively. Measurement of body composition can be included as a primary care strategy to motivate lifestyle modifications while managing metabolic derangements of type 2 diabetes.
Keywords: Bio-electrical impedance, body composition, obesity, type 2 diabetes, visceral fat
INTRODUCTION
Type 2 diabetes, a familial, and familiar disease, is now on the verge of becoming pandemic in developing countries like India[1] emerging as a challenge for family care physicians and obesity has gained state of serious health related issue in South Asian countries.[2] Obesity is often the precursor of type 2 diabetes and its measures are important to regulate even after the inception of the same. Body composition and pattern of distribution of body fat serve as both risk factor as well as the cause of type 2 diabetes mellitus (T2DM).[3] Measurement of body fat can be both quantitative as well as qualitative, with methods available in the form of anthropometric ones as well as those based on imaging techniques.[4] Qualitative analysis and determination of visceral fat (VF) is more important[5] and bio-electrical impedance analysis (BIA) is a simple, quick, cost-effective and objective method available to assess the same with proven efficacy in Indian population and can be used on large scale even by family physicians and primary care providers.[6] By this study, we tried to evaluate body composition in under-treatment type 2 diabetic patients in comparison to controls matched by age-sex, weight and body mass index (BMI) separately.
METHODS
Study design
The present cross-sectional observational study was carried out from January 2013 to April 2014 in clinical research lab Physiology Department of our Medical College.
Study sample
Sample size of 78 for current population of city 600,000 and prevalence of disease 7.33% in urban area of our state[7] yield us 90% confidence interval keeping margin for error 5% as calculated by RaoSoft sample size calculator software, (Raosoft, Inc. free online software, Seattle, WA, USA).
Study subjects
After getting approval from institutional review board and informed consent from participants, the study was carried out in under-treatment ambulatory type 2 diabetics. Subjects were recruited from medicine OPD of a tertiary care teaching hospital attached to our college and from private OPDs.
Inclusion criteria-case
Seventy-eight type 2 diabetics (44 males and 34 females) were undertaken in age group 30–80 years, not taking insulin, taking regular medicines, and having a recent investigation for glycemic or lipidemic control. We excluded to increase heterogeneity we took cases with and without hypertension, with and without statin therapy, with or without family history of type 2 diabetes, coming from various socioeconomic statuses, doing work with varying degrees as to make a fairly representative sample of the population.
Control
We recruited in total 179 healthy nondiabetic controls from the community which were grouped in number equal to cases matched by age-sex, weight, BMI individually.
Research method
Subjects meeting inclusion and exclusion criteria were registered for study with initial assessment in the form of informed consent, personal history, medical history, anthropometric measurement and recent reports of glycemic controls including fasting blood sugar, PP2BS and HbA1c and lipidemic control were taken.
Body composition measurement
After entering age, gender and height taken by stadio-meter subject was allowed to stand on the instrument after its calibration. A digital, portable noninvasive instrument Omron Karada Scan (Omron Karada Scan HBF-375 Body Fat Analyzer, Omron Health Care Pvt Ltd.China) working on principle of tetra polar BIA was used that passes electric current of 500 μA at frequency 5 kHz to scan the whole body to derive regional body composition. We enrolled ambulatory outdoor patients only and took the reading in the morning so as to avoid dehydration[8] that otherwise would affect the accuracy of this method.
Defining cut off norms
For qualitative analysis, we defined standard norms[9] as (1) BMI ≤25, (2) VF ≤10, (3) total body fat (TBF) and skeletal muscle mass as per standard guidelines.
Statistical analysis
The data were transferred on Excel spreadsheet, and descriptive analysis was expressed as mean ± standard deviation. All calculations were accomplished by GraphPad InStat 3 software (demo version free software of GraphPad Software, Inc. California, USA). We evaluated the difference between both groups for baseline data, body composition parameters by Student's t-test and risk calculation was done by odds ratio using defined cut off norms. Any observed difference was considered statistically significant with P < 0.05.
RESULTS
Table 1 shows baseline data of case group reflecting the participation of both sexes, the average duration of type 2 diabetes 7.5 years, high average BMI, good lipidemic control and poor glycemic control with respect to HbA1c. Table 2 shows three groups, where cases are matched for age-sex (a) weight (b) and BMI (c) with equal number of controls derived out of pool of 179.
Table 1.
Base line data of case group under study
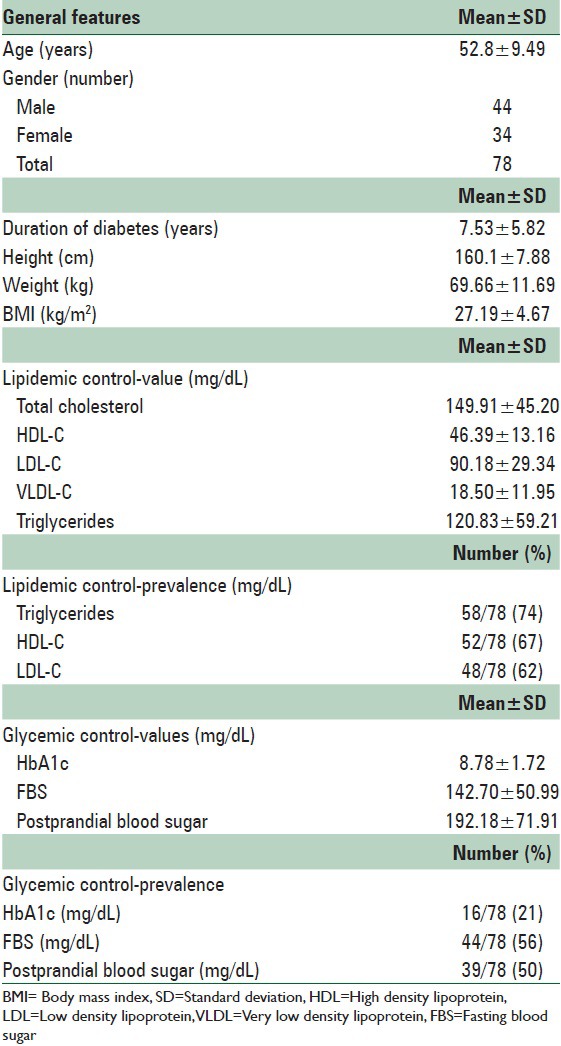
Table 2.
Matching patterns for comparison of parameters of body fat distribution case (n=78) versus control (n=78 for each out of pool of total 179 controls) groups
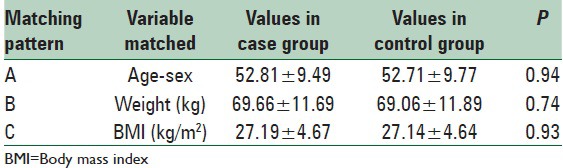
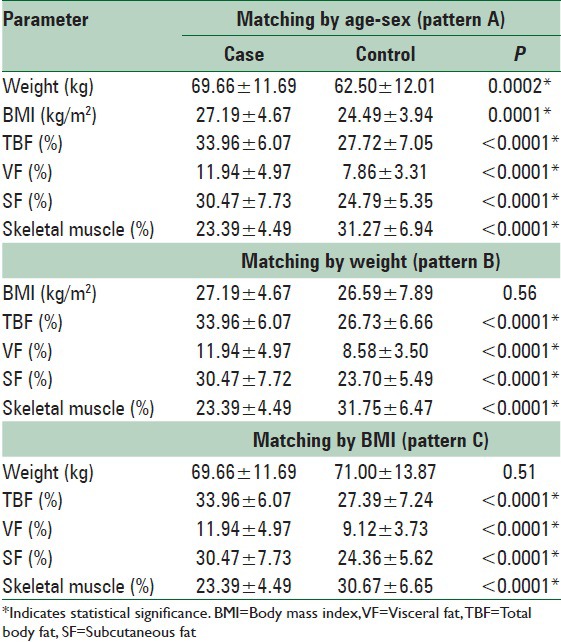
Table 3 shows comparison of various parameters of body composition among case and control groups reflecting significantly higher values of total, visceral and subcutaneous fat (SF) and lower values of skeletal muscle mass in type 2 diabetics as compared to controls for all three matching patterns.
Table 3.
Quantitative comparison of parameters of body fat distribution between case and 3 patterns of matched control groups (n=78 for each group)
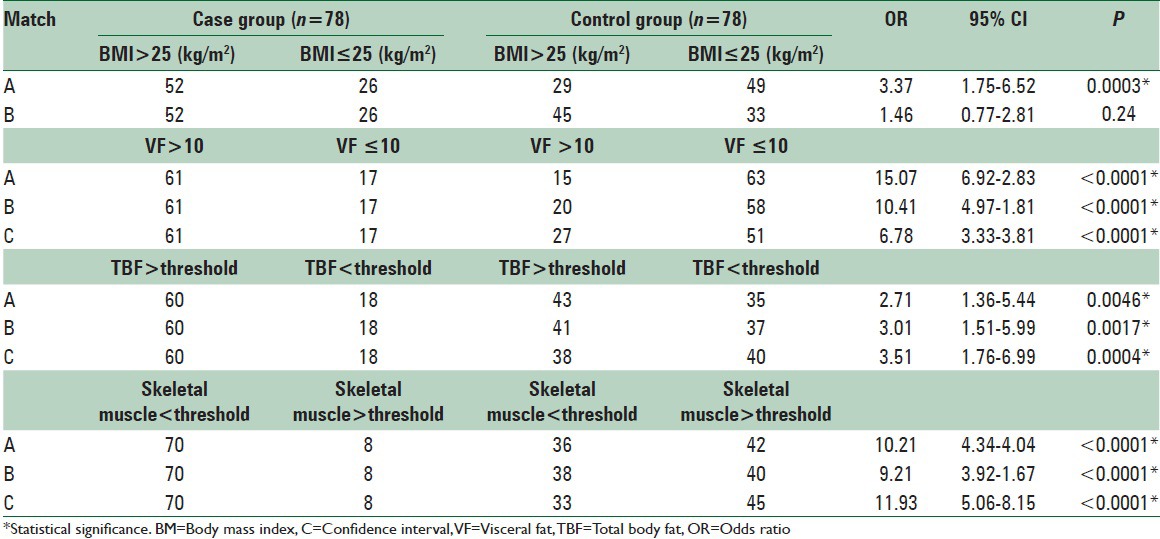
When parameters of body composition were compared qualitatively as per defined standard cut off norms, the high fat low muscle mass pattern of body composition in cases as compared to controls persisted, even after matching with weight and BMI, that too with statistical significance for all [Table 4].
Table 4.
Qualitative comparison of parameters of body fat distribution between case and 3 patterns of matched control groups (n=78 for each group)
DISCUSSION
Out of 135 million global diabetics nearly one-third are in India which is projected to reach 80.9 million by the year 2025.[10] It is further compounded by obesity doubling the cost of its management.[11] For given BMI, South Asians have greater adiposity and visceral and ectopic adipose tissue accumulation.[12] BMI, though used as a simple mean to define obesity, does not actually demarcate between fatty and fat-free mass[13] and do not give any qualitative inference. Few studies have revealed more adverse fat distribution at BMI > 21 kg/m2 in South Asians as compared to Caucasians in whom considerable dyslipidemia and dysglycemia are unseen until BMI exceeds 30 kg/m2.[14] With this propensity, it seems quite worthful to know body composition and body fat both quantitatively and qualitatively in high-risk obese subjects and type 2 diabetics.
We found excess TBF, SF and VF in type 2 diabetic as compared to age-sex matched controls. Even after matching the controls with cases for body weight and BMI, this pattern of excess fat and ectopic fat persisted. Body fat is more valuable parameter than BMI as we found the persistence of ectopic fat distribution after matching with BMI. BMI does not discriminate diabetes risk amongst obese and simple classification of obesity by BMI under classify few cases.[15] Anthropometrical methods like waist-hip ratio, waist circumference are simple means but subjective in nature, subjected to variation[16] and fails to differentiate between qualities of fat.[4] Imaging techniques like computed tomography (CT) scan, magnetic resonance imaging and dual X-ray absorptiometry are accurate[4] but not cost-effective more so in the Indian context. A study has revealed that CT scan is no better than simple methods to measure body fat[5] and availability of BIA serves the purpose of both quantitative and qualitative analysis whose validity has been proven in an Indian population.[6]
Obese type 2 diabetics have unfavorable body fat distribution than obese nondiabetics with an increase in VF and decrease in protective fat.[17] VF in excess has been linked to hepatic insulin resistance and TBF, SF to peripheral insulin resistance,[18] both of which can be measured by BIA method and in type 2 diabetics their excess was not nullified as compared to nondiabetics even after BMI matching, suggesting inclusion of these two in routine assessment. VF is more important than TBF or SF as excess VF is a risk factor for both prediabetes and diabetes[19] more so in Indians as compared to other Asian population.[20]
Glycemic control was poor being 20% for HbA1c, though lipidemic control was good (60%) and mean duration of diabetes being 7.5 years so it is obvious to look the results observed more cautiously as strict blood sugar control was not observed in most of the cases that is one of the features of Indian diabetics.[10] Therapy itself has a contributor to weight gain.[21] Diabetes is not merely a disease of disturbed glucose homeostasis and rather it is “more a disease of lipid than of carbohydrate.”[22] The phenomenon of ectopic fat deposition as seen in our case of obese diabetics is proven to be due to alteration of components of the immune system that damages adipose tissues, liver and pancreatic islets that ultimately leads to dyslipidemia and ectopic fat deposition,[23] most of which is hormone insensitive. Similarly protein wasting is one of the most serious of all the effects of severe diabetes mellitus (DM) that can lead to extreme weakness as well as many derangements in the functioning of organs.[24] The same immune alteration in type 2 diabetics that leads to fatty changes also induces activation of leucocytes, apoptosis and fibrosis that ends in muscle wasting and cachexia. Decreased skeletal muscle mass is also due to a higher percentage of intramuscular adipose tissue.[25]
Another part of this study about to be published[26] has proven that in the same subjects, deranged body fat distribution in T2DM measured by BIA correlated with BMI. These parameters are improved neither by lipidemic control nor by preventive pharmacotherapy. This suggests the use of other interventions like weight reduction and optimum use of BIA for monitoring utilizing primary health care resources.
The present study has few limitations like its cross-sectional nature, small sample size, presence of risk factors which cannot be eliminated and the method which is based on a predictive formula and tending to underestimate body fat. It is obvious that type 2 diabetes poses a significant risk to the body composition and balance between fat and protein both in quantity and quality. Skeletal muscle atrophy associated with enhanced SF deposition is an unwanted outcome of the disease, and severity of its progression correlates well with extent to which metabolic derangements are kept in check in type 2 diabetics. BIA method helps to know body composition in at-risk persons for DM to prevent the inception of disease as primary prevention, monitor the therapy to guide appropriate interventions. It helps to keep metabolic abnormality in check as a mean of secondary prevention and to keep life threatening events minimum by awareness of patients and doctors about body composition. This simple but objective method can be used by family physicians and primary care practitioners and patients themselves to monitor body composition and therapy would be something beyond doing exercise, taking oral hypoglycemic agents, diet restrictions and having hypolipidemic agents. Prevention is the key for diabetes in Indians, and simple prevention themes like “Eat less, eat on time, eat right, walk more, sleep well, and smile” are needed.[27] At least one can monitor the exact change in body composition and patient care may be improved by this.
CONCLUSIONS
Body mass index and body weight are short of qualitative inference when it comes to assessment of body composition and BIA offers a good cost-effective alternative. Type 2 diabetics must be assessed for qualitative body fat distribution and this method should be used optimally. Monitoring of body fat distribution by simple method of BIA offers a mean to be more precise in combating the challenge of treating type 2 diabetes patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 3.Heshka S, Ruggiero A, Bray GA, Foreyt J, Kahn SE, Lewis CE, et al. Altered body composition in type 2 diabetes mellitus. Int J Obes (Lond) 2008;32:780–7. doi: 10.1038/sj.ijo.0803802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wajchenberg BL. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 5.Bray GA, Jablonski KA, Fujimoto WY, Barrett-Connor E, Haffner S, Hanson RL, et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87:1212–8. doi: 10.1093/ajcn/87.5.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra S, Mercuri M, Anand SS. Measures of body fat in South Asian adults. Nutr Diabetes. 2013;3:e69. doi: 10.1038/nutd.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koria B, Kumar R, Nayak A, Kedia G. Prevalence of diabetes mellitus in urban population of ahmadabad city, Gujarat. Natl J Community Med. 2013;4:398–401. [Google Scholar]
- 8.Brown SP, Miller WC, Eason JM. VO2 max. In: Brown SP, Miller WC, Eason JM, editors. Exercise Physiology: Basis of Human Movement in Health and Disease. 2nd ed. China: Lippincott Williams and Wilkins; 2006. p. 324. [Google Scholar]
- 9.China: Omron Healthcare; 2008. Omron Instruction Manual. Full Body Sensor Body Composition Monitor and Scale Model HBF-510. [Google Scholar]
- 10.Sicree R, Shaw J, Zimmet P. Diabetes and impaired glucose tolerance. In: Gan D, editor. Diabetes Atlas. 3rd ed. Belgium: International Diabetes Federation; 2006. pp. 15–103. [Google Scholar]
- 11.Unnikrishnan AG, Bhattacharyya A, Baruah MP, Sinha B, Dharmalingam M, Rao PV. Importance of achieving the composite endpoints in diabetes. Indian J Endocrinol Metab. 2013;17:835–43. doi: 10.4103/2230-8210.117225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. Int J Obes (Lond) 2011;35:167–87. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–73. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razak F, Anand SS, Shannon H, Vuksan V, Davis B, Jacobs R, et al. Defining obesity cut points in a multiethnic population. Circulation. 2007;115:2111–8. doi: 10.1161/CIRCULATIONAHA.106.635011. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Gil MJ, et al. Body adiposity and type 2 diabetes: Increased risk with a high body fat percentage even having a normal BMI. Obesity (Silver Spring) 2011;19:1439–44. doi: 10.1038/oby.2011.36. [DOI] [PubMed] [Google Scholar]
- 16.Bosy-Westphal A, Booke CA, Blöcker T, Kossel E, Goele K, Later W, et al. Measurement site for waist circumference affects its accuracy as an index of visceral and abdominal subcutaneous fat in a Caucasian population. J Nutr. 2010;140:954–61. doi: 10.3945/jn.109.118737. [DOI] [PubMed] [Google Scholar]
- 17.Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, et al. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes. 2010;59:627–33. doi: 10.2337/db09-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki Y, DeFronzo RA. Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc Diabetol. 2009;8:44. doi: 10.1186/1475-2840-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng AC, Wai DC, Tai ES, Ng KM, Chan LL. Visceral adipose tissue, but not waist circumference is a better measure of metabolic risk in Singaporean Chinese and Indian men. Nutr Diabetes. 2012;2:e38. doi: 10.1038/nutd.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. Comparative effectiveness and safety of medications for type 2 diabetes: An update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–13. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganong WF. Fat metabolism in diabetes. In: Barret KE, Barman SM, Boitano S, Brooks HL, editors. Review of Medical Physiology. 24th ed. New York: McGraw Hill; 2012. p. 439. [Google Scholar]
- 23.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 24.Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Philadelphia: Elsevier Saunders; 2008. Effect of insulin on protein metabolism and on growth; pp. 966–7. [Google Scholar]
- 25.Karampinos DC, Baum T, Nardo L, Alizai H, Yu H, Carballido-Gamio J, et al. Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift-based water/fat separation. J Magn Reson Imaging. 2012;35:899–907. doi: 10.1002/jmri.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solanki JD, Makwana AH, Mehta HB, Desai CB, Gandhi PH. Body Mass Index, use of Statins or Current Lipidemic Control: Do they Affect Body Fat Distribution in Sedentary Type 2 Diabetes Mellitus? [Last cited on 2015 Mar 24];J Obes Metab Res. 2015 2:79–83. Available from: http://www.jomrjournal.org/text.asp?2015/2/2/79/151755 . [Google Scholar]
- 27.Joshi SR. Type 2 diabetes in Asian Indians. Clin Lab Med. 2012;32:207–16. doi: 10.1016/j.cll.2012.04.012. [DOI] [PubMed] [Google Scholar]


