Abstract
Background:
In Iran, bladder cancer is one of the most common malignancy sites among men, ranking as the fifth with age-specific incidence rate of about 11.2 per 100,000 males. It causes 8% of all malignancies in men and 3% of all malignancies in women.
Objectives:
The aim of this study was to report the epidemiological, clinical, and pathological features of bladder cancer in Western Iran compared to other studies.
Patients and Methods:
This is a retrospective study between 2003 and 2014 when forty-four patients with bladder cancer referred to Hematology Clinic of Kermanshah, Kermanshah, Iran. Transitional cell carcinoma (TCC) was in 39 patients.
Results:
In the patients with TCC, the mean age in diagnosis for them was 65.43 years (± 11.64), range of age 42 to 88 years , thirty-three patients (84.6%) were male, and six patients (15.4%) were female. Of 39 patients with TCC, 16 patients (41%) had metastasis. 21 patients (53.8%) were smoker and 16 patients (41%) had muscle invasive. 35 patients (89.7%) were histological high grade and the rest of patients were low grade. In the TCC patients with increasing age, metastasis and muscle invasive increased.
Conclusions:
The age presentation of TCC in West Iran was similar to other studies. Percentage of patients with high grade is more than other studies, and also the number of patients with bladder cancer has increased during last 4 years. For better results, studies must be conducted with more patients in this area, and other areas of Iran with checking of genetics, race and environmental factors.
Keywords: Bladder Cancer, Histological Grade, Transitional Cell Carcinoma
1. Background
Bladder cancer is a major health problem especially among men. It is estimated that in the year 2008, 150,000 cases lost their lives due to bladder cancer and 386,300 new cases were diagnosed throughout the world (1). In Iran, bladder cancer is one of the most common malignancy sites among men, ranking as the fifth with age-specific incidence rate of about 11.2 per 100,000 males (2). The bladder tumors were classified according to standard histopathological criteria as transitional cell carcinoma, adenocarcinoma or sarcoma. Mixed tumors consisted of both carcinomatous and sarcomatous or undifferentiated elements (3). In ultrasonic images, the normal muscle layer of bladder wall could be clearly distinguished into three layers, which were hyperechogenic mucosa, hypoechogenic muscle and hyperechogenic serosal. For non-muscle invasive tumors, the muscle layers were continuous, and distorted or discontinuous muscle layers could be seen in muscle-invasive case (4).
Muscle invasive bladder carcinoma is a complex, multifactorial disease caused by disruptions and alterations of several molecular pathways that result in heterogeneous phenotypes and variable disease outcome (5). The natural history of these bladder cancers is that of recurrence and progression to higher grades and stages. Urothelial (transitional cell) carcinoma is by far the most frequent type of bladder cancer. Bladder tumors are more common in industrial areas and their incidence increases with exposure to cigarette smoking and arylamines (6). Metastasis has been considered as an important clinical obstacle in the treatment of human cancer including bladder cancer (7).
2. Objectives
This study aimed to report the epidemiological, clinical, and pathological features of bladder cancer in West Iran compared to other studies.
3. Patients and Methods
3.1. Patients
This is a retrospective study between 2003 and 2014. Thirty-eight patients with bladder cancer referred to hematology clinic of Kermanshah, Kermanshah, Iran. We analyzed sex, age, metastasis, type of pathology, smoking, muscular involvement, and grade in the patients. In our study, grade Ι or ΙΙ was low grade, and grad ΙΙ high risk or grade ΙΙΙ or ΙV was high grade.
3.2. Statistical Analysis
Data were analyzed using IBM SPSS v.19 software. The p-value was calculated between smoking and bladder cancer with Chi-square test that P < 0.05 was significant. The figure was plotted by Excel 2007 software.
4. Results
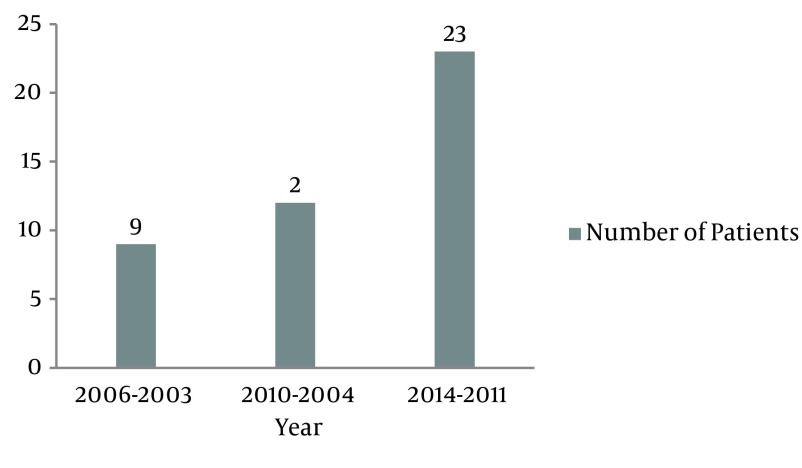
Between 2003 and 2014, around 5000 patients referred to hematology clinic of Kermanshah, 44 patients had bladder cancer whose pathology or histological cell types were: TCC (88.6%), squamous cell carcinoma (6.8%), undifferentiated carcinoma (2.3%) and adenocarcinomas (2.3%) (Table 1). In the patients with TCC, (Table 2) the mean age in diagnosis was 65.43 years (± 11.64) and range of age was 42 to 88 years, thirty -three patients (84.6%) were male and six patients (15.4%) were female. Of 39 patients with TCC, 16 patients (41%) had metastasis and we divided the patients based on histological grade (WHO) in two groups: Low grade (grade Ι or ΙΙ) and High grade (grade ΙΙ high risk or ΙΙΙ or ΙV). 21 patients (53.8%) were smoker and 16 patients (41%) had muscle invasive. The Figure 1 shows that referred patients with bladder cancer to Hematology Clinic of Kermanshah were more between of 2011 to 2014.
Table 1. Frequency of Various Histological Cell Types in 44 Patients With Primary Bladder Carcinomaa.
| Histological Cell Type | Values |
|---|---|
| Transitional cell carcinoma | 39 (88.6) |
| Squamous cell carcinoma | 3 (6.8) |
| Undifferentiated carcinoma | 1 (2.3) |
| Adenocarcinoma | 1 (2.3) |
aData are presented as No. (%).
Table 2. The Characteristics in 39 Patients With Primary Transitional Cell Carcinoma of the Urinary Bladdera,b.
| Characteristics | Values |
|---|---|
| Age (42 - 88), y | 65.43 ± 11.64 |
| Gender | |
| Male | 33 (84.6) |
| Female | 6 (15.4) |
| Metastasis | |
| Yes | 16 (41) |
| No | 23 (59) |
| Histological Grade (WHO) | |
| High | 35 (89.7) |
| Low | 4 (10.3) |
| Smoking | |
| Yes | 21 (53.8) |
| No | 18 (46.2) |
| Muscle Invasive | |
| Yes | 16 (41) |
| No | 23 (59) |
aData are presented as No. (%).
bLow grade: grade Ι or ΙΙ; High grade: grade ΙΙ high risk or ΙΙΙ or ΙV.
Figure 1. Number of Patients With Bladder Cancer Between of 2003 to 2014.
We divided the TCC patients to four groups (40 - 49, 50 - 59, 60 - 69 and ≥ 70 years). In these age groups, we studied metastasis and muscle invasive in them.
5. Discussion
Bladder cancer is the fourth most common malignancy in men and the eighth most common in women. It causes 8% of all malignancies in men and 3% of all malignancies in women (8). Effective treatment of TCC of the bladder requires early diagnosis. Identifying novel molecular markers in TCC would guide the development of diagnostic and therapeutic targets (9).
Incidence data from the United States indicate that TCC accounts for the vast majority (95%) of bladder tumors in this country, with squamous cell carcinoma (less than 3%) and adenocarcinoma (less than 2%) comprising nearly all the remaining cases (10). TCC constituted 95% of the cancers in Caucasians, compared with only 30% in Africans, whereas squamous cell carcinoma occurred in 53% of the African patients, but in only 2% of the Caucasians. Another study in East Africa (11) reported that squamous cell carcinoma was the most frequent histological type (55.1%), followed by conventional transitional cell carcinoma (40.5%) In Asian patients, 75% of the tumors were TCC and 18% were squamous cell carcinoma. Undifferentiated carcinoma occurred in 8% of African and only 1% of Caucasian patients, whereas adenocarcinoma, mixed tumors and sarcoma occurred in 9% of African patients and only 2% of Caucasian patients (3). Another study in East Asia (Japan) on 95 patients with primary urinary bladder cancer reported that TCC was in 87 cases (91.5%), squamous cell carcinoma in 5 cases (5.2%), adenocarcinoma in 2 cases (2.1%), and undifferentiated carcinoma in 1 case (1%) and also the overall 5-year actuarial survival rate was 36.0% (12). A study (13) in Southeast Asia, showed that the main histopathology was transitional cell carcinoma (TCC) (90.4%), followed by adenocarcinoma (6%), squamous cell carcinoma (1.2%), leiomyoma (1.2%) and myeloid sarcoma (1.2%). Matalka et al. (14) in West Asia (Jordan) reported that 95.6% of various histological cell types in the patients were TCC, 1.7% were squamous cell carcinoma, 2.6% were adenocarcinoma and there was no Undifferentiated carcinoma. A study (6) in Yemen (Southwest Asia) reported out of 316 urinary bladder cancers, 248 (78%) were urothelial neoplasms, 53 (17%) were squamous cell carcinoma, 7 (2%) were adenocarcinoma, and 3 (1%) were rhabdomyosarcoma. In our study in West Asia (Iran), 88.6% of tumors in the bladder cancer patients were TCC that is similar to results in the US, Caucasians and Asia but is different from results in Africa. Squamous cell carcinoma in our patients included 6.8% of patients that is close to results in East Asia but is upper than results in Jordan (West Asia) and the US and less than results in Yemen (Southwest Asia). Adenocarcinoma and undifferentiated carcinoma in our study and other studies are almost similar, except in Africa that are more.
Suo et al. (15) reported in twenty-one patients with bladder cancer, 12 patients (57.2%) were high grade and 9 patients (42.8%) were low grade and Sevcenco et al. (16) showed that muscle invasive bladder cancer was histologically confirmed in 10 out of 41 patients (24.4%). Snyder et al. (17) showed of the 669 patients, 154 had muscle invasive disease (23%). A study in Iran (18) reported that of 159 urothelial carcinomas including 96 (60%) low grade and 63 (40%) high grade carcinomas. In our study, 89.7% of patients were high grade and 41% had muscle invasive bladder cancer. In Malaysia, 41.4% of patients were muscle invasive and 32.5% were high grade tumors (13). Percentage of patients with high grade was more than other studies. This result from our study probably shows there are a few reasons why patients in West Iran cannot refer to clinics of oncology on time. These reasons can probably be lack of adequate awareness about cancer, embarrassment about bladder cancer especially in women and the fact that testing for cancer is not compulsory in our area. Studies showed that genetic factors such as P53 (19, 20), miR-21 (21), and OCT4B1 (22) can efficiently discriminate low grade tumors from high grade ones. Therefore, genetic factors, race (in this study, Kurdish), and environmental factors can affect the progression of bladder cancer. Kong et al. (13) showed that a total of 83 bladder tumour cases in Malaysia recorded that the incidence was the highest among the Chinese (56.6%), followed by Malays (34.9%), Indians (6%) and other races (2.4%). The male-to-female ratio was 9.4:1. The median age was 65 years (range 30 - 91 years). Mohseni et al. (23) in Iran reported that of 185 TCC patients, with a mean age of 65.1 ± 14.0 year, were included in this study, of whom 36 were females and 149 were males (male to female ratio of 4.1 to 1) and eighty-three patients were smokers (44.9%) that showed that smoking not only induces bladder cancer, but also, once it develops, it can increase the grade of tumor, resulting in worse prognosis.
In our study, 53.8% of patients were smoker and this shows that smoking can probably affect the progression of bladder cancer but it was not statistically significant (P > 0.05). A study on 32 patients by Sun et al. (24) showed that the mean age was 62.7 years consist of 22 males and 10 females. Kwon et al. (25) showed that in 746 patients (664 men and 82 women) mean age was 62.4 years. A total of 112 cases were analyzed by Ahmadi et al. (26) in North Iran where 98 (87.5%) were men and 14 (12.5%) women (mean age of 68.0 ± 14.6 years). A study in Jordan (14) showed that the mean age of TCC was 60.6 years. Rambau et al. (11) in Africa, reported that a total of 185 patients were diagnosed with cancer of the urinary bladder during the study period, where 90 (48.6%) were males and 95 (51.4) were females. The mean age at diagnosis was 54.3 years. Also in this study, the mean age was almost similar to other studies except for Africa, but male/female ratio for our study was 5.5 that based on previous studies, results in a lot of cases are close to each other. In older ages, metastasis and muscle invasive in the patients increase (Table 3), and the number of patients with bladder cancer increased in the last 4 years (Figure 1).
Table 3. Age Group Distribution by Metastasis and Muscle Invasive in 39 Patients With Primary Transitional Cell Carcinoma of the Urinary Bladder.
| Age Group, y | N | Metastasis | Muscle Invasive |
|---|---|---|---|
| 40 - 49 | 3 | 1 | 1 |
| 50 - 59 | 10 | 2 | 2 |
| 60 - 69 | 10 | 5 | 5 |
| ≥ 70 | 16 | 8 | 8 |
This calls for specialists’ attention to this uptrend and increasing of the risk of bladder cancer (metastasis) in this area.
The age presentation of TCC in West Iran was similar to other studies. Percentage of patients with high grade is more than other studies and the number of patients with bladder cancer increased in the last 4 years. For better results, studies must be carried out with more patients in this area, other areas of Iran and genetics, race and environmental factors must be taken into consideration.
Acknowledgments
None declared.
Footnotes
Authors’ Contribution:Study conception and design: Mehrdad Payandeh, Masoud Sadeghi; analysis and interpretation of data: Masoud Sadeghi, Edris Sadeghi; drafting of manuscript and revision: Mehrdad Payandeh, Masoud Sadeghi, Edris Sadeghi.
Conflict of Interest:None declared.
Financial Disclosure:None declared.
References
- 1.Karbakhsh M, Dabbagh N, Shabani A, Tabibi A, Akhavizadegan H. Age at diagnosis in bladder cancer: does opium addiction play a role? Asian Pac J Cancer Prev. 2013;14(8):4723–5. doi: 10.7314/apjcp.2013.14.8.4723. [DOI] [PubMed] [Google Scholar]
- 2.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13(2):143–6. [PubMed] [Google Scholar]
- 3.Groeneveld AE, Marszalek WW, Heyns CF. Bladder cancer in various population groups in the greater Durban area of KwaZulu-Natal, South Africa. Br J Urol. 1996;78(2):205–8. doi: 10.1046/j.1464-410x.1996.09310.x. [DOI] [PubMed] [Google Scholar]
- 4.Xu C, Zhang Z, Wang H, Song Q, Wei R, Yu Y, et al. A new tool for distinguishing muscle invasive and non-muscle invasive bladder cancer: the initial application of flexible ultrasound bronchoscope in bladder tumor staging. PLoS One. 2014;9(4):e4038. doi: 10.1371/journal.pone.0092385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat A, Heinzel A, Mayer B, Perco P, Muhlberger I, Husi H, et al. Protein interactome of muscle invasive bladder cancer. PLoS One. 2015;10(1):e4038. doi: 10.1371/journal.pone.0116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Samawi AS, Aulaqi SM. Urinary bladder cancer in yemen. Oman Med J. 2013;28(5):337–40. doi: 10.5001/omj.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu G, Yao W, Xiao W, Li H, Xu H, Lang B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J Exp Clin Cancer Res. 2014;33:779. doi: 10.1186/s13046-014-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezaianzadeh A, Mohammadbeigi A, Mobaleghi J, Mohammadsalehi N. Survival analysis of patients with bladder cancer, life table approach. J Midlife Health. 2012;3(2):88–92. doi: 10.4103/0976-7800.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Choi WW, Yan R, Yu H, Krasnoperov V, Kumar SR, et al. The differential expression of EphB2 and EphB4 receptor kinases in normal bladder and in transitional cell carcinoma of the bladder. PLoS One. 2014;9(8):e4038. doi: 10.1371/journal.pone.0105326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantor AF, Hartge P, Hoover RN, Fraumeni JF. Epidemiological characteristics of squamous cell carcinoma and adenocarcinoma of the bladder. Cancer Res. 1988;48(13):3853–5. [PubMed] [Google Scholar]
- 11.Rambau PF, Chalya PL, Jackson K. Schistosomiasis and urinary bladder cancer in North Western Tanzania: a retrospective review of 185 patients. Infect Agent Cancer. 2013;8(1):19. doi: 10.1186/1750-9378-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murase T, Takashi M, Aota Y, Shimoji T, Miyake K, Mitsuya H. [Total cystectomy for urinary bladder cancer: clinicopathological study of 95 cases]. Hinyokika Kiyo. 1985;31(4):615–21. [PubMed] [Google Scholar]
- 13.Kong CH, Singam P, Hong GE, Cheok LB, Azrif M, Tamil AM, et al. Clinicopathological features of bladder tumours in a single institution in Malaysia. Asian Pac J Cancer Prev. 2010;11(1):149–52. [PubMed] [Google Scholar]
- 14.Matalka I, Bani-Hani K, Shotar A, Bani Hani O, Bani-Hani I. Transitional cell carcinoma of the urinary bladder: a clinicopathological study. Singapore Med J. 2008;49(10):790–4. [PubMed] [Google Scholar]
- 15.Suo S, Chen X, Ji X, Zhuang Z, Wu L, Yao Q, et al. Investigation of the non-Gaussian water diffusion properties in bladder cancer using diffusion kurtosis imaging: a preliminary study. J Comput Assist Tomogr. 2015;39(2):281–5. doi: 10.1097/RCT.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 16.Sevcenco S, Haitel A, Ponhold L, Susani M, Fajkovic H, Shariat SF, et al. Quantitative apparent diffusion coefficient measurements obtained by 3-Tesla MRI are correlated with biomarkers of bladder cancer proliferative activity. PLoS One. 2014;9(9):e4038. doi: 10.1371/journal.pone.0106866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder C, Harlan L, Knopf K, Potosky A, Kaplan R. Patterns of care for the treatment of bladder cancer. J Urol. 2003;169(5):1697–701. doi: 10.1097/01.ju.0000056727.30546.b7. [DOI] [PubMed] [Google Scholar]
- 18.Keymoosi H, Gheytanchi E, Asgari M, Shariftabrizi A, Madjd Z. ALDH1 in combination with CD44 as putative cancer stem cell markers are correlated with poor prognosis in urothelial carcinoma of the urinary bladder. Asian Pac J Cancer Prev. 2014;15(5):2013–20. doi: 10.7314/apjcp.2014.15.5.2013. [DOI] [PubMed] [Google Scholar]
- 19.Kalantari MR, Ahmadnia H. P53 overexpression in bladder urothelial neoplasms: new aspect of World Health Organization/International Society of Urological Pathology classification. Urol J. 2007;4(4):230–3. [PubMed] [Google Scholar]
- 20.Lin HY, Yang MC, Huang CH, Wu WJ, Yu TJ, Lung FW. Polymorphisms of TP53 are markers of bladder cancer vulnerability and prognosis. Urol Oncol. 2013;31(7):1231–41. doi: 10.1016/j.urolonc.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Monfared H, Ziaee SA, Hashemitabar M, Khayatzadeh H, Kheyrollahi V, Tavallaei M, et al. Co-regulated expression of TGF-beta Variants and miR-21 in bladder cancer. Urol J. 2013;10(3):981–7. [PubMed] [Google Scholar]
- 22.Asadzadeh J, Asadi MH, Shakhssalim N, Rafiee MR, Kalhor HR, Tavallaei M, et al. A plausible anti-apoptotic role of up-regulated OCT4B1 in bladder tumors. Urol J. 2012;9(3):574–80. [PubMed] [Google Scholar]
- 23.Mohseni MG, Zand S, Aghamir SM. Effect of smoking on prognostic factors of transitional cell carcinoma of the bladder. Urol J. 2004;1(4):250–2. [PubMed] [Google Scholar]
- 24.Sun L, Zhang W, Liu H, Yuan J, Liu W, Yang Y. Computed tomography imaging-guided percutaneous argon-helium cryoablation of muscle-invasive bladder cancer: initial experience in 32 patients. Cryobiology. 2014;69(2):318–22. doi: 10.1016/j.cryobiol.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Kwon T, Jeong IG, Lee J, Lee C, You D, Hong B, et al. Adjuvant chemotherapy after radical cystectomy for bladder cancer: a comparative study using inverse-probability-of-treatment weighting. J Cancer Res Clin Oncol. 2015;141(1):169–76. doi: 10.1007/s00432-014-1793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadi M, Ranjbaran H, Amiri MM, Nozari J, Mirzajani MR, Azadbakht M, et al. Epidemiologic and socioeconomic status of bladder cancer in Mazandaran Province, northern Iran. Asian Pac J Cancer Prev. 2012;13(10):5053–6. doi: 10.7314/apjcp.2012.13.10.5053. [DOI] [PubMed] [Google Scholar]