Abstract
Background
During development, humans and other jawed vertebrates (Gnathostomata) express distinct hemoglobin genes, resulting in different hemoglobin tetramers. Embryonic and fetal hemoglobin have higher oxygen affinities than the adult hemoglobin, sustaining the oxygen demand of the developing organism. Little is known about the expression of hemoglobins during development of jawless vertebrates (Agnatha).
Results
We identified three hemoglobin switches in the life cycle of the sea lamprey. Three hemoglobin genes are specifically expressed in the embryo, four genes in the filter feeding larva (ammocoete), and nine genes correspond to the adult hemoglobin chains. During the development from the parasitic to the reproductive adult, the composition of hemoglobin changes again, with a massive increase of chain aHb1. A single hemoglobin chain is expressed constitutively in all stages. We further showed the differential expression of other globin genes: Myoglobin 1 is most highly expressed in the reproductive adult, myoglobin 2 expression peaks in the larva. Globin X1 is restricted to the embryo; globin X2 was only found in the reproductive adult. Cytoglobin is expressed at low levels throughout the life cycle.
Conclusion
Because the hemoglobins of jawed and jawless vertebrates evolved independently from a common globin ancestor, hemoglobin switching must also have evolved convergently in these taxa. Notably, the ontogeny of sea lamprey hemoglobins essentially recapitulates their phylogeny, with the embryonic hemoglobins emerging first, followed by the evolution of larval and adult hemoglobins.
Electronic supplementary material
The online version of this article (doi:10.1186/s12862-016-0597-0) contains supplementary material, which is available to authorized users.
Keywords: Agnatha, Ammocoete, Gene family, Hemoglobin switching, Myoglobin, Ontogeny, Oxygen, Phylogeny
Background
Hemoglobin (Hb) is a respiratory protein that facilitates the transport of oxygen (O2) from the respiratory surfaces (usually the skin, gills or lungs) to the inner organs [1]. Hb is present in almost all vertebrates, except some icefish species [2]. It is member of the globin protein family that is characterized by a conserved fold that includes a heme prosthetic group, by which the proteins reversibly bind O2 [1, 3]. In addition to Hb, other types of globins are present in the jawed vertebrates (Gnathostomata): myoglobin (Mb) [4], neuroglobin (Ngb) [5], cytoglobin (Cygb) [6–8], globin E (GbE) [9], globin X (GbX) [10], globin Y (GbY) [11] and androglobin (Adgb) [12]. A variety of functions other than O2 supply have been associated with these globins, including detoxification of reactive oxygen and nitrogen species (ROS/RNS) or signaling (for review, see [13]).
The Hb of the jawed vertebrates is a hetero-tetramer that is composed of two α- and two β-chains. The interaction of the chains leads to cooperative O2 binding [3]. Further modulation of the O2 affinity according to the physiological requirements is brought about by the interaction with organic phosphates (ATP, GTP, 2,3-diphosphoglycerate), CO2, and protons (Bohr effect), or by changing temperatures. Multiple, paralogous α- and β-genes have originated in evolution by gene duplication and divergence. During ontogeny, the O2 demand changes and, consequently, in many vertebrates distinct Hb chains are expressed in certain developmental stages [11, 14, 15]. For example, humans possess six Hb genes (α, β, γ, δ, ε, and ζ) [1]. Their differential expression results in embryonic, fetal, and adult forms of hemoglobin tetramers [1, 16]. The embryonic Hb consists of two α or ζ chains, respectively, plus two ε chains; the fetal Hb is composed of two α and two γ chains, which change to the adult Hb form (2 × α, 2 × β) during the first year after birth [1]. Embryonic and fetal Hb have higher oxygen affinities than adult Hb, which is essential to overcome the placental barrier in mammals [17].
The lamprey harbors five distinct globins: Adgb, GbX and Cygb, and functionally analogous Hbs and Mbs that evolved convergently from a common globin ancestor [18]. Lampreys, along with hagfishes, constitute the cyclostomes, the sole survivors of a lineage that diverged from the ancestor to the jawed vertebrates more than 500 million years ago [19, 20]. Like its counterpart in the jawed vertebrates, the lamprey Mb (aMb) is preferentially expressed in the skeletal muscle and presumably supports O2 to this tissue. The agnathan Hb (aHb) is structurally distinct from the gnathostome Hb, although it carries out similar functions. aHb is a monomer in its oxygenated form and associates into homodimers or tetramers when deoxygenated [21, 22]. Like the gnathostome Hbs, aHbs display cooperative O2 binding and a pH-dependent regulation of O2 affinity [23]. In the sea lamprey Petromyzon marinus, four distinct chains have been identified on the protein level that are components of the adult aHb [24–27]. However, analysis of the P. marinus genome revealed at least 14 additional aHb genes plus two pseudogenes [18]. Four of these closely resemble the known adult chains and probably are recent gene duplicates that cannot be distinguished from the main chain on the protein level. The expression patterns of the other nine aHbs remain unclear, leading to the speculation that they represent globin chains expressed in early developmental stages [18].
Sea lampreys (P. marinus) spend most of their life as filter-feeding larvae (ammocoetes), burrowed in the sediments of freshwater rivers [28]. After a dramatic metamorphosis involving major modifications to the morphology, physiology and behavior of the animal [29], the adult anadromous lampreys migrate to the sea, where they have a free-swimming hematophagous lifestyle. At the completion of the feeding phase, lampreys become sexually mature and return to fresh water, undergoing an upstream migration prior to spawning and death.
Early electrophoretic studies reported a shift from larval to adult aHb proteins during metamorphosis in a number of lamprey species [30–35]. More recently, Lanfranchi et al. [36, 37] demonstrated differential expression of one larval and two adult aHb genes before and after metamorphosis in the Po (Lombardy) brook lamprey (Lampetra zanandreai). No further studies, however, have examined the expression of other globins during ontogenesis of lampreys, and little attention has been paid to other developmental stages (e.g., embryogenesis, sexual maturation). The presence of at least 18 aHbs genes in the genome of P. marinus suggests intricate developmental regulation of expression [18]. To trace the hemoglobin switch in lampreys, we employed an RNA-seq and a qRT-PCR approach and studied the changes of the mRNA levels of aHbs and the other globins during four developmental stages of P. marinus: embryo, larval, parasitic adult, and reproductive adult.
Results
Expression pattern of aHb during development
We quantified the mRNA levels of the aHb genes of the sea lamprey (P. marinus) in different developmental stages by RNA-seq and qRT-PCR. First, a selection of putative housekeeping genes was evaluated in RNA-seq datasets for quality control and possible normalization. While the data indicate the integrity of the RNA, none of the putative housekeeping genes showed constant expression levels. Therefore, for RNA-seq normalization was done relative to the maximum expression level of aHb1 in the adult reproductive datasets (Table 1), which was set to 100 arbitrary units (AU). All other expression levels were calculated as RPKM (reads per kilobase of transcript per million reads) and were related to that value. In qRT-PCR analyses, we estimated the mRNA copy numbers.
Table 1.
Expression levels of globin genes in different developmental stages of the sea lamprey
| Gene | Embryo | Larva | Adult-parasitic | Adult- reproductive |
|---|---|---|---|---|
| aHb1 | 0.0000 | 0.0767 | 8.9347 | 100.00 |
| aHb2a | 0.0100 | 0.0153 | 0.6641 | 0.2205 |
| aHb2b | 0.0353 | 0.0154 | 20.7233 | 10.4043 |
| aHb2c | 0.0200 | 0.0000 | 0.6943 | 0.2069 |
| aHb2d | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| aHb3 | 0.0000 | 0.0000 | 12.4060 | 7.9237 |
| aHb5a | 0.0150 | 0.0000 | 7.3651 | 1.1106 |
| aHb5b | 0.0050 | 0.0000 | 6.9953 | 1.1677 |
| aHb5d | 0.0000 | 0.0000 | 12.6550 | 11.0104 |
| aHb6 | 3.1568 | 4.1841 | 0.0425 | 0.1838 |
| aHb7 | 0.8887 | 3.4145 | 2.7205 | 12.6774 |
| aHb8 | 0.0200 | 0.0000 | 2.5884 | 0.8084 |
| aHb9 | 2.5544 | 0.1310 | 0.0000 | 0.0000 |
| aHb10 | 2.0739 | 0.0000 | 0.0000 | 0.0052 |
| aHb11 | 1.5650 | 0.0291 | 0.0000 | 0.0646 |
| aHb12 | 0.1424 | 1.1575 | 0.0000 | 0.9181 |
| aHb13 | 0.7416 | 4.1488 | 0.0074 | 0.0747 |
| aHb14 | 1.1001 | 7.0349 | 0.0074 | 0.0721 |
| aMb1 | 0.4822 | 4.8613 | 13.1111 | 45.9467 |
| aMb2 | 0.0893 | 1.9698 | 0.2619 | 0.0996 |
| Cygb | 1.1232 | 0.2356 | 0.2934 | 0.1723 |
| GbX1 | 0.0164 | 0.0000 | 0.0000 | 0.0000 |
| GbX2 | 0.0000 | 0.0000 | 0.0000 | 0.0038 |
The values were derived from the RNA-seq data and related to the highest expression level of aHb1, which was set to 100 arbitrary units (AU)
Both methods indicated differential expression of the globins throughout development. Analysis by RNA-seq showed that aHb9, aHb10, and aHb11 are almost exclusively expressed in the embryo (Fig. 1a; Table 1). Compared to the high levels of aHb in the adult stage, the overall expression levels in the total embryo were comparably low (AU < 3). aHb6, aHb12, aHb13, and aHb14 are mainly expressed in the larval stage, with the highest levels for aHb14 (7 AU) (Fig. 1b); notable amounts of aHb6 were also detectable in the embryo (1.1 AU). The other NGS datasets were separated into an adult-parasitic stage and an adult-reproductive stage. The genes representing the known chains of P. marinus aHb [24–27], aHb2a, b, c, aHb3, aHb5a, b, d and aHb8 are most highly expressed in the adult-parasitic stage (Fig. 1c). Notable amounts of aHb2b, aHb5d and aHb3 are also detectable in the later adult stage. aHb1 is the most highly expressed globin gene of P. marinus. While it is not expressed in the embryo and only traces could be found in the larva (AU = 0.076; Table 1), it is a component of the adult aHb and reaches 100 AU in the reproductive stage (Fig. 1d). This finding is supported by the qRT-PCR results employing adult blood (Fig. 2a). In the adult, notable amounts of mRNA were also detected for aHb5, while the other analyzed aHbs display low levels. The qRT-PCR further showed that aHb6, aHb11 and aHb12 are mainly expressed in the ammocoete (Fig. 2b). The pattern of aHb7 differs from that of the other aHb genes, being expressed with increasing levels throughout ontogeny (AU = 0.88 to 12.7) (Figs. 1e; 2a, b; Table 1).
Fig. 1.
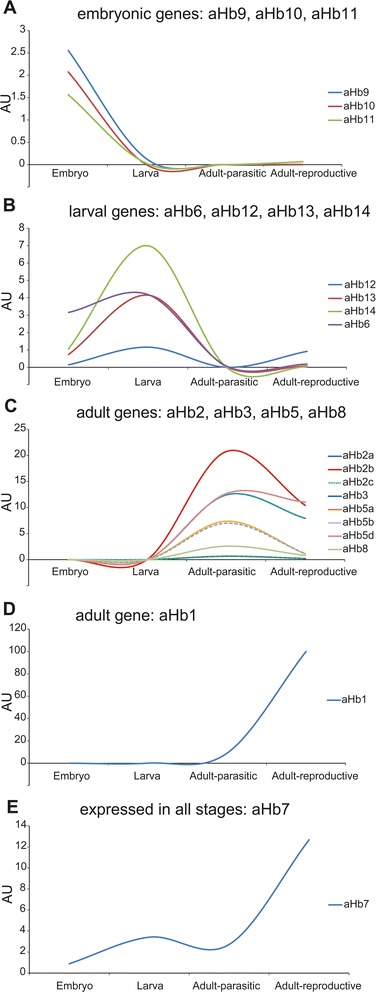
Expression profiles of sea lamprey aHbs quantified by RNA-seq. The aHb genes were displayed as predominantly expressed in the embryonic (a), larval (b), adult-parasitic (c) and adult-reproductive (d) stages. aHb7 (e) was not assigned to any specific developmental stage. The expression level is indicated as arbitrary units (AU), relative to the highest RPKM of the pool of adult-reproductive aHb1 expression, which is set to 100. Note that the expression levels of aHb2a and aHb2c, and aHb5a and aHb5b, respectively, were almost identical
Fig. 2.
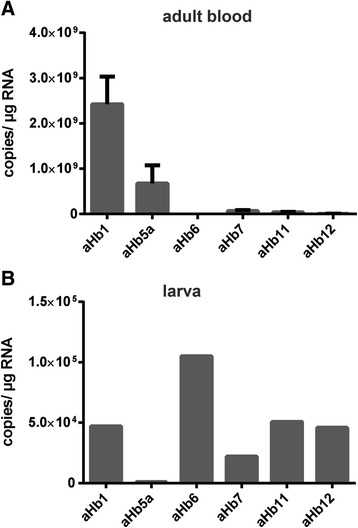
Quantification of mRNA levels of selected sea lamprey globins. Adult blood (a) or whole ammocoete (b) were used. The transcript abundance was quantified by qRT-PCR of aHb1, aHb5a, aHb6, aHb7, aHb11, aHb12 (a, b)
Ontogeny of the tissue globins
In addition to the aHbs, which code for globin chains that transport O2 in the blood, the globin repertoire of the sea lamprey includes Cygb, aMb1, aMb2, GbX1 and GbX2, which reside in the tissues [18]. We traced the expression changes of these genes by RNA-seq. Among them, the mRNA level of aMb1 is the highest; while only 0.482 AU were found in the embryo, its expression constantly increases until it reaches 46 AU in the adult stage (Fig. 3; Table 1). The level of aMb2 and Cygb are low throughout all developmental stages. The aMb2 transcript is mainly expressed in the larva (AU = 1.97), and Cygb is highest in the embryo (AU = 1.12) (Fig. 3, inset). The two GbX transcripts showed a low but differential expression: while GbX1 was found exclusively in the embryo (0.016 AU), GbX2 is only expressed in the adult (0.0038 AU) (Fig. 3, inset; Table 1).
Fig. 3.

Expression profiles of sea lamprey globins quantified by RNA-seq. The expression level of aMb1, aMb2, Cygb, GbX1 and GbX2 are indicated as AU (see above). The mRNA levels of aMb2, Cygb, GbX1, GbX2 were additionally displayed in the inset
Evolution of stage-specific hemoglobins in sea lamprey
We mapped the expression patterns onto a Bayesian phylogenetic tree of the agnathan globins. For simplification, only globins of the sea lamprey were considered. The basic topology of the tree was similar to that retrieved in a previous analysis [18]. The aHbs build a single clade, which forms the sister group of the two aMbs (Fig. 4). In most cases, the aHbs that are predominantly expressed during a certain phase of the life cycle, cluster together in specific clades (Fig. 4). Only aHb6, which is mainly expressed in both the embryo and the larva, and aHb7, which is expressed throughout the lifecycle, do not match this pattern. Among the sea lamprey aHbs, aHb6 diverged first, followed by aHb7. Next comes a clade composed of the embryonic aHbs (aHb9-11), which forms the sister group of the larval aHbs (aHb12-14) and adult aHbs (aHb1-3, 5, 8).
Fig. 4.

Mapping of the stage specific expression patterns onto a Bayesian phylogenetic tree of sea lamprey globins. The developmental stages are shaded in different grey scales. The numbers at the nodes are posterior probabilities. The bar represents 0.3 PAM distance
Discussion
Changes in hemoglobin composition during the ontogeny of the sea lamprey
Protein studies identified four distinct aHb chains in adult sea lamprey [24–27], which correspond to aHb1- aHb3 and aHb5 [18]. In fact, the mRNAs of these aHbs (including the aHb2 and aHb3 variants that represent recent gene duplicates) are the most strongly expressed adult aHbs. aHb8 represents an additional adult chain, which has been missed in previous protein sequencing studies, probably due to its similarity with aHb3. The composition of the aHb appears to change during the transition of the adult lamprey from the parasitic to the reproductive stage (Fig. 1). While the mRNA levels of aHb2, 3, 5, and 8 slightly decrease, aHb1 markedly rises to the highest levels measured for any globin in the sea lamprey. Thus, the composition of the aHb changes during adult life. In contrast, electrophoretic studies on the hemoglobin of the Austalian parasitic anadromous lamprey Mordacia mordax found no difference between small adults just prior to parasitic feeding and large adults returning on their spawning migration [34]. Thus, although it has long been known that a hemoglobin switch occurs in lampreys at metamorphosis, a further change in Hb composition at maturation has not been previously characterized. It remains uncertain which physiological constraints alter the O2 demands in the reproductive form of the sea lamprey requiring such a change in aHb composition.
While protein sequencing supports the interpretation of aHb1- aHb3, and aHb5 (and their variants) as the main components of the adult aHb, the nature of the other aHbs can only be inferred by tracing their stage-specific expression. The embryonic aHb probably consists of at least aHb9, aHb10, and aHb11 encoded chains. Traces of mRNA of these aHbs can be also found in the adult reproductive (but not parasitic) stage, which may be interpreted as eggs that were included in the mRNA preparation. The larval aHb at least consists of chains encoded by aHb12, aHb13, and aHb14 transcripts. This interpretation is further backed by the aHbA of the Po brook lamprey [37], which cluster in phylogenetic analyses with a clade formed by aHb13 and aHb14 of the sea lamprey [18]. The nature of aHb6 and aHb7 transcripts is less clear. aHb6 is most likely component of both the embryonic and the larval aHbs. The levels of aHb7 do not show indications of a clear switch but rather constantly increase during development, which hints at a more specific role.
Convergent evolution of hemoglobin switching in jawed and jawless vertebrates
The data demonstrated three hemoglobin switches in the sea lamprey, which can be considered as functionally analogous to the Hb switch of the jawed vertebrates [1, 38]. The first switch occurs from the embryonic to the larval stage. An analogous Hb switch during early development occur in fish [14, 15] and in amphibians [39]; it can also be considered analogous to the switch from embryonic to fetal Hb during mammalian development [1, 16]. The second switch takes place during metamorphosis from the larval to the adult lamprey. Again, analogous switches occur in the jawed vertebrates [11, 40–42]. A third, minor switch occurs during the transition of the parasitic to the reproductive adults (see above).
However, the Hb genes of jawed (Gnathostomata) and jawless (Agnatha) vertebrates are not homologous but emerged convergently [18, 43]. Thus, also the hemoglobin switch in these taxa must have emerged convergently and the scenario of an ancient common origin of the vertebrate hemoglobin switch can be excluded. The expression of different Hb genes that probably result in changes of O2 affinities may be instrumental to adapt to different O2 requirements of different developmental stages.
Ontogeny recapitulates phylogeny of lamprey hemoglobins
The phylogenetic tree (Fig. 4) remarkably mirrors the ontogeny of aHb expression: embryonic aHbs diverged first, followed by larval and adult aHbs. Only aHb6 and aHb7 deviate from this pattern. aHb6 is an embryonic/larval aHb that forms the earliest branching clade among the agnathan aHbs; in the tree, it is followed by aHb7, which is expressed throughout the development of the sea lamprey, with increasing levels in later life. This tree topology may have emerged by chance; however, it is also conceivable that it reflects the different changes in lifestyles that have occurred during evolution. The embryonic aHbs may mirror the hemoglobin in the agnathan ancestor; the aHbs of the filter-feeding lamprey larva may reflect the aHbs that emerged with the first agnathans, which had a similar life style (for example, Haikouichthys and Myllokunmingia; [44, 45]. With the evolution of a free-living, hematophagous lamprey, the adult forms of hemoglobin may have emerged. This interpretation is supported by the fact that the differentially expressed aHbs diverged before the hagfish and lampreys diverged [18].
Regulation of hemoglobin expressions
The expression of Hb in the Gnathostomata is controlled by sequential activation and repression of Hb genes that are chromosomally linked in clusters. In amphibians and Teleostei, the ancestral arrangement of the tandemly arranged Hbα and Hbβ is well preserved [11, 40, 46–49]. In the Amniota, the genes that code for Hbα and Hbβ are arranged on different chromosomes; this arrangement is the result of independent translocations of the Hbβ clusters [50, 51]. Currently, little is known about the arrangement of aHb genes in the lamprey [18] due to the fragmentary nature of the genome assemblies of P. marinus [52] and the Arctic lamprey Lethenteron camtschaticum [53]. However, the adult genes aHb3, aHb5a, aHb5b, aHb5c, and aHb8 are tandemly arranged in the same orientation in a single cluster, which may be an indicator for a coupled regulation. The same applies for the larval genes aHb12, aHb13 and aHb14 [18].
Switches in myoglobin and GbX expression in the sea lamprey
In addition to the aHbs, also aMb and GbX show differential expression throughout development. The sea lamprey possesses two aMb genes, which are – according to qRT-PCR studies – mainly expressed in the heart tissue, but were also found in muscle and brain [18]. aMb1 mRNA levels constantly increase from the embryo to the adult stages, which is probably associated with an enhanced heart function that requires a better O2 supply. It remains uncertain why aMb2 peaks in the larval stage. The expression patterns of the two GbX genes show a clear-cut difference, which restricts GbX1 to the embryo and GbX2 to the adult reproductive stage (Table 1). This pattern suggests that the divergence of GbX1 and GbX2 has led to a subfunctionalization of these genes, which probably have specific roles in each developmental stage.
Conclusions
The expression of distinct genes during animal development allows for the emergence of specific structures and the adaptation to specific metabolic requirements. The developmentally controlled expression of Hbs is probably the most prominent example of a switch in gene expression during the ontogeny of humans and other jawed vertebrates [1, 11, 14–16]. Here we have shown that in the sea lamprey, three hemoglobin switches occur, accounting for specific sets of aHb chains in the embryo, the larva, the parasitic adult and the reproductive adult. Because Hbs evolved convergently in gnathostomes and agnathans [18], the Hb switching in jawed and jawless vertebrates must also have evolved convergently. Surprisingly, the ontogeny of sea lamprey aHbs recapitulates their phylogeny. This has not been observed for the gnathostome Hbs.
Methods
Data collection and gene expression analyses with RNA-seq data
Next-generation sequencing (NGS, i.e. 454 or Illumina technologies) reads from four different development stages of P. marinus were obtained from NCBI (http://www.ncbi.nlm.nih.gov/sra). A total of 22 datasets were used: seven from embryonic stages (Illumina), four from the larval stages (two 454, two Illumina), six of adult-parasitic stages (two 454, four Illumina) and five of adult stages (three LS 454, two Illumina). Information on the datasets, including developmental stages and tissue, respectively, are given in Table 2. The transcript abundance of all annotated sea lamprey globin genes [18] were estimated using the RNA-seq analysis tool of CLC Genomics Workbench. The globin cDNAs and putative housekeeping genes were used as reference sequences with adjusted mapping options due to the high similarity among the aHb sequences (mismatch cost: 2, insert cost: 3, deletion cost: 3, length fraction: 0.99, similarity fraction: 0.99) in a global alignment with the SRA datasets. Each RNA-Seq dataset (Table 2) was individually mapped against the reference sequences and normalized according to the transcript length and the size of the dataset. Because none of the tested putative housekeeping genes showed constant expression throughout ontogeny, the resulting RPKM values were used. Due to the small reference dataset, the highest aHb expression level (aHb1 in the adult reproductive datasets) in RPKM was set to 100 arbitrary units (AU). All other RPKMs were related to that value.
Table 2.
RNA-seq data sets used in expression analyses. For each given stage, the datasets were combined; the analyses of the individual samples are given in Additional file 3: A3
| Developmental stage | Accession number | Tissue and specific stage | Sequencing method |
|---|---|---|---|
| Embryo | SRX110035 | Neural Crest Migration, Stage 24c2 | Illumina |
| SRX110034 | Neural Crest Migration, Stage 24c1 | Illumina | |
| SRX110033 | Neural Crest Migration, Stage 23 | Illumina | |
| SRX110032 | Neurula, Stage 22b | Illumina | |
| SRX110031 | Neurula, Stage 22a | Illumina | |
| SRX110030 | Gastrula, Stage 20 | Illumina | |
| SRX110029 | Late Blastula, Stage 18 | Illumina | |
| Larva | SRX109766 | Liver | 454 |
| SRX109765 | Brain | 454 | |
| SRX110023 | Kidney | Illumina | |
| SRX109770 | Intestine | Illumina | |
| Adult-parasitic | SRX110026 | Distal intestine | Illumina |
| SRX110025 | Proximal intestine | Illumina | |
| SRX110024 | Kidney | Illumina | |
| SRX109767 | Liver | 454 | |
| SRX109769 | Liver | Illumina | |
| SRX109761 | Olfactory epithelium | 454 | |
| Adult-reproductive | SRX109768 | Brain | Illumina |
| SRX109764 | Brain | 454 | |
| SRX109762 | Olfactory epithelium | 454 | |
| SRX110028 | Kidney | Illumina | |
| SRX110027 | Intestine | Illumina |
Animals
Two adult sea lampreys (63 cm, 731.1 g and 58 cm, 535.3 g) were collected from the Elbe estuary in June 2013 with the permission of the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit. Animals were handled according to regulations of the German Animal Welfare Act (Tierschutzgesetz). Samples of blood (each about 1 ml), heart and gonads were harvested, immediately placed on dry ice and stored at -80 °C. One larva (ammocoete) of the sea lamprey was provided by the U.S. Geological Survey, Hammond Bay Biological Station in Ray Road, Millersburg, Michigan. The larva (7.2 cm, 0.48 g) was collected in May 2014 from the Chippewa River, Canada. The collection, storage, and use of sea lamprey followed the guidelines of the U.S. Geological Survey. The animal was cut into pieces of 0.5 cm and immediately stored in RNAlater (Qiagen, Hilden, Germany), shipped at room temperature and stored subsequently at -20 °C.
RNA extraction
Total RNA from each sample was extracted using peqGOLD Trifast (PEQLAB, Erlangen, Germany) and the Crystal RNA Mini Kit (BiolabProducts, Gödenstorf) according to manufacturer’s instructions. Two blood samples were thawed on ice and homogenized with a pestle in 750 μl peqGOLD Trifast. The heart, gonads and larval samples were rinsed in PBS (140 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH = 7.1) that had been treated with diethylpyrocarbonate, ground in liquid nitrogen with mortar and pestle and homogenized in 1 ml peqGOLD Trifast. After the addition of one fifth volume of chloroform, the aqueous phase was purified using the silica column of the Crystal RNA Mini Kit with on-column DNase treatment (RNase-free DNase, Qiagen).
Reverse transcription and cDNA cloning
The RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Bonn) was used for reverse transcription. For cDNA cloning and quantitative real-time expression analyses, 1 μg and 750 ng, respectively, isolated RNA were reverse transcribed with oligo-(dT)18 primer in a total volume of 20 μl. Gene-specific oligonucleotides (Additional file 1: Table A1) were used for amplification of selected sea lamprey globin genes. The genes were chosen to cover each aHb branch of the phylogenetic tree [18], thus representing putative embryonic, larval and adult hemoglobins. The PCR products were cloned into standard cloning vectors (pGEM-T, Promega, or pJET1.2, Thermo Scientific) and sequenced by a commercial service (GATC, Konstanz, Germany).
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
The expression of selected globin mRNAs (aHb1, aHb5a, aHb6, aHb7, aHb11, aHb12, Cygb, aMb1 and aMb2) was estimated by qRT-PCR on an ABI 7500 real-time PCR system. Amplification was performed using the ABI Power SYBR Green master mix (Applied Biosystems, Darmstadt, Germany) with 40 cycles (95 °C for 15 s, 60 °C for 15 s, 72 °C for 30 s, detection at last step), employing intron-spanning primers (Additional file 1: Table A1). A cDNA amount equivalent to 18.75 ng RNA was used per reaction; the experiments were carried out as triplicates. Negative controls without cDNA were run as single experiments. The specificity of the amplifications was controlled by dissociation curve analyses. The standard curve method with recombinant plasmids in tenfold serial dilution was used to calculate the total mRNA copy number. The samples were normalized according to 1 μg total RNA.
Phylogenetic analyses of sea lamprey globins
The sea lamprey globin repertoire was extracted from a published dataset [18]. In the new dataset, all partial sequences and pseudogenes were excluded but closely related proteins with identical amino acid sequences were retained, thus a set of 21 sequences was used (GbX1, Cygb, two aMbs, 17 aHbs) (Additional file 2: Figure A1). The phylogenetic relationship among these globins was estimated using MrBayes on XSEDE 3.2.6 [54, 55] (https://www.phylo.org/) with the LG model of amino acid evolution. The multiple alignment of amino acid sequences was generated by MAFFT with L-INS-I strategy [56, 57]. Two independent runs with one cold and three heated chains were performed for 5x106 iterations and trees were sampled every 1000th generation. Posterior probabilities were estimated on the final 3,000 trees. GbX1 were used as outgroup because of its divergence from the other globin lineages before the separation of Protostomia and Deuterostomia [10, 58].
Availability of data and materials
All supporting data are available in the Additional file 1, Additional file 2 and Additional file 3.
Acknowledgments
We thank Miriam Götting, Walter Zeeck and Claus Zeeck for their help with the collection of adult lampreys, and Nicholas Johnson from the U.S. Geological Survey Hammond Bay Biological Station (Millersburg, MI, US) for providing the sea lamprey ammocoetes. We thank Kevin L. Campbell for support and Andrej Fabrizius for critical reading of the manuscript. This project is supported by the Deutsche Forschungsgemeinschaft (Bu956/18).
Abbreviations
- Adgb
androglobin
- aHb
agnathan hemoglobin
- aMb
agnathan myoglobin
- AU
arbitrary units
- Cygb
cytoglobin
- GbE
eye-specific globin
- GbX
globin X
- GbY
globin Y
- Hb
hemoglobin
- Mb
myoglobin
- Ngb
neuroglobin
- RPKM
reads per kilobase of transcript per million reads
Additional files
Oligonucleotides used for RT-PCR and qRT-PCR. (PDF 26 kb)
Sequence alignment (FASTA format) of the globin sequences of P. marinus. (PDF 8 kb)
Analyses of individual sequence datasets. (XLSX 51 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KR and FS collected the data. MD provided the ammocoete. KR and TB analyzed the data and wrote the manuscript, supported by MD. All authors read and approved the final manuscript.
Contributor Information
Kim Rohlfing, Email: kim.rohlfing@uni-hamburg.de.
Friederike Stuhlmann, Email: fstuhlmann@gmail.com.
Margaret F. Docker, Email: margaret.docker@umanitoba.ca
Thorsten Burmester, Email: thorsten.burmester@uni-hamburg.de.
References
- 1.Dickerson RE, Geis I. Hemoglobin: structure, function, evolution, and pathology: Benjamin/Cummings Pub. Co.; 1983.
- 2.Sidell BD, O’Brien KM. When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J Exp Biol. 2006;209:1791–802. doi: 10.1242/jeb.02091. [DOI] [PubMed] [Google Scholar]
- 3.Perutz MF. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–86. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- 4.Wittenberg BA, Wittenberg JB. Transport of oxygen in muscle. Annu Rev Physiol. 1989;51:857–78. doi: 10.1146/annurev.ph.51.030189.004233. [DOI] [PubMed] [Google Scholar]
- 5.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–3. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 6.Burmester T, Ebner B, Weich B, Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol. 2002;19:416–21. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- 7.Kawada N, Kristensen DB, Asahina K, Nakatani K, Minamiyama Y, Seki S, et al. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem. 2001;276:25318–23. doi: 10.1074/jbc.M102630200. [DOI] [PubMed] [Google Scholar]
- 8.Trent JT, 3rd, Hargrove MS. A ubiquitously expressed human hexacoordinate hemoglobin. J Biol Chem. 2002;277:19538–45. doi: 10.1074/jbc.M201934200. [DOI] [PubMed] [Google Scholar]
- 9.Kugelstadt D, Haberkamp M, Hankeln T, Burmester T. Neuroglobin, cytoglobin, and a novel, eye-specific globin from chicken. Biochem Biophys Res Commun. 2004;325:719–25. doi: 10.1016/j.bbrc.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 10.Roesner A, Fuchs C, Hankeln T, Burmester T. A globin gene of ancient evolutionary origin in lower vertebrates: evidence for two distinct globin families in animals. Mol Biol Evol. 2005;22:12–20. doi: 10.1093/molbev/msh258. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs C, Burmester T, Hankeln T. The amphibian globin gene repertoire as revealed by the Xenopus genome. Cytogenet Genome Res. 2006;112:296–306. doi: 10.1159/000089884. [DOI] [PubMed] [Google Scholar]
- 12.Hoogewijs D, Ebner B, Germani F, Hoffmann FG, Fabrizius A, Moens L, et al. Androglobin: a chimeric globin in metazoans that is preferentially expressed in mammalian testes. Mol Biol Evol. 2012;29:1105–14. doi: 10.1093/molbev/msr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burmester T, Hankeln T. Function and evolution of vertebrate globins. Acta Physiol. 2014;211:501–14. doi: 10.1111/apha.12312. [DOI] [PubMed] [Google Scholar]
- 14.Brownlie A, Hersey C, Oates AC, Paw BH, Falick AM, Witkowska HE, et al. Characterization of embryonic globin genes of the zebrafish. Dev Biol. 2003;255:48–61. doi: 10.1016/S0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 15.Tiedke J, Gerlach F, Mitz SA, Hankeln T, Burmester T. Ontogeny of globin expression in zebrafish (Danio rerio) J Comp Physiol B. 2011;181:1011–21. doi: 10.1007/s00360-011-0588-9. [DOI] [PubMed] [Google Scholar]
- 16.Stamatoyannopoulos G. Human hemoglobin switching. Science. 1991;252:383. doi: 10.1126/science.2017679. [DOI] [PubMed] [Google Scholar]
- 17.Bauer C, Tamm R, Petschow D, Bartels R, Bartels H. Oxygen affinity and allosteric effects of embryonic mouse haemoglobins. Nature. 1975;257:333–4. doi: 10.1038/257333a0. [DOI] [PubMed] [Google Scholar]
- 18.Schwarze K, Campbell KL, Hankeln T, Storz JF, Hoffmann FG, Burmester T. The globin gene repertoire of lampreys: convergent evolution of hemoglobin and myoglobin in jawed and jawless vertebrates. Mol Biol Evol. 2014;31:2708–21. doi: 10.1093/molbev/msu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Docker MF, Hume JB, Clemens BJ. Introduction: a surfeit of lampreys. In: Docker MF, editor. Lampreys: biology, conservation and control, vol. 1. Dordrecht: Springer; 2015. pp. 1–34. [Google Scholar]
- 20.Hedges SB, Marin J, Suleski M, Paymer M, Kumar S. Tree of life reveals clock-like speciation and diversification. Mol Biol Evol. 2015;32:835–45. doi: 10.1093/molbev/msv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behlke J, Scheler W. Zur Wirkung von Liganden auf das Assoziations-Dissoziations-Gleichgewicht des Methämoglobins der Flußneunaugen (Lampetra fluviatilis L.) Eur J Biochem. 1970;15:245–9. doi: 10.1111/j.1432-1033.1970.tb01001.x. [DOI] [PubMed] [Google Scholar]
- 22.Fago A, Giangiacomo L, D’Avino R, Carratore V, Romano M, Boffi A, et al. Hagfish hemoglobins: structure, function, and oxygen-linked association. J Biol Chem. 2001;276:27415–23. doi: 10.1074/jbc.M100759200. [DOI] [PubMed] [Google Scholar]
- 23.Wald G, Riggs A. The hemoglobin of the sea lamprey, Petromyzon marinus. J Gen Physiol. 1951;35:45–53. doi: 10.1085/jgp.35.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li SL, Riggs A. The amino acid sequence of hemoglobin V from the lamprey, Petromyzon marinus. J Biol Chem. 1970;245:6149–69. [PubMed] [Google Scholar]
- 25.Hombrados I, Rodewald K, Allard M, Neuzil E, Braunitzer G. Primary structure of the minor haemoglobins from the sea lamprey (Petromyzon marinus, Cyclostomata) Biol Chem Hoppe Seyler. 1987;368:145–54. doi: 10.1515/bchm3.1987.368.1.145. [DOI] [PubMed] [Google Scholar]
- 26.Hombrados I, Rodewald K, Neuzil E, Braunitzer G. Haemoglobins, LX. Primary structure of the major haemoglobin of the sea lamprey Petromyzon marinus (var. Garonne, Loire) Biochimie. 1983;65:247–57. doi: 10.1016/S0300-9084(83)80276-4. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Y, Maillett DH, Knapp J, Olson JS, Riggs AF. Lamprey hemoglobin. Structural basis of the bohr effect. J Biol Chem. 2000;275:13517–28. doi: 10.1074/jbc.275.18.13517. [DOI] [PubMed] [Google Scholar]
- 28.Hardisty MW, Potter IG. The general biology of adult lampreys. In: Hardisty MW, Potter IG, editors. The biology of lampreys, volume 1. New York: Academic Press London; 1971. pp. 127–206. [Google Scholar]
- 29.Manzon RG, Youson JH, Holmes JA. Lamprey metamorphosis. In: Docker MF, editor. Lampreys: Biology, conservation and control, vol. 1. Dordrecht: Springer; 2015. pp. 139–214. [Google Scholar]
- 30.Adinolfi M, Chieffi G. Larval and adult haemoglobins of the cyclostome Petromyzon planeri. Nature. 1958;182:730. doi: 10.1038/182730a0. [DOI] [PubMed] [Google Scholar]
- 31.Adinolfi M, Chieffi G, Siniscalco M. Haemoglobin pattern of the cyclostome Petromyzon planeri during course of development. Nature. 1959;184:1325–6. doi: 10.1038/1841325b0. [DOI] [PubMed] [Google Scholar]
- 32.Bird DJ, Lutz PL, Potter IC. Oxygen dissociation curves of the blood of larval and adult lampreys (Lampetra fluviatilis) J Exp Biol. 1976;65:449–58. doi: 10.1242/jeb.65.2.449. [DOI] [PubMed] [Google Scholar]
- 33.Potter IC, Brown ID. Changes in haemoglobin electorpherograms during the life cycle of two closely related lampreys. Comp Biochem Physiol B. 1975;51(4):517–9. doi: 10.1016/0305-0491(75)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Potter IC, Nicol PI. Electrophoretic studies on the haemoglobins of Australian lampreys. Aust J Exp Biol Med Sci. 1968;46:639–41. doi: 10.1038/icb.1968.170. [DOI] [PubMed] [Google Scholar]
- 35.Uthe JF, Tsuyuki H. Cornparative zone electropherograms of muscle myogens and blood proteins of adult and ammocoete larnprey. J Fish Res Bd Can. 1967;24:1269–73. doi: 10.1139/f67-108. [DOI] [Google Scholar]
- 36.Lanfranchi G, Odorizzi S, Laveder P, Valle G. Different globin messenger RNAs are present before and after the metamorphosis in Lampetra zanandreai. Dev Biol. 1991;145:367–73. doi: 10.1016/0012-1606(91)90135-P. [DOI] [PubMed] [Google Scholar]
- 37.Lanfranchi G, Pallavicini A, Laveder P, Valle G. Ancestral hemoglobin switching in lampreys. Dev Biol. 1994;164:402–8. doi: 10.1006/dbio.1994.1210. [DOI] [PubMed] [Google Scholar]
- 38.Peterson KR. Hemoglobin switching: new insights. Curr Opin Hematol. 2003;10:123–9. doi: 10.1097/00062752-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Kobel HR, Wolff J. Two transitions of haemoglobin expression in Xenopus: from embryonic to larval and from larval to adult. Differentiation. 1983;24:24–6. doi: 10.1111/j.1432-0436.1983.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 40.Hosbach HA, Widmer HJ, Andres AC, Weber R. Expression and organization of the globin genes in Xenopus laevis. Prog Clin Biol Res. 1982;85 Pt A:115–25. [PubMed] [Google Scholar]
- 41.Banville D, Williams JG. Developmental changes in the pattern of larval β-globin gene expression in Xenopus laevis. Identification of two early larval β-globin mRNA sequences. J Mol Biol. 1985;184:611–20. doi: 10.1016/0022-2836(85)90307-9. [DOI] [PubMed] [Google Scholar]
- 42.Banville D, Williams JG. The pattern of expression of the Xenopus laevis tadpole α-globin genes and the amino acid sequence of the three major tadpole α-globin polypeptides. Nucleic Acids Res. 1985;13:5407–21. doi: 10.1093/nar/13.15.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann FG, Opazo JC, Storz JF. Gene cooption and convergent evolution of oxygen transport hemoglobins in jawed and jawless vertebrates. Proc Natl Acad Sci U S A. 2010;107:14274–9. doi: 10.1073/pnas.1006756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris SC, Caron JB. A primitive fish from the Cambrian of North America. Nature. 2014;512:419–22. doi: 10.1038/nature13414. [DOI] [PubMed] [Google Scholar]
- 45.Shu DG, Morris SC, Han J, Zhang ZF, Yasui K, Janvier P, et al. Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature. 2003;421:526–9. doi: 10.1038/nature01264. [DOI] [PubMed] [Google Scholar]
- 46.Jeffreys AJ, Wilson V, Wood D, Simons JP, Kay RM, Williams JG. Linkage of adult α- and β-globin genes in X. laevis and gene duplication by tetraploidization. Cell. 1980;21:555–64. doi: 10.1016/0092-8674(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 47.Hosbach HA, Wyler T, Weber R. The Xenopus laevis globin gene family: chromosomal arrangement and gene structure. Cell. 1983;32:45–53. doi: 10.1016/0092-8674(83)90495-6. [DOI] [PubMed] [Google Scholar]
- 48.Chan FY, Robinson J, Brownlie A, Shivdasani RA, Donovan A, Brugnara C, et al. Characterization of adult α- and β-globin genes in the zebrafish. Blood. 1997;89:688–700. [PubMed] [Google Scholar]
- 49.Pisano E, Cocca E, Mazzei F, Ghigliotti L, di Prisco G, Detrich HW, 3rd, et al. Mapping of α- and β-globin genes on Antarctic fish chromosomes by fluorescence in-situ hybridization. Chromosome Res. 2003;11:633–40. doi: 10.1023/A:1024961103663. [DOI] [PubMed] [Google Scholar]
- 50.Hardison RC. Globin genes on the move. J Biol. 2008;7:35. doi: 10.1186/jbiol92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel VS, Cooper SJ, Deakin JE, Fulton B, Graves T, Warren WC, et al. Platypus globin genes and flanking loci suggest a new insertional model for β-globin evolution in birds and mammals. BMC Biol. 2008;6:34. doi: 10.1186/1741-7007-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, et al. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat Genet. 2013;45:415–21. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta TK, Ravi V, Yamasaki S, Lee AP, Lian MM, Tay BH, et al. Evidence for at least six Hox clusters in the Japanese lamprey (Lethenteron japonicum) Proc Natl Acad Sci U S A. 2013;110:16044–9. doi: 10.1073/pnas.1315760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayres DL, Darling A, Zwickl DJ, Beerli P, Holder MT, Lewis PO, et al. BEAGLE: an application programming interface and high-performance computing library for statistical phylogenetics. Syst Biol. 2012;61:170–3. doi: 10.1093/sysbio/syr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–5. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 56.Katoh K, Asimenos G, Toh H. Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol. 2009;537:39–64. doi: 10.1007/978-1-59745-251-9_3. [DOI] [PubMed] [Google Scholar]
- 57.Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9:286–98. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- 58.Blank M, Burmester T. Widespread occurrence of N-terminal acylation in animal globins and possible origin of respiratory globins from a membrane-bound ancestor. Mol Biol Evol. 2012;29:3553–61. doi: 10.1093/molbev/mss164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data are available in the Additional file 1, Additional file 2 and Additional file 3.


