Abstract
A number of transcription factors, including En1/2, Foxa1/2, Lmx1a/b, Nurr1, Otx2, and Pitx3, with key roles in midbrain dopaminergic (mDA) neuron development, also regulate adult mDA neuron survival and physiology. Mouse models with targeted disruption of some of these genes display several features reminiscent of Parkinson disease (PD), in particular the selective and progressive loss of mDA neurons in the substantia nigra pars compacta (SNpc). The characterization of these animal models has provided valuable insights into various mechanisms of PD pathogenesis. Therefore, the dissection of the mechanisms and survival signalling pathways engaged by these transcription factors to protect mDA neuron from degeneration can suggest novel therapeutic strategies. The work on En1/2-mediated neuroprotection also highlights the potential of protein transduction technology for neuroprotective approaches in PD.
1. Introduction
Parkinson disease (PD) is the second most common neurodegenerative disorder. The disease is characterized by a loss of midbrain dopaminergic (mDA) neurons in the substantia nigra pars compacta (SNpc) and the presence of α-synuclein-containing protein aggregates and termed Lewy bodies (and/or Lewy neurites) in affected neurons [1, 2]. Apart from certain familial monogenic forms of the disease, in which mutated genes (e.g., SNCA, LRRK22, PINK1, PARKIN, DJ-1, and ATP13a2) have been identified, the molecular bases of sporadic idiopathic PD remain largely unknown [3, 4]. As for other neurodegenerative diseases, such as Alzheimer's disease and Huntington's disease, ageing is considered a major risk factor for PD development [5].
The current view is that the slow and progressive death of SNpc mDA neurons remains asymptomatic until 30% of mDA neuron cell bodies and 50–60% of axonal terminals are lost [6]. Over time, this loss results in severe dopamine (DA) deficiency in the striatum, leading to the cardinal motor symptoms including rest tremor, bradykinesia, rigidity, and postural instability. No therapies are yet available to prevent the loss of mDA neurons or even delay the course of the disease [7]. One remarkable feature of the disease is that nonnigral dopaminergic neurons including mDA neurons in the ventral tegmental area (VTA), located in the vicinity of the SNpc mDA neurons, are relatively spared. The molecular determinants for the selective vulnerability of the SNpc mDA neurons in PD are not known [8, 9].
A number of studies have pointed to oxidative stress, mitochondrial dysfunction, protein misfolding and aggregation, impaired proteasomal and lysosomal degradation pathways, altered vesicular trafficking, and neuroinflammation as possible culprits in PD pathogenesis [1, 2, 8]. Many PD-linked genes affect mitochondrial activity or integrity [10] and a potential link between mitochondrial dysfunction and PD is supported by the ability of complex I-specific neurotoxins such as MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) to induce PD-like symptoms in rodents and primates, including humans. Recent studies also suggest that DNA damage or repair dysfunction [11–13] and nucleolar stress [14–17] play important roles in PD pathogenesis and other neurodegenerative diseases [18–23]. Mouse lines expressing PD-linked gene mutations recapitulate several features of PD pathogenesis but most of them do not present the selective and progressive loss of mDA neurons in the SNpc [24–26].
Major progress has recently been made in dissecting the genetic and signalling networks that control the generation of mDA neurons [27–29]. These studies have revealed the crucial role of several transcription factors. Interestingly, a number of these transcription factors (e.g., Engrailed-1/Engrailed-2, Foxa1/2, Lmx1a/b, Nurr1, Otx2, and Pitx3) remain present in adult mDA neurons and are required for their maintenance throughout life [30–32]. The elucidation of the roles and mechanisms of action of these transcription factors during development and adulthood could bring important insights into PD pathogenesis and suggest new therapeutic strategies. It is noteworthy that possible genetic links between these transcription factors and PD have been reported [33–46]. This review summarizes some aspects of the function of these developmental transcription factors in relation to PD.
2. Transcription Factors as Key Players in mDA Neuron Development
Midbrain DA neuron development starts around embryonic day E8 in the mouse with the induction of neurons in the floor plate and the specification of mDA progenitors. These progenitors give rise to mature mDA neurons following successive steps of proliferation, maturation, migration, axonal pathfinding, and synaptogenesis [27–29]. Midbrain DA neurons of the SNpc project mainly to the dorsal striatum to form the nigrostriatal pathway involved in the control of voluntary movements, whereas VTA mDA neurons project to the nucleus accumbens, the amygdala, the hippocampus, and the prefrontal cortex to form the mesolimbic and mesocortical pathways involved in motivation, reward, addiction, cognition, and memory. Mature SNpc mDA neurons express elevated levels of the highly active glycosylated form of the dopamine transporter (DAT) glyco-DAT and the inwardly rectifying potassium channel GIRK2, whereas VTA mDA neurons are more enriched in the calcium binding protein calbindin D28K (CALB1) [47].
Induction and specification of DA progenitors are governed by the concerted action of secreted factors (e.g., SHH, FGF8, Wnt1, and TGF-β) and of key transcription factors including En1/2, Otx2, Lmx1a/b, and Foxa1/2 [27–29]. Early loss of function of any of these transcription factors has dramatic consequences in the ontogenesis of mDA neurons. The subsequent steps of mDA neuron development are accompanied by the expression of additional transcription factors such as Nurr1 and Pitx3, which participate in the differentiation of mDA progenitors into mature mDA neurons. Hence, Nurr1 and Pitx3 are required for the expression of several genes encoding proteins that determine mature mDA neuron identity such as TH (tyrosine hydroxylase), DAT and VMAT2 (vesicular monoamine transporter 2), AADC (dopa decarboxylase), DRD2 (dopamine receptor D2), or ALDH1A1 (aldehyde dehydrogenase 1 family, member A1). Furthermore, Nurr1/Pitx3 are persistently required for maintaining the adult expression of these genes and mDA neurons with a conditional adult ablation of Nurr1 degenerate progressively [48]. Interestingly, functional interactions between pairs of transcription factors (e.g., Nurr1 and Pitx3, Pitx3 and En1/2, or Nurr1 and Foxa1/2) have been reported [49–51].
Earlier work demonstrated that En1/2 are required for the survival of mature mDA neurons during late embryonic life in a dose-dependent manner [52–54]. This might be achieved through the activation of the Erk1/2 MAPK survival pathways and suppression of the proapoptotic activity of the proneurotrophin receptor p75NTR [55]. It was also shown that En1/2 is involved in the acquisition of a mature mDA neuron identity [50]. For example, in En1 homozygous mutants (viable on a C57BL/6 background), expression at E13.5 of Pitx3, Th, Dat, Vmat2, and Ddc (encoding AADC) is reduced in the rostral-lateral mDA domain [50]. Otx2, together with Sox6, also controls mDA neuron subtype identity [56] and in the course of development, its expression becomes restricted to a specific subset of dorsal-lateral VTA mDA neurons [47]. In addition to transcription regulation, the importance of epigenetic mechanisms in all these processes must also be recalled [57].
3. Developmental Transcription Factors Required in Adult mDA Neuron Maintenance
As mentioned above, many developmental transcription factors remain expressed in mDA neurons throughout life and are required for their survival and physiological functions. We shall now briefly describe the effects of loss or gain of function of some of these transcriptions factors and their relevance to PD in adult mDA neurons.
3.1. Manipulating the Expression of Nurr1, Otx2, Foxa1/2, and Pitx3 in Adult mDA Neurons
Nurr1 expressed in adult mDA neurons of the SNpc and VTA is critical for the maintenance of their phenotype [58, 59]. Nurr1-deficient mice die shortly after birth. Nurr1 haplodeficient young animals present a normal number of mDA neurons and have no abnormal motor phenotype, but the number of mDA neurons decreases in old mice (after 15 months) in parallel with a decreased locomotor activity [48]. Nurr1+/− mice also exhibit increased vulnerability to MPTP [60] and show an exacerbated sensitivity to the toxicity of repeated methamphetamine exposure [61]. Nurr1 ablation in adult mDA neurons using AAV-Cre leads to mDA neuron dysfunction and to the progressive loss of mDA neuron markers [48]. Finally, tamoxifen-induced conditional deletion of Nurr1 in mDA neurons in 5-week-old mice results in a progressive pathology, associated with loss of or reduced striatal DA, impaired motor behaviour, and dystrophic axons and fragmented dendrites containing varicosities [62]. However, no major loss of mDA neurons was reported in these mice.
Otx2 is expressed in a subset of mDA neurons in the central and mediolateral area of the VTA in the adult [47]. Conditional knockout of Otx2 in the adult leads to selective loss of the axonal projections from VTA mDA neurons [63, 64]. Otx2 is also a negative regulator of DAT and there is an inverse correlation between Otx2 expression and glyco-DAT levels in mDA neurons [47]. Otx2 gain of function in SNpc mDA neurons decreases glyco-DAT levels, thus conferring protection against MPTP toxicity [47, 65]. Foxa1/2 also continue to be expressed in adult mDA neurons and Foxa2 heterozygous mice present late-onset, spontaneous degeneration of mDA neurons [66]. Conditional tamoxifen-inducible deletion of both Foxa1 and Foxa2 in early adulthood results in a decline of striatal DA content along with locomotor deficits and progressive loss of ALDH1A1, AADC, and DAT, ultimately leading to a reduction of mDA neurons in the SNpc of aged animals [67]. Finally, the spontaneous deletion of Pitx3 in the Aphakia mouse or global Pitx3 gene inactivation leads to rapid and preferential loss of mDA neurons in the SNpc of neonatal mice [68, 69]. Dorsal SNpc mDA neurons, which do not express Pitx3, are spared in mutant mice similar to what is observed in PD [70].
3.2. En1 Heterozygous Mice as a Model for PD
En1/2 are expressed in SNpc and VTA mDA neurons from early development on into adulthood [52]. Although En1−/− pups die (OF1 background) at birth [71], En1 heterozygous mice are viable. En1+/− mice display a normal number of mDA neurons until 6 weeks after birth when SNpc mDA neurons start to die progressively [72]. The extent of cell death reaches about 40% in the SNpc at 48 weeks of age and is correlated with a decreased DA content in the striatum. Midbrain DA neurons in the VTA are affected to a much lesser extent. En1+/− mice present PD-like motor symptoms such as decreased spontaneous locomotor activity (distance travelled, rearing), increased amphetamine-sensitization, and decreased motor coordination and sensorimotor learning (rotarod). The loss of mDA neurons in the VTA, albeit less pronounced, also leads to some nonmotor behaviour alterations such as increased depressive-like behaviour (forced swimming test), increased anhedonic-like behaviour (saccharine preference), and poor social interaction [72]. This suggests that the mesolimbic system is also affected in these mutants. The death of adult mDA neurons from En1 haploinsufficiency has now been observed in several independent studies [50, 73, 74] and follows the retrograde degeneration of axons [50, 73, 74]. En1+/−; En2−/− mice (C57BL/6 background) are normal at birth but present a massive loss of mDA neurons in the SNpc of young adult, illustrating an En1/2 dosage effect on survival [75]. Midbrain DA neurons in these mice are also more responsive to MPTP-induced cell death.
A more detailed characterization of En1 heterozygous mice revealed early signs of degeneration of mDA axon terminals in the striatum [74], prior to neuronal cell loss in the SNpc. Dopaminergic terminals become dystrophic and swollen, contain autophagic vacuoles, and present deficits in DA release and uptake. The nigral dopaminergic cell bodies exhibit signs of decreased autophagy accompanied by an increase in mTOR activity and a decrease of the autophagic marker LC3B [74]. These findings illustrate a retrograde degeneration of the nigrostriatal system in En1+/− mice, akin to what occurs in PD [6, 76, 77]. Retrograde degeneration may be a common feature of many progressive neurodegenerative disorders [78]. Individual axons in the nigrostriatal pathway of En1+/− mice undergo fragmentation supporting the idea that axonal transport failure might be an early feature of PD [79]. The possible role of autophagy in PD pathogenesis [80, 81] was recently assessed in a mouse model generated by the conditional deletion of the autophagy-related gene Atg7, which recapitulates many pathologic features of PD, including age-related loss of mDA neurons [82]. En1 heterozygous mice thus represent a valuable model to gain further insights into PD pathogenesis.
A recent study shows that mDA neurons in Lmx1b conditional knockout mice are progressively lost, in both the SNpc and the VTA. These mice also present abnormally large nerve terminals in the striatum and these terminals are filled with autophagic and lysosomal vesicles, before the onset of mDA cell loss. Very much in analogy with the En1+/− mouse phenotype, these findings suggest a retrograde degeneration of mDA neurons. Alteration of the autophagy/lysosomal pathway could be due to increased mTOR activity of mDA neurons in Lmx1b mutants and in En1 heterozygous mice. In this context it is of note that rapamycin treatment of conditional Lmx1b knockout mice normalizes the phenotypic alterations [83]. Finally, gene expression profiling in the MN9D dopaminergic cell line identified nuclear-encoded mitochondrial subunits of the respiratory chain as potential Lmx1a targets, suggesting a possible link also between Lmx1a and mitochondria [84].
4. Developmental Transcription Factors and Neuroprotective Approaches for PD
4.1. Protection of mDA Neurons by En1/2 Protein Transduction in Experiential PD Models
It is now well established that several homeoproteins, including En1/2 and Otx2, are endowed with the ability to transduce cells [85, 86]. This property was exploited to examine the therapeutic potential of En1/2. It was first shown that mDA neuronal loss in En1+/− mice can be stopped by infusing recombinant En1/2 proteins (En1 and En2 are biochemically equivalent) in the SNpc [72]. Subsequently, En1/2 protein transduction was shown to protect mDA neurons in various experimental models of PD in vitro and in vivo, including the MPTP, rotenone, 6-OHDA (6-hydroxydopamine), and mutated a-synuclein (A30P) models [87]. Interestingly, unilateral Engrailed infusion in naive mice increases ipsilateral striatal DA content. This results in amphetamine-induced turning behaviour contralateral to the side of infusion indicating an activation of the nigrostriatal pathway upon Engrailed infusion in the SNpc [87]. Thus, En1/2 is able not only to protect mDA neurons against various PD-related insults but also to increase their physiological activity. Finally, it was shown that forced expression of Otx2 in mDA neurons in the SNpc of En1+/− mice can prevent the progressive loss of mDA neurons caused by En1 haploinsufficiency [73]. Ectopic expression of Otx2 in SNpc mDA neurons also protects them against MPTP toxicity (see above). Otx2 protein transduction could thus also be of potential therapeutic interest for neuroprotection in PD. It is noteworthy that Otx2 protein transduction has previously been shown to protect retinal ganglion cells (RGCs) against NMDA (N-methyl-D-aspartate) toxicity in a mouse model of glaucoma [88].
4.2. Use of Developmental Transcription Factors for Cell Replacement Strategies
Although the feasibility of cell replacement therapy for PD has been demonstrated with embryonic ventral midbrain tissue transplantation [89], the scarcity of the material for transplantation remains a major hurdle [90]. The knowledge gained from the genes and mechanisms involved in mDA neuron development has been very valuable for the generation of midbrain DA progenitors from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) [91]. ESCs and iPSCs converted into DA progenitors expressing Lmx1a and Foxa2 by exposure to SHH and FGF8 (with or without Wnt1/TGF-β1/retinoic acid) can then differentiate into DA neurons in vitro and/or in vivo [92–94]. Similarly, Nurr1 expression together with that of the transcription factor Ascl1 (also important for mDA progenitor specification) is sufficient to drive DA differentiation of forebrain embryonic rat neural precursors [95–97]. Proper innervation of target areas and functional recovery upon transplantation of in vitro generated DA progenitors has now been demonstrated in both rodents and nonhuman primate PD models [98–101]. To further analyse the functionality of transplanted cells, two recent studies used optogenetic tools or “designer receptor exclusively activated by designer drug” (DREADD) technology to stimulate the function of engrafted DA neurons in vivo by illumination or by injecting specific drugs, respectively [102–104]. Such approaches will be particularly useful to assess the long-term function of transplanted cells in PD models.
From a safety point of view, as an alternative to DNA or RNA mediated gene delivery, a few studies have considered protein transduction as a means for reprogramming through the delivery of cocktails of recombinant proteins fused to cell penetrating peptides (CPPs) [85, 105]. It was reported that protein-based human iPSCs can efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease [106]. The neuroprotection achieved by transduction of En1/2 or Otx2 [72, 87, 88], two homeoproteins naturally containing the “penetratin” sequence, should encourage more direct protein delivery-based strategies for neuroprotection or neurorepair.
5. Mechanisms of Action of Developmental Transcription Factors in Adult mDA Neurons
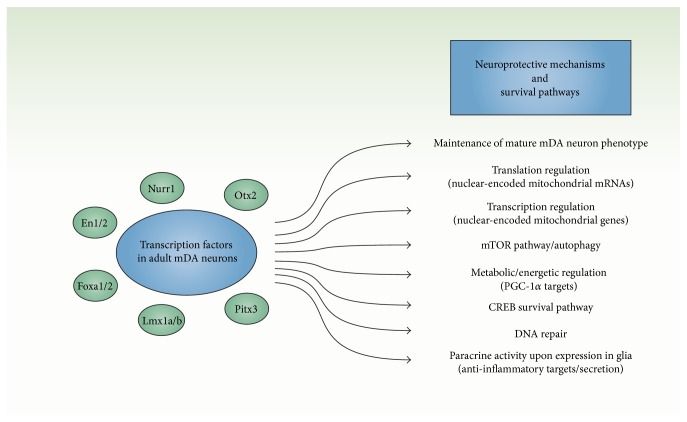
Our knowledge concerning the mechanisms of action of these developmental transcription factors in the survival and maintenance of adult mDA neurons is still limited. However, it has emerged from recent studies that these transcription factors engage several neuroprotective mechanisms and are linked to several survival pathways in adult mDA neurons (Figure 1). Since Otx2 gain of function in the SNpc can prevent cell loss in En1+/− mice [73], it is likely that Otx2 and En1/2 share some common neuroprotective mechanisms.
Figure 1.
Multiple mechanisms and signalling pathways engaged by transcription factors for neuroprotection of adult mDA neurons.
Engrailed survival activity relies on several mechanisms and signalling pathways. It is noteworthy that in addition to being a transcription factor Engrailed is also a translation regulator [31] that guides retinal axons through the translation of local mRNAs [107–110]. This property is explained by the fact that Engrailed, like many other homeoproteins, interacts with the translation initiation factor eIF4E and regulates cap-dependent translation [111, 112]. In the context of neuron survival, it was shown that Engrailed protects mDA neurons against MPTP by upregulating the translation of a subset of nuclear-encoded mitochondrial complex I subunits (e.g., Ndufs1/Ndufs3), thus enhancing complex I activity and ATP synthesis [87, 109]. The importance of translational regulation of nuclear-encoded mitochondrial mRNAs coding for respiratory chain components has also been underscored recently for the function of some PD-linked genes [113]. All these studies support the idea that a failure to sustain the high-energy demand of mDA neurons may be critical in PD pathogenesis [114, 115].
Mitochondria are critically required for long-term axonal survival and maintenance [116]. Thus, decreased mitochondrial activity might contribute to retrograde cell death in En1 heterozygous mice. As mentioned above, Engrailed plays a role in the activation of the mTOR pathway and the regulation of autophagy [74, 107]. The search for En1/2 translation targets in retinal axons of the Xenopus also identified Lamin B2, which is a major constituent of the nuclear envelope. It was shown that Lamin B2 translation in axons regulates mitochondrial size and mitochondrial membrane potential and supports axon survival [110]. En1/2 might thus play a role in mitochondrial activity and axon maintenance throughout adulthood.
Nurr1-mediated survival might involve neurotrophic GDNF/Ret signalling since Ret is a Nurr1 target gene [117, 118]. Nurr1 is downregulated by mutated α-synuclein [119, 120] and this could compromise GDNF/Ret survival signalling in PD. Absence of Ret signalling in mice causes progressive and late degeneration of the nigrostriatal system [121]. A recent study shows that Parkin cooperates with GDNF/Ret signalling to improve mitochondrial function through activation of the prosurvival NF-κB pathway and prevents mDA neuron degeneration [122]. Gene expression profiling in adult conditional Nurr1 knockout mice also identified several nuclear-encoded mitochondrial genes as potential Nurr1 transcriptional targets [62]. Thus the mitochondria appear to be a target of Nurr1 activity for mDA neuron maintenance in the adult. In addition, Nurr1 was reported to be a downstream target of the cAMP response element binding protein (CREB) mediated neuroprotection [123]. Another function of Nurr1 in the nucleus might be related to DNA double strand break repair [124]. Interestingly, Nurr1 expression is induced in microglia and astrocytes under inflammatory conditions. Nurr1 activity in these cells suppresses proinflammatory NF-κB target gene expression through recruitment of the CoREST corepressor complex [125]. A more recent study shows that forced expression of Nurr1 and Foxa2 in glial cells markedly protects mDA neurons in the MPTP mouse model of PD [126]. Nurr1 and Foxa2 act synergistically in microglia to decrease the production and release of proinflammatory cytokines and enhance the synthesis and secretion of neurotrophic factors (e.g., GDNF, BDNF, NT3, SHH, erythropoietin, thioredoxin, TGF-β, and IGF-1) with paracrine action on mDA neurons [126]. In view of the ability of homeoproteins to be secreted and internalized, it will be interesting to examine if En1/2 and Otx2 play similar roles in glial cells in a non-cell-autonomous manner. The role of non-cell-autonomous signalling by these homeoproteins has been extensively demonstrated in axon guidance in the visual system for En1/2 [107–109] and in visual cortex plasticity for Otx2 [127, 128].
Pitx3 targets are also linked to several survival pathways in mDA neurons. The Pitx3 target Aldh1a1 is crucial for the production of retinoic acid that exerts antiapoptotic and antioxidant activities. Aldh1a1 is expressed in a subpopulation of mDA neurons in the SNpc and VTA. As already mentioned, the dependence on Pitx3 is not uniform for all mDA neurons. In Pitx3 hypomorphic Aphakia mutants, a subpopulation of Pitx3-deficient neurons persists and these neurons are less vulnerable to MPTP-induced degeneration [129]. It has been suggested that striatal uptake and retrograde axonal transport of GDNF maintains proper expression of Pitx3 and its target Bdnf in SNpc mDA neurons [130]. BDNF functions in synaptic transmission, plasticity, and growth and might contribute to synaptic maintenance of the nigrostriatal mDA neurons throughout adulthood [131]. A potential link between Pitx3 and PGC-1α (peroxisome proliferator-activated receptor gamma, coactivator 1 α), which is a positive regulator of genes required for mitochondrial biogenesis and cellular antioxidant responses, has also been recognized [132]. Overexpression of PGC-1α disrupts mitochondrial activity end energy balance and this might partly be due to downregulation of Pitx3 by PGC-1α [133]. Finally, Pitx3 also regulates microRNA miR-133b expression, which in turn downregulates Pitx3 [134]. miR-133b was shown to be downregulated in PD patients but the exact role of miR-133b in mDA neuron survival is not known [134].
6. Perspectives: From Basic Science to Potential New Therapeutic Avenues for PD
The characterization of mDA neuron populations in the SNpc and VTA, based on the expression of selected markers [9] and more recent single cell gene expression profiling [135], has revealed a substantial heterogeneity across neurons. This suggests that selected sets of transcription factors expressed in mDA neuron subpopulations might determine the degrees of vulnerability in PD. These developmental transcription factors also have adult functions through the regulation of mitochondrial activity and several survival signalling pathways. Disruption of postmitotic neuron maintenance through an alteration of their transcriptional/translational regulation may lead to neurodegeneration [136, 137], often marked by cell cycle entry prior to death [138, 139]. As suggested above, many physiological processes participating in neuronal health and survival are controlled by developmental transcription factors. Possible sites of action are DNA repair and chromatin remodelling as well as pathways controlling genome stability. A recent example is Tau-mediated promotion of neurodegeneration through global heterochromatin relaxation [140]. Indeed these are homeostatic processes that can be regulated by classical signalling pathways as shown in the case of SHH [141]. From a more practical point of view, the successful use of homeoproteins, which have the innate ability to transduce, in the protection of mDA neurons has emphasized the potential of protein transduction-based strategies to deliver proteins directly into the cells of interest [142, 143]. The development of therapeutic proteins endowed with their own transduction domain, as is the case for homeoproteins, or made cell-permeable by the addition of a CPP-tag, could thus be seen as an alternative to cell grafting or gene therapy, provided that their effects be long-lasting.
Acknowledgments
The authors thank Michel Volovitch, Ariel Di Nardo, and Alain Joliot for useful discussions. The work was supported by Région Ile de France, Fondation Bettencourt Schueller, GRL Program no. 2009-00424, and ERC Advanced Grant HOMEOSIGN no. 339379.
Abbreviations
- AADC:
Dopa decarboxylase (aromatic L-amino acid decarboxylase)
- AAV:
Adenoassociated virus
- ALDH1A1:
Aldehyde dehydrogenase 1 family, member A1
- Ascl1:
Achaete-scute family bHLH transcription factor 1
- Atg7:
Autophagy-related 7
- ATP13a2:
ATPase type 13A2
- BDNF:
Brain-derived neurotrophic factor
- CALB1:
Calbindin 1, 28 kDa
- CPP:
Cell penetrating peptide
- CREB:
cAMP-responsive-element-binding protein
- DA:
Dopamine
- DAT:
Dopamine transporter (SLC6A3)
- DDC:
Gene encoding AADC
- DJ-1:
Parkinson protein 7 (PARK7)
- DRD2:
Dopamine receptor D2
- DREADD:
Designer receptors exclusively activated by designer drugs
- eiF4E:
Eukaryotic translation initiation factor 4E
- En1/2:
Engrailed-1/Engrailed-/2
- Erk1/2:
Extracellular signal-regulated-kinase 1/2
- ESC:
Embryonic stem cell
- FGF8:
Fibroblast growth factor 8
- Foxa1/2:
Forkhead box A1/2
- GDNF:
Glial cell derived neurotrophic factor
- GIRK2:
Potassium inwardly rectifying channel, subfamily J, member 6 (KCNJ6)
- IGF-1:
Insulin-like growth factor 1
- iPSC:
Induced pluripotent stem cells
- LC3B:
Microtubule-associated protein 1 light chain 3 beta (MAP1LC3B)
- Lmx1a/b:
LIM homeobox transcription factor 1, alpha/beta
- LRRK2:
Leucine-rich repeat kinase 2
- MAPK:
Mitogen-activated protein kinase
- mDA:
Midbrain dopaminergic
- MPTP:
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mTOR:
Mechanistic target of rapamycin (serine/threonine kinase)
- Ndufs1/3:
NADH dehydrogenase (ubiquinone) Fe-S protein 1/3
- NF-κB:
Nuclear factor of kappa light polypeptide gene enhancer in B-cells
- NMDA:
N-Methyl-D-aspartate
- Nurr1:
Nuclear receptor subfamily 4, group A, member 2 (Nr4a2)
- NT3:
Neurotrophin 3 (NTF3)
- 6-OHDA:
6-Hydroxydopamine
- Otx2:
Orthodenticle homeobox 2
- p75NTR:
Nerve growth factor receptor (NGFR)
- PARKIN:
Parkin RBR E3 ubiquitin protein ligase (PARK2)
- PD:
Parkinson disease
- PGC-1α:
Peroxisome proliferator-activated receptor gamma, coactivator 1 α
- PINK1:
PTEN induced putative kinase 1
- Pitx3:
Paired-like homeodomain 3
- Ret:
Ret protooncogene
- RGC:
Retinal ganglion cells
- SHH:
Sonic hedgehog
- SNCA:
Synuclein, α
- SNpc:
Substantia nigra pars compacta
- Sox6:
SRY- (sex determining region Y-) box 6
- Tfam:
Transcription factor A, mitochondrial
- TGF-β:
Transforming growth factor, β 1
- TH:
Tyrosine hydroxylase
- VMAT2:
Vesicular monoamine transporter 2 (SLC18A2)
- VTA:
Ventral tegmental area
- Wnt1:
Wingless-type MMTV integration site family, member 1.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Dawson T. M., Dawson V. L. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 2.Schapira A. H. V. Aetiopathogenesis of Parkinson's disease. Journal of Neurology. 2011;258(supplement 2):S307–S310. doi: 10.1007/s00415-011-6016-y. [DOI] [PubMed] [Google Scholar]
- 3.Lesage S., Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Human Molecular Genetics. 2009;18(1):R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 4.Verstraeten A., Theuns J., van Broeckhoven C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends in Genetics. 2015;31(3):140–149. doi: 10.1016/j.tig.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Collier T. J., Kanaan N. M., Kordower J. H. Ageing as a primary risk factor for Parkinson's disease: evidence from studies of non-human primates. Nature Reviews Neuroscience. 2011;12(6):359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng H.-C., Ulane C. M., Burke R. E. Clinical progression in Parkinson disease and the neurobiology of axons. Annals of Neurology. 2010;67(6):715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brichta L., Greengard P., Flajolet M. Advances in the pharmacological treatment of Parkinson's disease: targeting neurotransmitter systems. Trends in Neurosciences. 2013;36(9):543–554. doi: 10.1016/j.tins.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 9.Brichta L., Greengard P. Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Frontiers in Neuroanatomy. 2014;8, article 152 doi: 10.3389/fnana.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schapira A. H. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. The Lancet Neurology. 2008;7(1):97–109. doi: 10.1016/s1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 11.Eilam R., Peter Y., Elson A., et al. Selective loss of dopaminergic nigro-striatal neurons in brains of Atm- deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(21):12653–12656. doi: 10.1073/pnas.95.21.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirshner M., Galron R., Frenkel D., et al. Malfunctioning DNA damage response (DDR) leads to the degeneration of nigro-striatal pathway in mouse brain. Journal of Molecular Neuroscience. 2012;46(3):554–568. doi: 10.1007/s12031-011-9643-y. [DOI] [PubMed] [Google Scholar]
- 13.Cardozo-Pelaez F., Sanchez-Contreras M., Nevin A. B. C. Ogg1 null mice exhibit age-associated loss of the nigrostriatal pathway and increased sensitivity to MPTP. Neurochemistry International. 2012;61(5):721–730. doi: 10.1016/j.neuint.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieker C., Engblom D., Kreiner G., et al. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. Journal of Neuroscience. 2011;31(2):453–460. doi: 10.1523/jneurosci.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parlato R., Kreiner G. Nucleolar activity in neurodegenerative diseases: a missing piece of the puzzle? Journal of Molecular Medicine. 2013;91(5):541–547. doi: 10.1007/s00109-012-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang H., Shin J.-H. Repression of rRNA transcription by PARIS contributes to Parkinson's disease. Neurobiology of Disease. 2015;73C:220–228. doi: 10.1016/j.nbd.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Vilotti S., Codrich M., dal Ferro M., et al. Parkinson's disease DJ-1 l166p alters rRNA biogenesis by exclusion of TTRAP from the nucleolus and sequestration into cytoplasmic aggregates via TRAF6. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035051.e35051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suberbielle E., Sanchez P. E., Kravitz A. V., et al. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β . Nature Neuroscience. 2013;16(5):613–621. doi: 10.1038/nn.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madabhushi R., Pan L., Tsai L.-H. DNA damage and its links to neurodegeneration. Neuron. 2014;83(2):266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeppesen D. K., Bohr V. A., Stevnsner T. DNA repair deficiency in neurodegeneration. Progress in Neurobiology. 2011;94(2):166–200. doi: 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsoi H., Lau T. C.-K., Tsang S.-Y., Lau K.-F., Chan H. Y. E. CAG expansion induces nucleolar stress in polyglutamine diseases. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(33):13428–13433. doi: 10.1073/pnas.1204089109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J., Hwang Y. J., Ryu H., Kowall N. W., Ryu H. Nucleolar dysfunction in Huntington's disease. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2014;1842(6):785–790. doi: 10.1016/j.bbadis.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeusler A. R., Donnelly C. J., Periz G., et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature. 2014;507(7491):195–200. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson T. M., Ko H. S., Dawson V. L. Genetic animal models of Parkinson's disease. Neuron. 2010;66(5):646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezard E., Yue Z., Kirik D., Spillantini M. G. Animal models of Parkinson's disease: limits and relevance to neuroprotection studies. Movement Disorders. 2013;28(1):61–70. doi: 10.1002/mds.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blesa J., Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Frontiers in Neuroanatomy. 2014;8, article 155 doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakash N., Wurst W. Genetic networks controlling the development of midbrain dopaminergic neurons. The Journal of Physiology. 2006;575(2):403–410. doi: 10.1113/jphysiol.2006.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smidt M. P., Burbach J. P. H. How to make a mesodiencephalic dopaminergic neuron. Nature Reviews Neuroscience. 2007;8(1):21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- 29.Blaess S., Ang S.-L. Genetic control of midbrain dopaminergic neuron development. Wiley Interdisciplinary Reviews: Developmental Biology. 2015;4(2):113–134. doi: 10.1002/wdev.169. [DOI] [PubMed] [Google Scholar]
- 30.Alavian K. N., Scholz C., Simon H. H. Transcriptional regulation of mesencephalic dopaminergic neurons: the full circle of life and death. Movement Disorders. 2008;23(3):319–328. doi: 10.1002/mds.21640. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs J., Stettler O., Alvarez-Fischer D., Prochiantz A., Moya K. L., Joshi R. L. Engrailed signaling in axon guidance and neuron survival. European Journal of Neuroscience. 2012;35(12):1837–1845. doi: 10.1111/j.1460-9568.2012.08139.x. [DOI] [PubMed] [Google Scholar]
- 32.Alavian K. N., Jeddi S., Naghipour S. I., Nabili P., Licznerski P., Tierney T. S. The lifelong maintenance of mesencephalic dopaminergic neurons by Nurr1 and engrailed. Journal of Biomedical Science. 2014;21(1, article 27) doi: 10.1186/1423-0127-21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le W.-D., Xu P., Jankovic J., et al. Mutations in NR4A2 associated with familial Parkinson disease. Nature Genetics. 2003;33(1):85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- 34.Xu P.-Y., Liang R., Jankovic J., et al. Association of homozygous 7048G7049 variant in the intron six of Nurr1 gene with Parkinson's disease. Neurology. 2002;58(6):881–884. doi: 10.1212/wnl.58.6.881. [DOI] [PubMed] [Google Scholar]
- 35.Zheng K., Heydari B., Simon D. K. A common NURR1 polymorphism associated with Parkinson disease and diffuse Lewy body disease. Archives of Neurology. 2003;60(5):722–725. doi: 10.1001/archneur.60.5.722. [DOI] [PubMed] [Google Scholar]
- 36.Grimes D. A., Han F., Panisset M., et al. Translated mutation in the Nurr1 gene as a cause for Parkinson's disease. Movement Disorders. 2006;21(7):906–909. doi: 10.1002/mds.20820. [DOI] [PubMed] [Google Scholar]
- 37.Bergman O., Håkansson A., Westberg L., et al. PITX3 polymorphism is associated with early onset Parkinson's disease. Neurobiology of Aging. 2010;31(1):114–117. doi: 10.1016/j.neurobiolaging.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y., Ding H., Gu Z., Ma J., Chan P. Genetic variants of the PITX3 gene are not associated with late-onset sporadic Parkinson's disease in a Chinese population. Neuroscience Letters. 2011;498(2):124–126. doi: 10.1016/j.neulet.2011.04.073. [DOI] [PubMed] [Google Scholar]
- 39.Cai Y., Ding H., Gu Z., Baskys A., Ma J., Chan P. PITX3 polymorphism is not associated with Parkinson's disease in a Chinese population. Neuroscience Letters. 2011;505(3):260–262. doi: 10.1016/j.neulet.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y., Le W.-D., Jankovic J., et al. Systematic genetic analysis of the PITX3 gene in patients with Parkinson disease. Movement Disorders. 2011;26(9):1729–1732. doi: 10.1002/mds.23693. [DOI] [PubMed] [Google Scholar]
- 41.Liu J., Sun Q.-Y., Tang B.-S., et al. PITX3 gene polymorphism is associated with Parkinson's disease in Chinese population. Brain Research. 2011;1392:116–120. doi: 10.1016/j.brainres.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 42.Tang L., Zhao S., Wang M., et al. Meta-analysis of association between PITX3 gene polymorphism and Parkinson's disease. Journal of the Neurological Sciences. 2012;317(1-2):80–86. doi: 10.1016/j.jns.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 43.Haubenberger D., Reinthaler E., Mueller J. C., et al. Association of transcription factor polymorphisms PITX3 and EN1 with Parkinson's disease. Neurobiology of Aging. 2011;32(2):302–307. doi: 10.1016/j.neurobiolaging.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs J., Mueller J. C., Lichtner P., et al. The transcription factor PITX3 is associated with sporadic Parkinson's disease. Neurobiology of Aging. 2009;30(5):731–738. doi: 10.1016/j.neurobiolaging.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Rissling I., Strauch K., Höft C., Oertel W. H., Möller J. C. Haplotype analysis of the engrailed-2 gene in Young-Onset Parkinson's disease. Neurodegenerative Diseases. 2009;6(3):102–105. doi: 10.1159/000207796. [DOI] [PubMed] [Google Scholar]
- 46.Bergman O., Håkansson A., Westberg L., et al. Do polymorphisms in transcription factors LMX1A and LMX1B influence the risk for Parkinson's disease? Journal of Neural Transmission. 2009;116(3):333–338. doi: 10.1007/s00702-009-0187-z. [DOI] [PubMed] [Google Scholar]
- 47.Di Salvio M., Di Giovannantonio L. G., Acampora D., et al. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nature Neuroscience. 2010;13(12):1481–1489. doi: 10.1038/nn.2661. [DOI] [PubMed] [Google Scholar]
- 48.Kadkhodaei B., Ito T., Joodmardi E., et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. Journal of Neuroscience. 2009;29(50):15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs F. M. J., van Erp S., van der Linden A. J. A., von Oerthel L., Burbach P. H., Smidt M. P. Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development. 2009;136(4):531–540. doi: 10.1242/dev.029769. [DOI] [PubMed] [Google Scholar]
- 50.Veenvliet J. V., dos Santos M. T. M. A., Kouwenhoven W. M., et al. Specification of dopaminergic subsets involves interplay of En1 and Pitx3. Development. 2013;140(16):3373–3384. doi: 10.1242/dev.094565. [DOI] [PubMed] [Google Scholar]
- 51.Yi S.-H., He X.-B., Rhee Y.-H., et al. Foxa2 acts as a co-activator potentiating expression of the Nurr1-induced DA phenotype via epigenetic regulation. Development. 2014;141(4):761–772. doi: 10.1242/dev.095802. [DOI] [PubMed] [Google Scholar]
- 52.Simon H. H., Saueressig H., Wurst W., Goulding M. D., O'Leary D. D. M. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. Journal of Neuroscience. 2001;21(9):3126–3134. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon H. H., Thuret S., Alberi L. Midbrain dopaminergic neurons: control of their cell fate by the engrailed transcription factors. Cell and Tissue Research. 2004;318(1):53–61. doi: 10.1007/s00441-004-0973-8. [DOI] [PubMed] [Google Scholar]
- 54.Albéri L., Sgadò P., Simon H. H. Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development. 2004;131(13):3229–3236. doi: 10.1242/dev.01128. [DOI] [PubMed] [Google Scholar]
- 55.Alavian K. N., Sgadó P., Alberi L., Subramaniam S., Simon H. H. Elevated P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression. Neural Development. 2009;4(11) doi: 10.1186/1749-8104-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panman L., Papathanou M., Laguna A., et al. Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Reports. 2014;8(4):1018–1025. doi: 10.1016/j.celrep.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 57.van Heesbeen H. J., Mesman S., Veenvliet J. V., Smidt M. P. Epigenetic mechanisms in the development and maintenance of dopaminergic neurons. Development. 2013;140(6):1159–1169. doi: 10.1242/dev.089359. [DOI] [PubMed] [Google Scholar]
- 58.Zetterström R. H., Solomin L., Jansson L., Hoffer B. J., Olson L., Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276(5310):248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 59.Decressac M., Volakakis N., Björklund A., Perlmann T. NURR1 in Parkinson disease—from pathogenesis to therapeutic potential. Nature Reviews Neurology. 2013;9(11):629–636. doi: 10.1038/nrneurol.2013.209. [DOI] [PubMed] [Google Scholar]
- 60.Le W.-D., Conneely O. M., He Y., Jankovic J., Appel S. H. Reduced Nurr1 expression increases the vulnerability of mesencephalic dopamine neurons to MPTP-induced injury. Journal of Neurochemistry. 1999;73(5):2218–2221. [PubMed] [Google Scholar]
- 61.Luo Y., Wang Y., Kuang S. Y., Chiang Y.-H., Hoffer B. Decreased level of Nurr1 in heterozygous young adult mice leads to exacerbated acute and long-term toxicity after repeated methamphetamine exposure. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0015193.e15193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadkhodaei B., Alvarsson A., Schintu N., et al. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(6):2360–2365. doi: 10.1073/pnas.1221077110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borgkvist A., Puelles E., Carta M., et al. Altered dopaminergic innervation and amphetamine response in adult Otx2 conditional mutant mice. Molecular and Cellular Neuroscience. 2006;31(2):293–302. doi: 10.1016/j.mcn.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 64.Chung C. Y., Licznerski P., Alavian K. N., et al. The transcription factor orthodenticle homeobox 2 influences axonal projections and vulnerability of midbrain dopaminergic neurons. Brain. 2010;133(7):2022–2031. doi: 10.1093/brain/awq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simeone A., Di Salvio M., Di Giovannantonio L. G., Acampora D., Omodei D., Tomasetti C. The role of Otx2 in adult mesencephalic-diencephalic dopaminergic neurons. Molecular Neurobiology. 2011;43(2):107–113. doi: 10.1007/s12035-010-8148-y. [DOI] [PubMed] [Google Scholar]
- 66.Kittappa R., Chang W. W., Awatramani R. B., McKay R. D. G. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biology. 2007;5(12) doi: 10.1371/journal.pbio.0050325.e325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Domanskyi A., Alter H., Vogt M. A., Gass P., Vinnikov I. A. Transcription factors Foxa1 and Foxa2 are required for adult dopamine neurons maintenance. Frontiers in Cellular Neuroscience. 2014;8, article 275 doi: 10.3389/fncel.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwang D.-Y., Ardayfio P., Kang U. J., Semina E. V., Kim K.-S. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Molecular Brain Research. 2003;114(2):123–131. doi: 10.1016/S0169-328X(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 69.van den Munckhof P., Luk K. C., Ste-Marie L., et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130(11):2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- 70.Nunes I., Tovmasian L. T., Silva R. M., Burke R. E., Goff S. P. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wurst W., Auerbach A. B., Joyner A. L. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994;120(7):2065–2075. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- 72.Sonnier L., Le Pen G., Hartmann A., et al. Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. The Journal of Neuroscience. 2007;27(5):1063–1071. doi: 10.1523/jneurosci.4583-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Giovannantonio L. G., Di Salvio M., Acampora D., Prakash N., Wurst W., Simeone A. Otx2 selectively controls the neurogenesis of specific neuronal subtypes of the ventral tegmental area and compensates En1-dependent neuronal loss and MPTP vulnerability. Developmental Biology. 2013;373(1):176–183. doi: 10.1016/j.ydbio.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 74.Nordström U., Beauvais G., Ghosh A., et al. Progressive nigrostriatal terminal dysfunction and degeneration in the engrailed1 heterozygous mouse model of Parkinson's disease. Neurobiology of Disease. 2015;73:70–82. doi: 10.1016/j.nbd.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sgadò P., Albéri L., Gherbassi D., et al. Slow progressive degeneration of nigral dopaminergic neurons in postnatal Engrailed mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(41):15242–15247. doi: 10.1073/pnas.0602116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burke R. E., O'Malley K. Axon degeneration in Parkinson's disease. Experimental Neurology. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kordower J. H., Olanow C. W., Dodiya H. B., et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136(8):2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coleman M. P., Perry V. H. Axon pathology in neurological disease: a neglected therapeutic target. Trends in Neurosciences. 2002;25(10):532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 79.Chu Y., Morfini G. A., Langhamer L. B., He Y., Brady S. T., Kordower J. H. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson's disease. Brain. 2012;135(7):2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sánchez-Pérez A. M., Claramonte-Clausell B., Sánchez-Andrés J. V., Herrero M. T. Parkinson's disease and autophagy. Parkinson's Disease. 2012;2012:6. doi: 10.1155/2012/429524.429524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y., Coleman M., Zhang L., Zheng X., Yue Z. Autophagy in axonal and dendritic degeneration. Trends in Neurosciences. 2013;36(7):418–428. doi: 10.1016/j.tins.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmed I., Liang Y., Schools S., Dawson V. L., Dawson T. M., Savitt J. M. Development and characterization of a new Parkinson's disease model resulting from impaired auto phagy. Journal of Neuroscience. 2012;32(46):16503–16509. doi: 10.1523/jneurosci.0209-12.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laguna A., Schintu N., Nobre A., et al. Dopaminergic control of autophagic-lysosomal function implicates Lmx1b in Parkinson's disease. Nature Neuroscience. 2015;18(6):826–835. doi: 10.1038/nn.4004. [DOI] [PubMed] [Google Scholar]
- 84.Hoekstra E. J., von Oerthel L., van der Linden A. J. A., et al. Lmx1a is an activator of Rgs4 and Grb10 and is responsible for the correct specification of rostral and medial mdDA neurons. European Journal of Neuroscience. 2013;37(1):23–32. doi: 10.1111/ejn.12022. [DOI] [PubMed] [Google Scholar]
- 85.Prochiantz A. Messenger proteins: homeoproteins, TAT and others. Current Opinion in Cell Biology. 2000;12(4):400–406. doi: 10.1016/s0955-0674(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 86.Prochiantz A., Joliot A. Can transcription factors function as cell-cell signalling molecules? Nature Reviews Molecular Cell Biology. 2003;4(10):814–819. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- 87.Alvarez-Fischer D., Fuchs J., Castagner F., et al. Engrailed protects mouse midbrain dopaminergic neurons against mitochondrial complex I insults. Nature Neuroscience. 2011;14(10):1260–1266. doi: 10.1038/nn.2916. [DOI] [PubMed] [Google Scholar]
- 88.Ibad R. T., Rheey J., Mrejen S., et al. Otx2 promotes the survival of damaged adult retinal ganglion cells and protects against excitotoxic loss of visual acuity in vivo. The Journal of Neuroscience. 2011;31(14):5495–5503. doi: 10.1523/jneurosci.0187-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kefalopoulou Z., Politis M., Piccini P., et al. Long-term clinical outcome of fetal cell transplantation for parkinson disease: two case reports. JAMA Neurology. 2014;71(1):83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petit G. H., Olsson T. T., Brundin P. The future of cell therapies and brain repair: Parkinson's disease leads the way. Neuropathology and Applied Neurobiology. 2014;40(1):60–70. doi: 10.1111/nan.12110. [DOI] [PubMed] [Google Scholar]
- 91.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 92.Fasano C. A., Chambers S. M., Lee G., Tomishima M. J., Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6(4):336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Cooper O., Hargus G., Deleidi M., et al. Differentiation of human ES and Parkinson's disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Molecular and Cellular Neuroscience. 2010;45(3):258–266. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sánchez-Danés A., Consiglio A., Richaud Y., et al. Efficient generation of A9 midbrain dopaminergic neurons by lentiviral delivery of LMX1A in human embryonic stem cells and induced pluripotent stem cells. Human Gene Therapy. 2012;23(1):56–69. doi: 10.1089/hum.2011.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caiazzo M., Dell'Anno M. T., Dvoretskova E., et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 96.Pfisterer U., Wood J., Nihlberg K., et al. Efficient induction of functional neurons from adult human fibroblasts. Cell Cycle. 2011;10(19):3311–3316. doi: 10.4161/cc.10.19.17584. [DOI] [PubMed] [Google Scholar]
- 97.Addis R. C., Hsu F.-C., Wright R. L., Dichter M. A., Coulter D. A., Gearhart J. D. Efficient conversion of astrocytes to functional midbrain dopaminergic neurons using a single polycistronic vector. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028719.e28719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wernig M., Zhao J.-P., Pruszak J., et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(15):5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hallett P. J., Deleidi M., Astradsson A., et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell. 2015;16(3):269–274. doi: 10.1016/j.stem.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim J., Su S. C., Wang H., et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9(5):413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kriks S., Shim J.-W., Piao J., et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480(7378):547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steinbeck J. A., Choi S. J., Mrejeru A., et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nature Biotechnology. 2015;33(2):204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vazey E. M., Aston-Jones G. New tricks for old dogmas: optogenetic and designer receptor insights for Parkinson's disease. Brain Research. 2013;1511:153–163. doi: 10.1016/j.brainres.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dell'Anno M. T., Caiazzo M., Leo D., et al. Remote control of induced dopaminergic neurons in parkinsonian rats. The Journal of Clinical Investigation. 2014;124(7):3215–3229. doi: 10.1172/jci74664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou H., Wu S., Joo J. Y., et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rhee Y.-H., Ko J.-Y., Chang M.-Y., et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. The Journal of Clinical Investigation. 2011;121(6):2326–2335. doi: 10.1172/jci45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brunet I., Weinl C., Piper M., et al. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438(7064):94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wizenmann A., Brunet I., Lam J. S. Y., et al. Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo. Neuron. 2009;64(3):355–366. doi: 10.1016/j.neuron.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stettler O., Joshi R. L., Wizenmann A., et al. Engrailed homeoprotein recruits the adenosine A1 receptor to potentiate ephrin A5 function in retinal growth cones. Development. 2012;139(1):215–224. doi: 10.1242/dev.063875. [DOI] [PubMed] [Google Scholar]
- 110.Yoon B. C., Jung H., Dwivedy A., O'Hare C. M., Zivraj K. H., Holt C. E. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148(4):752–764. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nédélec S., Foucher I., Brunet I., Bouillot C., Prochiantz A., Trembleau A. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(29):10815–10820. doi: 10.1073/pnas.0403824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Topisirovic I., Borden K. L. B. Homeodomain proteins and eukaryotic translation initiation factor 4E (elF4E): an unexpected relationship. Histology and Histopathology. 2005;20(4):1275–1284. doi: 10.14670/HH-20.1275. [DOI] [PubMed] [Google Scholar]
- 113.Gehrke S., Wu Z., Klinkenberg M., et al. PINK1 and parkin control localized translation of respiratory chain component mRNAs on mitochondria outer membrane. Cell Metabolism. 2015;21(1):95–108. doi: 10.1016/j.cmet.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Court F. A., Coleman M. P. Mitochondria as a central sensor for axonal degenerative stimuli. Trends in Neurosciences. 2012;35(6):364–372. doi: 10.1016/j.tins.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Exner N., Lutz A. K., Haass C., Winklhofer K. F. Mitochondrial dysfunction in Parkinson′s disease: molecular mechanisms and pathophysiological consequences. The EMBO Journal. 2012;31(14):3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pissadaki E. K., Bolam J. P. The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson's disease. Frontiers in Computational Neuroscience. 2013;7, article 13 doi: 10.3389/fncom.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wallén Å., Castro D. S., Zetterström R. H., et al. Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Molecular and Cellular Neuroscience. 2001;18(6):649–663. doi: 10.1006/mcne.2001.1057. [DOI] [PubMed] [Google Scholar]
- 118.Galleguillos D., Fuentealba J. A., Gómez L. M., et al. Nurr1 regulates RET expression in dopamine neurons of adult rat midbrain. Journal of Neurochemistry. 2010;114(4):1158–1167. doi: 10.1111/j.1471-4159.2010.06841.x. [DOI] [PubMed] [Google Scholar]
- 119.Decressac M., Kadkhodaei B., Mattsson B., Laguna A., Perlmann T., Björklund A. α-synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Science Translational Medicine. 2012;4(163) doi: 10.1126/scitranslmed.3004676.163ra156 [DOI] [PubMed] [Google Scholar]
- 120.Lin X., Parisiadou L., Sgobio C., et al. Conditional expression of Parkinson's disease-related mutantα-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. Journal of Neuroscience. 2012;32(27):9248–9264. doi: 10.1523/jneurosci.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kramer E. R., Aron L., Ramakers G. M. J., et al. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biology. 2007;5(3, article e39) doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meka D. P., Müller-Rischart A. K., Nidadavolu P., et al. Parkin cooperates with GDNF/RET signaling to prevent dopaminergic neuron degeneration. Journal of Clinical Investigation. 2015;125(5):1873–1885. doi: 10.1172/jci79300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Volakakis N., Kadkhodaei B., Joodmardi E., et al. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(27):12317–12322. doi: 10.1073/pnas.1007088107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Malewicz M., Kadkhodaei B., Kee N., et al. Essential role for DNA-PK-mediated phosphorylation of NR4A nuclear orphan receptors in DNA double-strand break repair. Genes and Development. 2011;25(19):2031–2040. doi: 10.1101/gad.16872411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saijo K., Winner B., Carson C. T., et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oh S. M., Chang M. Y., Song J. J., et al. Combined Nurr1 and Foxa2 roles in the therapy of Parkinson's disease. EMBO Molecular Medicine. 2015;7(5):510–525. doi: 10.15252/emmm.201404610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sugiyama S., Di Nardo A. A., Aizawa S., et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134(3):508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 128.Spatazza J., Lee H. H. C., Di Nardo A. A., et al. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Reports. 2013;3(6):1815–1823. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luk K. C., Rymar V. V., van den Munckhof P., et al. The transcription factor Pitx3 is expressed selectively in midbrain dopaminergic neurons susceptible to neurodegenerative stress. Journal of Neurochemistry. 2013;125(6):932–943. doi: 10.1111/jnc.12160. [DOI] [PubMed] [Google Scholar]
- 130.Peng C., Aron L., Klein R., et al. Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. The Journal of Neuroscience. 2011;31(36):12802–12815. doi: 10.1523/jneurosci.0898-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu B., Nagappan G., Guan X., Nathan P. J., Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nature Reviews Neuroscience. 2013;14(6):401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 132.Zheng B., Liao Z., Locascio J. J., et al. PGC-1α, a potential therapeutic target for early intervention in Parkinson's disease. Science Translational Medicine. 2010;2(52):52ra–73ra. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Clark J., Silvaggi J. M., Kiselak T., et al. Pgc-1alpha overexpression downregulates Pitx3 and increases susceptibility to MPTP toxicity associated with decreased Bdnf. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0048925.e48925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim J., Inoue K., Ishii J., et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317(5842):1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Poulin J.-F., Zou J., Drouin-Ouellet J., Kim K.-Y. A., Cicchetti F., Awatramani R. B. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Reports. 2014;9(3):930–943. doi: 10.1016/j.celrep.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deneris E. S., Hobert O. Maintenance of postmitotic neuronal cell identity. Nature Neuroscience. 2014;17(7):899–907. doi: 10.1038/nn.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holmberg J., Perlmann T. Maintaining differentiated cellular identity. Nature Reviews Genetics. 2012;13(6):429–439. doi: 10.1038/nrg3209. [DOI] [PubMed] [Google Scholar]
- 138.Herrup K., Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nature Reviews Neuroscience. 2007;8(5):368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- 139.Höglinger G. U., Breunig J. J., Depboylu C., et al. The pRb/E2F cell-cycle pathway mediates cell death in Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3585–3590. doi: 10.1073/pnas.0611671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frost B., Hemberg M., Lewis J., Feany M. B. Tau promotes neurodegeneration through global chromatin relaxation. Nature Neuroscience. 2014;17(3):357–366. doi: 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gonzalez-Reyes L. E., Verbitsky M., Blesa J., et al. Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron. 2012;75(2):306–319. doi: 10.1016/j.neuron.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Joshi R. L., Ibad R. T., Rheey J., Castagner F., Prochiantz A., Moya K. L. Cell non-autonomous functions of homeoproteins in neuroprotection in the brain. FEBS Letters. 2011;585(11):1573–1578. doi: 10.1016/j.febslet.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 143.Prochiantz A., Di Nardo A. A. Homeoprotein signaling in the developing and adult nervous system. Neuron. 2015;85(5):911–925. doi: 10.1016/j.neuron.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]