Abstract
Transplanted stem cells (SCs), owing to their regenerative capacity, represent one of the most promising methods to restore erectile dysfunction (ED). However, insufficient source, invasive procedures, ethical and regulatory issues hamper their use in clinical applications. The endogenous SCs/progenitor cells resident in organ and tissues play critical roles for organogenesis during development and for tissue homeostasis in adulthood. Even without any therapeutic intervention, human body has a robust self-healing capability to repair the damaged tissues or organs. Therefore, SCs-for-ED therapy should not be limited to a supply-side approach. The resident endogenous SCs existing in patients could also be a potential target for ED therapy. The aim of this review was to summarize contemporary evidence regarding: (1) SC niche and SC biological features in vitro; (2) localization and mobilization of endogenous SCs; (3) existing evidence of penile endogenous SCs and their possible mode of mobilization. We performed a search on PubMed for articles related to these aspects in a wide range of basic studies. Together, numerous evidences hold the promise that endogenous SCs would be a novel therapeutic approach for the therapy of ED.
Keywords: endogenous stem cell, erectile dysfunction, homing, niche
INTRODUCTION
The morbidity of erectile dysfunction (ED) is about 50% in adult men which means this disease bothers >300 million male in worldwide. Although oral phosphodiesterase type 5 inhibitors (PDE5-Is) is a commonly used as first-line treatment for ED. The clinical efficacy of PDE5-Is reaches to 70% with mild side effects, however, the clinical efficacy of PDE5-Is for the severe ED caused by diabetes, surgery, and severe cardiovascular disease is limited.1 Although recent data have revealed that daily intake of tadalafil (5 mg) help to improve the function of endothelial cells in ED patients, but this is only a preliminary research, and its mechanism is still unknown.2 The second line treatments for ED include vacuum devices and intracavernosal injection therapy. Penile erection is usually induced after 5–10 min post the drug injection with an effective rate of about 70%, but accompanied with common complications such as pain, priapism, and fibration.3 For patients without a response to the first-line and second line treatment, prosthesis implantation is usually as the last choice. Though its effective rate is about 90%, high cost, risk of infection, erosion, and equipment failure are the significant limitations.4
All these methods of current therapies on ED are mainly used as on demanded treatment to temporarily enhance erectile function to relieve the symptoms, which could not reverse the pathological changes in erectile tissues,5,6 and the most of patients are expected an ideal method to restore their natural erectile function, which provide important scientific issue on future research on ED.
It is found that stem cells (SCs) are unspecialized precursor cells that are uniquely capable of both differentiation and self-renewal. Recently, SCs including adipose-derived SC (ADSC),7 bone marrow SC (BMSC),8 embryonic SC (ESC),9 endothelial progenitor cell (EPC),10 and skeletal muscle SC11 have drawn great attention in reversing the pathological changes in erectile tissues of ED in animal model. Increasing evidences have shown that SCs improve pathological changes in the ED mainly by secreting some cytokines.7,12,13 Notably, the using of ESC has many limitations because of the ethical issues and immunogenicity. Recently, new technologies are springing up to reprogram adult somatic cells to enter a pluripotent state.14 These cells, normally termed “induced pluripotent stem (iPS) cells,” enable the transplantation without the need for immunosuppression. But we also should bear in mind that iPS cells have a risk of tumorigenicity and in some circumstances may cause immunological reaction. On the other hands, many postnatal tissues contain SCs which normally termed “adult SCs (ASCs)” or “tissue-specific SCs.” ASCs have recently developed as a foundation for regenerative therapy because they are easily available and used for therapeutics after ex vivo purification and expansion. However, time/cost-intensive, invasive procedures, and low retention rate in the injected site have limited the clinical application of ASCs.15
Stem/progenitor cells reside in every adult organ and tissue, which might play an important role in tissue homeostasis and injury repair. Although increasing evidences suggest the presence of penile endogenous SCs, the regenerative potential that rely on endogenous SCs/progenitor cells thus offer new insights into ED therapy,16,17,18 while underline mechanisms about their mobilization and recruitment are far from well understood. This review presents a brief outline on the detection methods and possible regulatory mechanisms of endogenous SCs, with particular emphasis placed upon the characterization and possible regulatory mechanisms of activating and mobilizing penile endogenous SCs.
STEM CELLS RESIDE IN NICHES
Stem cell niche, consisting signaling molecules, intercellular communication and the interaction between SCs and their in vivo milieu, is highly organized microenvironment. Within a niche, SCs are in contact with supporting cells, which provide short-range signals via soluble factors as well as via transmembrane proteins. SCs also keep closely contacting with extracellular matrix, a chemically and physically cross-linked complex network that provides biochemical and mechanical signals. Blood vessels are believed to constitute niches, transport long-range signals, and recruit circulating cells into the bone marrow (BM) or tissue-specific niches.19,20 Moreover, metabolic signals such as reactive oxygen species can also influence niche function.21 The pivotal function of SC niche is to maintain the dynamic balance of quiescent and activated SCs. SCs physiologically resident in tissue-specific niches are capable of sustaining/replenishing the SC pool and differentiating into multiple matching cell lineages.22
It is now well-documented that SC niches are present in many adult organs and tissues, including BM, brain, skin, skeletal muscle, heart, gut, and ovarian epithelium.23,24,25 The BM is the main reservoir of many types of SCs. Under steady state, a small quantity of SCs constantly leaves the BM, enters the blood or tissues, and travels back to the BM or peripheral tissue-specific niches. Specific cellular components within the BM niche, including vascular/perivascular cells,26 nestin positive MSCs,27 osteoblasts,28 macrophages,29,30 and neurons of the sympathetic nervous system31 have been identified as important regulators of SCs maintenance and function. As demonstrated in the hematopoietic SC (HSC) niche, interstitial cells express adhesion molecules such as vascular cellular adhesion molecule 1 (VCAM-1) and SC factor (SCF), which binds to HSC receptor α4 β1and c-kit respectively.32 In addition, the binding of stromal derived factor-1 (SDF-1, also called CXCL-12) to its receptor (cxc chemokine receptor 4, CXCR4) on HSC surface also plays a key role in the HSC retention within the BM.33
The SC microenvironment (niche) is thought to influence/control the “stemness” of SCs, that is, self-maintenance or differentiation.34 The importance of the niche as an SC regulator for cell fate determination is exemplified by the fact that SCs tend to quickly differentiate when removed from their microenvironment and cultured in vitro. An interesting theory is that SCs can undergo various patterns of differentiation depending on appropriate epigenetic signals in mature tissues. For example, Galli et al.35 demonstrated that human neural SCs could generate skeletal muscle cells in vitro and in vivo, following direct exposure to myoblasts and transplantation into skeletal muscle respectively. Zhao et al.36 found that human BMSCs implanted into the brain can differentiate into neural cells expressed markers for astrocytes (GFAP(+)), oligodendroglia (GalC(+)), and neurons (beta III(+), NF160(+), NF200(+), hNSE(+), and hNF70(+)). These findings support that SCs can generate terminal differentiated cells specific to the host niche in which they reside. Nevertheless, the mechanism of SC differentiation regulated by specific niche still warrants further investigation.
QUIESCENT AND ACTIVATION OF ENDOGENOUS STEM CELLS
The tissue-specific ASCs are crucial for physiological tissue turnover and regeneration. Mammalian ASCs are generally in a predominantly quiescent (out of cell cycle and in a lower metabolic level) state,28 but are able to exit quiescence and rapidly expand and differentiate in response to stress. In principle, SCs in their niches undergo different fates: SCs (i) remain in a relatively quiescent (nondividing) state, (ii) undergo apoptosis or death, and (iii) trigger self-renewal divisions that result in two daughter SCs (termed symmetric divisions), one daughter SC and one committed progenitor (termed asymmetric divisions), or two differentiated cells.37 In general, rapidly growing tissues co-exist two states of activation and quiescent SCs, and states can convert to each other. An improved understanding of the regulatory mechanisms that direct SC fate determination has great significance to modulate and activate existing SCs within tissues.
The quiescent state appears to be necessary for preserving long-term reconstituting capacity of SCs. This is best exemplified by HSC, it remains quiescent under steady state and only a rare set of HSC undergo a massive expansion to produce mature blood cells, which provides a lifelong blood supply without exhausting the HSC pool.38 However, HSC can exit quiescence and rapidly proliferate to re-establish homeostatic conditions in response to stress.39 Defects in the regulation of the quiescent state will result in premature exhaustion of the HSC pool.40 SCs quiescence and activation are likely controlled by cooperation of intrinsic mechanisms and extrinsic signals.
Recent researches have shown that p53 is critical for SC self-renewal and quiescence. Meletis et al.41 found that p53 suppresses neural SC proliferation and self-renewal. Liu et al.42 demonstrated that two p53 target genes, Gfi-1, and Necdin, are important regulators of HSC quiescence. Foxo 3a is a forkhead-type transcription factor that is essential for SC quiescence.43 Without Foxo 3a, NSC may lose their ability to re-enter a state of relative quiescence, which may lead to the amplification of progenitors and the exhaustion of the NSC in vivo.44 Hypoxia-inducible factor-1α (HIF-1 α) is a master transcriptional regulator under low oxygen conditions. HSCs from HIF-1α-deficient model reduced quiescence and decreased the number during various stresses including bone marrow transplantation, myelosuppression, or aging.45
It has been reported that nuclear factor of activated T cells c1 (NFATc1) is preferentially expressed by hair follicle SCs in their niche, where its expression is activated by bone morphogenic proteins (BMPs) signaling upstream and it acts downstream to transcriptionally repress CDK4 and maintain SC quiescence. When NFATc1 is suppressed pharmacologically (e.g., cyclosporine A), hair follicle SCs are activated to proliferate and form the new hair follicle.46 Goldstein et al.47 showed that NFATc1 up-regulates the expression of prolactin receptor which drives quiescence of hair follicle SCs via JAK/STAT5 signaling. Interactions between SCs and their in vivo milieu are crucial for adult hematopoiesis. Members of the transforming growth factor-beta (TGF-β) family involves in a wide range of cell functions, such as cell cycle regulation, neuronal differentiation, and survival. Neutralization of TGF-β1 releaseearly human hematopoietic progenitors from quiescence of early human hematopoietic progenitors from quiescence into cycling in vitro.48 Kandasamy et al.49 demonstrated that TGF-β1 can both promote SC quiescence and neuronal survival. BMP have been implicated as key regulators of hematopoietic development. In addition, the interaction between BMP and Wnt signaling pathway takes part in the hair follicle SC activation by inducing the entry of β-catenin into nucleus.50,51 Arai et al.28 demonstrated that the Tie2/Angiopoietin-1 signaling pathway plays a critical role in the maintenance of HSCs in a quiescent state in the BM niche. Other extrinsic micro-environmental regulatory mechanisms include adhesion molecules (e.g., N-cadherin and β1-integrin), thrombopoietin, osteopontin (Opn), and more.
STME CELL MOBILIZATION
Appropriate SC functions require SC trafficking regardless of whether the cell is endogenous or exogenous administered.52,53 In the field of SC research, SC homing refers to the capability to find its way to a particular anatomic destination. In response to diseases or tissue injuries, the body's self-healing via host cells likely involves recruiting endogenous SCs from either the BM through bloodstream or a tissue-specific niche.54,55,56 Asahara et al.55 found that EPCs exist in the human peripheral blood, and they could incorporate into the sites of active angiogenesis. Rochefort et al.57 reported that multipotential mesenchymal SCs could be mobilized into peripheral blood when animals are exposed to chronic hypoxia. In addition, SCs/progenitor cells residing within surrounding healthy tissues may also be recruited to an injury site for therapeutics. For example, neural precursors in the subventricular zone (SVZ) play an important role in the repairing of focal brain lesion.58 In this regard, therapeutic interventions by endogenous SC homing hold great promise in the clinical aspect.
Granulocyte colony-stimulating factor (CSF) is the most commonly used agent for clinical HSC mobilization. It has been used to mobilize CD34(+) cells into peripheral blood which has largely replaced BM as a source of SCs for both autologous and allogeneic cell transplantation.56 Possible mechanisms underlying its function include the promotion of granulocyte expansion, clearance of VCAM-1, also called CD10659 and disruption of the SDF-1/CXCR4 axis.60 SDF-1/CXCR4 axis plays a pivotal role in HSC quiescence and retention within the BM. Plerixafor (Mozobil, AMD3100), a CXCR4 antagonist, can inhibit the binding of SDF-1α to its receptor CXCR4. It facilitates rapid mobilization of CD34(+) cells from the BM into the peripheral blood.61 Other agents clinical used for SC mobilization includes cyclophosphamide, granulocyte-macrophage CSF, and recombinant human SCF.
In addition, there are also some novel and experimental strategies for SC mobilization. These ways mainly target cell adhesion molecules (VCAM/VLA4 inhibitors and proteasome inhibitors), redox signaling (stabilization of HIF), chemokines (CCL2, CXCL-1, CXCL-7, CXCL-12) and their receptors (CCR2, CXCR3, CXCR4). Shen et al.62 developed a knitted silk-collagen sponge scaffold by incorporating of SDF-1α to encourage recruiting of endogenous SCs for in situ tendon regeneration. After 4 weeks, SDF-1α treatment group had increased expression of tendon repair gene markers and exhibited a better therapeutic effect than the control group. Meanwhile, Chim et al.63 used SDF-1α in combination with BMP or TGF-β1 to induce cell migration in a rat model. The results showed that SDF-1α promotes cell migration into the scaffolds and can result in osteogenic and chondrogenic differentiation. In both studies, no exogenous SCs were used before scaffold implantation. Rather, they all relied on endogenous SC recruitment and local tissue responses to achieve tendon regeneration, osteogenesis, and chondrogenesis.
CLASSIFICAITON LOCALIZATION OF ENDOGENOUS STEM CELLS
It is generally believed that tissue-specific SCs exist in most postnatal tissues. BM is the biggest SC pool that contains at least two types of SCs: HSC and mesenchymal SCs; the former gives the entire hematopoietic system, and the latter is a subpopulation of perivascular cells.27,64,65 Neural SCs mainly reside in their specialized microenvironments: the SVZ of the lateral ventricles and the subgranular zone of the dentate gyrus within the hippocampus.66,67 Skeletal muscle has a tremendous ability to regenerate due to the mobilization of its own tissue-specific SCs, known as satellite cells, which are located between the basal lamina and the sarcolemma of the muscle fiber.68 In 2008, Lin et al.69 firstly defined the location of ADSCs within human adipose tissue, and they found that ADSC are likely vascular SCs at various stages of differentiation. A study conducted by Bussolati et al.70 showed that adult renal progenitor cells expressing PAX2 and the antigen CD133 (a marker of HSC) could be isolated from human renal cortex samples. In adulthood, tissue homeostasis and repair are critically dependent on both the self-renewal and the differentiation capacity of these SCs.
To identify such cells, various approaches have been employed, including the use of SC markers. Leucine-rich G protein-coupled receptor 5 (Lgr5) is a 7-transmembrane receptor which has recently gained prominence as a marker of ASC populations in the hair-follicle and intestine.71,72,73 A closely-related protein, Lgr6 marks ASCs in the hair follicle that generate all cell lineages of the skin.74 However, it is worth to note that CD34 is the classical marker for HSC, while it also expresses in some non-SCs such as endothelial cells75 as well as c-kit, another HSC marker, is also expressed in the interstitial cells of Cajal and urinary tracts.76,77 Stro-1 is one of the most well-known markers for MSCs, but it is not universally expressed in all reported types of MSCs.78
Due to the lack of specific markers for tissue-specific SCs, potential SCs in some researches have been identified using the “label-retaining cell (LRC)” strategy.79,80 Quiescent SCs have some characteristics in common, such as low amount of RNA,81 scarce proliferative cell markers,82 and retaining of some cell labels.83 LRCs strategy is frequently explored for identification of quiescent SCs. The underlying mechanism is based on the principle that the rapidly proliferating cells will lose cell label in a short time while quiescent cells and slow-cycling cells will retain the label in a longer period. A label-retaining assay contains two essential parts: a pulse period and a chase period. BrdU, EdU or radiolabel led DNA analogs can be administrated into the animals for a certain time (the pulse) to label all of the proliferating cells. The labeling reagents will be then taken away for a prolonged period (the chase) before the tissues to be examined. Fast-cycling cells are constantly dividing and dilute the label through each round of division. Therefore, after the chase, their original label is diluted to a degree in which it can no longer be detected. Conversely, slow-cycling cells divide infrequently during the chase period. Therefore, they retain significant amounts of the label and appear as LRCs. EdU labeling was first established in 2008 and is one of the most effective and important labeling techniques in researching endogenous quiescent SCs, as it does not interfere cellular duplication, differentiation, secretion, and mobilization.84
PENILE ENDOGENOUS STEM CELLS
The penis is composed of multiple types of tissues, such as skin, tunica albuginea (TA), corpora cavernosa, corpus spongiosum, blood vessels, nerves, and urethra. Two types of foreskin SCs have been isolated to date, including skin-derived progenitors (SKPs) and MSCs. Toma et al. have isolated, expanded, and characterized SKPs from mammalian dermis.85 Then, they found that human SKPs grew in suspension as spheres in the presence of the mitogens fibroblast growth factor 2 and expressed nestin. Clonal analysis indicated that single SKPs were multipotent and could give rise to both neural and mesodermal progeny.86 Meanwhile, MSCs were also isolated from human postnatal dermal tissues. The isolated cells could clonal grow and be differentiated into adipogenic, osteogenic, and myogenic lineages.87 All these results indicated that penile skin may provide an accessible, autologous source of SCs.
Vernet et al.16 investigated whether cells from normal TA and Peyronie's disease (PD) plaques undergo osteogenesis, express SCs markers, or give rise to other cell lineages via processes modulated by TGF-β1. In this study, osteogenic markers (alkaline phosphatase and Opn) and calcification were found in the TA and PD cells in osteogenic medium. Both cultures expressed SC marker CD34, but none of the cultures underwent adipogenesis in adipogenic medium. Incubation with TGF-β1 increased osteogenesis and myofibroblast differentiation and reduced CD34 antigen expression in both cultures. In addition, putative endogenous SCs were shown in the penile shaft tissue sections from murine by detecting CD34 and a possible Sca1 variant.17
Very recently, Lin et al.18 used LRC strategy to identify potential SCs/progenitor cells in the penis. In this study, they found that numerous cells in the penis of neonatal rat were labeled by EdU, but the number of labeled cells dropped sharply within 1 week. In addition, the labeled cells were mainly distributed in the subtunic and perisinusoidal spaces (Figure 1). After isolation and culture in vitro, the EdU-labeled LRCs could form cell clones, which is a one of the SC properties. However, they also found that labeling of penis cells by EdU occurred randomly, and label-retaining was not associated with some SC markers, such as c-kit or PCNA, except A2B5. The results suggest that penile endogenous SC modulation might be viable therapeutic approaches for ED.
Figure 1.
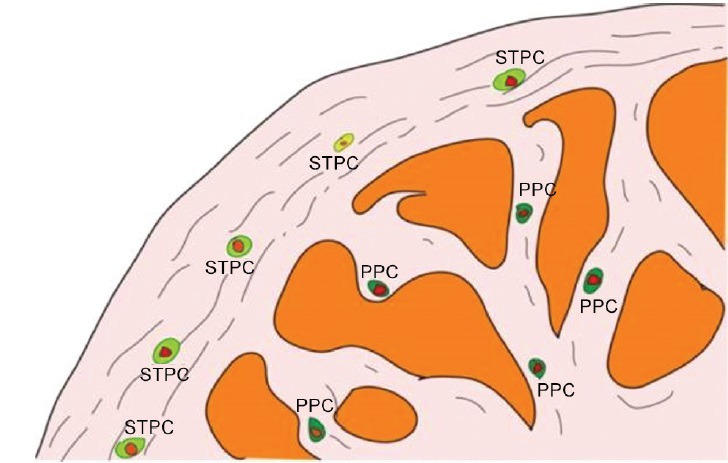
Hypothetical scheme of the EdU labeled penile label-retaining cells18 (PPC: perisinusoidal penile progenitor cell; STPCs: subtunic penile progenitor cell).
POSSIBLE APPROACHES TO UTILIZE ENDOGENOUS STEM CELLS FOR ERECTILE DYSFUNCTION
The conversion of physical medical signal into biomedical signal is regarded as the third revolution in biomedicine. Currently, low energy extracorporeal shock wave therapy (LESWT) has been applied widely as a novel noninvasive strategy for wound healing,88 bone regeneration,89 ameliorating inflammatory,90 osteochondritis dissecans,91 chronic hind limb ischemia,92 plantar fasciitis,93 ED94,95 and many other diseases. Potential mechanisms discussed herein mainly include initial angiogenesis, stimulated cell proliferation and differentiation, anti-inflammatory as well as suppression of nociception.
Interestingly, some of these studies pointed out that the beneficial tissue effects were likely mediated by recruitment of mesenchymal SCs. In one study of a rat model of chronic hind limb ischemia, LESWT was found to enhance the recruitment of circulating EPCs into nonischemic as well as in chronic ischemic tissue.92 In another study, Chen et al.96 demonstrated that LESWT treatment could enhance recruitment of MSCs to improve bone repair by up-regulating the expression of TGF-β1 and vascular endothelial growth factor. More importantly, Qiu et al.97 also found that LESWT can ameliorate diabetes mellitus (DM)-associated ED by promoting regeneration of erectile components (nNOS-positive nerves, endothelium, and smooth muscle) in the penis. In this study, they have used LRC strategy for the identification of putative SCs, and found that LESWT treatment could significantly increase the EdU labeled cell in the penis. However, it is still unclear whether these therapeutic effects of LESWT on ED are achieved by endogenous SCs or other mechanisms.
ACTIVATION OF ENDOGENOUS STEM CELLS
p38 pathway is essential for the differentiation of various SCs/progenitor cells. Jones et al.98 found that somatic SCs could be activated from the quiescent state and undergo myogenic differentiation via activation of p38 pathway. In addition, adult neural differentiation also can be promoted via activation of p38 pathway.99 Herba Epimedii, a traditional Chinese medicine, has been traditionally used for the treatment of ED for centuries in East Asian countries. Several studies100,101 have demonstrated that icariside II (ICA II, C27H32O10, 514.54) possess notable erectogenic effects in the treatment of ED. Interestingly, a recently published study reported that ICA II can activate p38 pathway.102 Recently, Xu et al.100 conducted a research, in which LCR strategy were used for tracking the putative endogenous SCs, to investigate the underlying mechanisms of ICA II in treating ED. The results showed that ICA II could effectively restore erectile function and prevent distortion of penile histopathological changes in a rat model of bilateral cavernous nerve injury. What's more, the results indicated that these therapeutic effects of ICA II involve enhanced endogenous SCs differentiation, which may be regulated by p38 pathway.
On the other hand, the EPCs play an important role in vascular repair,103 and previous study has showed that the number of circulating EPCs is reduced in patients with ED.104 Moreover, the EPCs resident in the vascular wall are capable to differentiate into mature endothelial cells, hematopoietic, and local immune cells.105 These results indicate that EPCs in the penis may also serve as a source for progenitor cells for postnatal vasculogenesis. Increasing evidences have demonstrated that the pathophysiological mechanisms of diabetic ED are associated with oxidative stress.106 Melatonin, an antioxidant, can reduce the level of oxidative stress induced by diabetes.107 Qiu et al.108 found that melatonin has a beneficial effect on preventing ED in a rat model of DM. In this study, chronic administration of melatonin increased the superoxide dismutase level and decreased the malondialdehyde level in BM accompanied with an increased number of circulating EPCs. These results indicated that the salutary effect of melatonin on ED may result from the mobilization of EPCs.
CONCLUSIONS
Endogenous SCs exist in a certain place of different tissues or organs, which is an alternative choice for SCs therapy compared to the exogenous SCs. Endogenous SCs possess the ability of self-renewal and are always in a quiescent state with low level of metabolism, but under some pathological condition, they will be activated and differentiated into various types of cells. As a part of a preventive medicine, the maintenance of SCs fitness represents the most obvious method for conservation of initial healing capacity of a healthy organism. Likewise, in ED patients, reactivation of endogenous SC potential might help the rejuvenation of damaged erectile function. Therefore, the enhanced proliferation of penile endogenous SCs from these niches, as well as their commitment differentiation into damaged cell population should be a novel therapeutic approach for future ED therapy.
COMPETING INTERESTS
Nothing to declare.
AUTHOR CONTRIBUTIONS
YDX drafted the manuscript. ZCX, GTL, FTL, YLG revised the manuscript. All authors approved the final version.
REFERENCES
- 1.Hellstrom WJ, Gittelman M, Karlin G, Segerson T, Thibonnier M, et al. Sustained efficacy and tolerability of vardenafil, a highly potent selective phosphodiesterase type 5 inhibitor, in men with erectile dysfunction: results of a randomized, double-blind, 26-week placebo-controlled pivotal trial. Urology. 2003;61:8–14. doi: 10.1016/s0090-4295(03)00115-8. [DOI] [PubMed] [Google Scholar]
- 2.Wrishko R, Sorsaburu S, Wong D, Strawbridge A, McGill J. Safety, efficacy, and pharmacokinetic overview of low-dose daily administration of tadalafil. J Sex Med. 2009;6:2039–48. doi: 10.1111/j.1743-6109.2009.01301.x. [DOI] [PubMed] [Google Scholar]
- 3.Hatzichristou DG, Apostolidis A, Tzortzis V, Ioannides E, Yannakoyorgos K, et al. Sildenafil versus intracavernous injection therapy: efficacy and preference in patients on intracavernous injection for more than 1 year. J Urol. 2000;164:1197–200. [PubMed] [Google Scholar]
- 4.Mulcahy JJ, Wilson SK. Current use of penile implants in erectile dysfunction. Curr Urol Rep. 2006;7:485–9. doi: 10.1007/s11934-006-0059-0. [DOI] [PubMed] [Google Scholar]
- 5.McMahon CN, Smith CJ, Shabsigh R. Treating erectile dysfunction when PDE5 inhibitors fail. BMJ. 2006;332:589–92. doi: 10.1136/bmj.332.7541.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein I, Kim E, Steers WD, Pryor JL, Wilde DW, et al. Efficacy and safety of tadalafil in men with erectile dysfunction with a high prevalence of comorbid conditions: results from MOMENTUS: multiple observations in men with erectile dysfunction in National Tadalafil Study in the US. J Sex Med. 2007;4:166–75. doi: 10.1111/j.1743-6109.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Guan R, Lei H, Li H, Wang L, et al. Therapeutic potential of adipose-derived stem cells-based micro-tissues in a rat model of postprostatectomy erectile dysfunction. J Sex Med. 2014;11:2439–48. doi: 10.1111/jsm.12636. [DOI] [PubMed] [Google Scholar]
- 8.He Y, He W, Qin G, Luo J, Xiao M. Transplantation KCNMA1 modified bone marrow-mesenchymal stem cell therapy for diabetes mellitus-induced erectile dysfunction. Andrologia. 2014;46:479–86. doi: 10.1111/and.12104. [DOI] [PubMed] [Google Scholar]
- 9.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–9. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 10.Gou X, He WY, Xiao MZ, Qiu M, Wang M, et al. Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl. 2011;13:332–8. doi: 10.1038/aja.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovanecz I, Rivera S, Nolazco G, Vernet D, Segura D, et al. Separate or combined treatments with daily sildenafil, molsidomine, or muscle-derived stem cells prevent erectile dysfunction in a rat model of cavernosal nerve damage. J Sex Med. 2012;9:2814–26. doi: 10.1111/j.1743-6109.2012.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ, et al. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184:1560–6. doi: 10.1016/j.juro.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albersen M, Fandel TM, Lin G, Wang G, Banie L, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–40. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamzon JL, Kokorowski P, Koh CJ. Stem cells and tissue engineering applications of the genitourinary tract. Pediatr Res. 2008;63:472–7. doi: 10.1203/PDR.0b013e31816a704a. [DOI] [PubMed] [Google Scholar]
- 15.Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk Lymphoma. 2005;46:1531–44. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- 16.Vernet D, Nolazco G, Cantini L, Magee TR, Qian A, et al. Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for peyronie disease. Biol Reprod. 2005;73:1199–210. doi: 10.1095/biolreprod.105.041038. [DOI] [PubMed] [Google Scholar]
- 17.Nolazco G, Kovanecz I, Vernet D, Gelfand RA, Tsao J, et al. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 2008;101:1156–64. doi: 10.1111/j.1464-410X.2008.07507.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin G, Alwaal A, Zhang X, Wang J, Wang L, et al. Presence of stem/progenitor cells in the rat penis. Stem Cells Dev. 2015;24:264–70. doi: 10.1089/scd.2014.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Kwon CH, Nakano I. Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development. J Neurosci Res. 2014;92:1419–24. doi: 10.1002/jnr.23431. [DOI] [PubMed] [Google Scholar]
- 22.Smart N, Riley PR. The stem cell movement. Circ Res. 2008;102:1155–68. doi: 10.1161/CIRCRESAHA.108.175158. [DOI] [PubMed] [Google Scholar]
- 23.Leri A, Rota M, Hosoda T, Goichberg P, Anversa P. Cardiac stem cell niches. Stem Cell Res. 2014;13:631–46. doi: 10.1016/j.scr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 25.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–43. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–71. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–28. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 31.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 32.Coskun S, Chao H, Vasavada H, Heydari K, Gonzales N, et al. Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 2014;9:581–90. doi: 10.1016/j.celrep.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 34.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 35.Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–91. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- 36.Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, et al. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 37.Lutolf MP, Blau HM. Artificial stem cell niches. Adv Mater. 2009;21:3255–68. doi: 10.1002/adma.200802582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beerman I, Rossi DJ. Epigenetic regulation of hematopoietic stem cell aging. Exp Cell Res. 2014;329:192–9. doi: 10.1016/j.yexcr.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20:1321–6. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 40.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–5. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meletis K, Wirta V, Hede SM, Nistér M, Lundeberg J, et al. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–9. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–39. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein J, Fletcher S, Roth E, Wu C, Chun A, et al. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev. 2014;28:983–94. doi: 10.1101/gad.236554.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatzfeld J, Li ML, Brown EL, Sookdeo H, Levesque JP, et al. Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor beta 1 or Rb oligonucleotides. J Exp Med. 1991;174:925–9. doi: 10.1084/jem.174.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kandasamy M, Lehner B, Kraus S, Sander PR, Marschallinger J, et al. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J Cell Mol Med. 2014;18:1444–59. doi: 10.1111/jcmm.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmid B, Fürthauer M, Connors SA, Trout J, Thisse B, et al. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–67. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, He XC, Tong WG, Johnson T, Wiedemann LM, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–39. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 52.Sieveking DP, Ng MK. Cell therapies for therapeutic angiogenesis: back to the bench. Vasc Med. 2009;14:153–66. doi: 10.1177/1358863X08098698. [DOI] [PubMed] [Google Scholar]
- 53.Assis AC, Carvalho JL, Jacoby BA, Ferreira RL, Castanheira P, et al. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19:219–30. doi: 10.3727/096368909X479677. [DOI] [PubMed] [Google Scholar]
- 54.Kocher AA, Schuster MD, Bonaros N, Lietz K, Xiang G, et al. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol. 2006;40:455–64. doi: 10.1016/j.yjmcc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 56.Deng J, Zou ZM, Zhou TL, Su YP, Ai GP, et al. Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci. 2011;32:641–51. doi: 10.1007/s10072-011-0608-2. [DOI] [PubMed] [Google Scholar]
- 57.Rochefort GY, Delorme B, Lopez A, Hérault O, Bonnet P, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–8. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 58.Goodus MT, Guzman AM, Calderon F, Jiang Y, Levison SW. Neural stem cells in the immature, but not the mature, subventricular zone respond robustly to traumatic brain injury. Dev Neurosci. 2015;37:29–42. doi: 10.1159/000367784. [DOI] [PubMed] [Google Scholar]
- 59.Lévesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, et al. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–9. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 60.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 61.Pusic I, DiPersio JF. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010;17:319–26. doi: 10.1097/MOH.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- 62.Shen W, Chen X, Chen J, Yin Z, Heng BC, et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–49. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 63.Chim H, Miller E, Gliniak C, Alsberg E. Stromal-cell-derived factor (SDF) 1-alpha in combination with BMP-2 and TGF-ß1 induces site-directed cell homing and osteogenic and chondrogenic differentiation for tissue engineering without the requirement for cell seeding. Cell Tissue Res. 2012;350:89–94. doi: 10.1007/s00441-012-1449-x. [DOI] [PubMed] [Google Scholar]
- 64.Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35:32–7. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 67.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 68.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin G, Garcia M, Ning H, Banie L, Guo YL, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–63. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–55. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu C, Xie Y, Gao F, Wang Y, Guo Y, et al. Lgr5 expression as stem cell marker in human gastric gland and its relatedness with other putative cancer stem cell markers. Gene. 2013;525:18–25. doi: 10.1016/j.gene.2013.04.067. [DOI] [PubMed] [Google Scholar]
- 72.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 73.da Silva-Diz V, Solé-Sánchez S, Valdés-Gutiérrez A, Urpí M, Riba-Artés D, et al. Progeny of Lgr5-expressing hair follicle stem cell contributes to papillomavirus-induced tumor development in epidermis. Oncogene. 2013;32:3732–43. doi: 10.1038/onc.2012.375. [DOI] [PubMed] [Google Scholar]
- 74.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–9. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 75.Lin CS, Xin ZC, Deng CH, Ning H, Lin G, et al. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–15. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 76.Loera-Valencia R, Wang XY, Wright GW, Barajas-López C, Huizinga JD. Ano1 is a better marker than c-Kit for transcript analysis of single interstitial cells of Cajal in culture. Cell Mol Biol Lett. 2014;19:601–10. doi: 10.2478/s11658-014-0214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H, Lin G, Qiu X, Ning H, Banie L, et al. Label retaining and stem cell marker expression in the developing rat urinary bladder. Urology. 2012;79:746.e1–6. doi: 10.1016/j.urology.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–19. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 79.Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol. 2008;294:F1415–21. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- 80.Huang YL, Tao X, Xia J, Li CY, Cheng B. Distribution and quantity of label-retaining cells in rat oral epithelia. J Oral Pathol Med. 2009;38:663–7. doi: 10.1111/j.1600-0714.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 81.Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–59. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 82.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 83.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 84.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–20. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–84. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 86.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 87.Bartsch G, Yoo JJ, De Coppi P, Siddiqui MM, Schuch G, et al. Propagation, expansion, and multilineage differentiation of human somatic stem cells from dermal progenitors. Stem Cells Dev. 2005;14:337–48. doi: 10.1089/scd.2005.14.337. [DOI] [PubMed] [Google Scholar]
- 88.Mittermayr R, Antonic V, Hartinger J, Kaufmann H, Redl H, et al. Extracorporeal shock wave therapy (ESWT) for wound healing: technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012;20:456–65. doi: 10.1111/j.1524-475X.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- 89.Lai JP, Wang FS, Hung CM, Wang CJ, Huang CJ, et al. Extracorporeal shock wave accelerates consolidation in distraction osteogenesis of the rat mandible. J Trauma. 2010;69:1252–8. doi: 10.1097/TA.0b013e3181cbc7ac. [DOI] [PubMed] [Google Scholar]
- 90.Shao PL, Chiu CC, Yuen CM, Chua S, Chang LT, et al. Shock wave therapy effectively attenuates inflammation in rat carotid artery following endothelial denudation by balloon catheter. Cardiology. 2010;115:130–44. doi: 10.1159/000262331. [DOI] [PubMed] [Google Scholar]
- 91.Moretti B, Notarnicola A, Moretti L, Giordano P, Patella V. A volleyball player with bilateral knee osteochondritis dissecans treated with extracorporeal shock wave therapy. Chir Organi Mov. 2009;93:37–41. doi: 10.1007/s12306-009-0022-6. [DOI] [PubMed] [Google Scholar]
- 92.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, et al. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114:2823–30. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 93.Wilner JM, Strash WW. Extracorporeal shockwave therapy for plantar fasciitis and other musculoskeletal conditions utilizing the Ossatron – An update. Clin Podiatr Med Surg. 2004;21:441–7. doi: 10.1016/j.cpm.2004.03.002. viii. [DOI] [PubMed] [Google Scholar]
- 94.Vardi Y, Appel B, Kilchevsky A, Gruenwald I. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187:1769–75. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 95.Liu J, Zhou F, Li GY, Wang L, Li HX, et al. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci. 2013;14:10661–73. doi: 10.3390/ijms140510661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, et al. Recruitment of mesenchymal stem cells and expression of TGF-beta 1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526–34. doi: 10.1016/j.orthres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 97.Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–46. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones NC, Tyner KJ, Nibarger L, Stanley HM, Cornelison DD, et al. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol. 2005;169:105–16. doi: 10.1083/jcb.200408066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oh JE, Bae GU, Yang YJ, Yi MJ, Lee HJ, et al. Cdo promotes neuronal differentiation via activation of the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23:2088–99. doi: 10.1096/fj.08-119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu Y, Guan R, Lei H, Gao Z, Li H, et al. Implications for differentiation of endogenous stem cells: therapeutic effect from icariside II on a rat model of postprostatectomy erectile dysfunction. Stem Cells Dev. 2015;24:747–55. doi: 10.1089/scd.2014.0380. [DOI] [PubMed] [Google Scholar]
- 101.Zhang J, Li AM, Liu BX, Han F, Liu F, et al. Effect of icarisid II on diabetic rats with erectile dysfunction and its potential mechanism via assessment of AGEs, autophagy, mTOR and the NO-cGMP pathway. Asian J Androl. 2013;15:143–8. doi: 10.1038/aja.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song J, Shu L, Zhang Z, Tan X, Sun E, et al. Reactive oxygen species-mediated mitochondrial pathway is involved in Baohuoside I-induced apoptosis in human non-small cell lung cancer. Chem Biol Interact. 2012;199:9–17. doi: 10.1016/j.cbi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413–21. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 104.Foresta C, Caretta N, Lana A, Cabrelle A, Palù G, et al. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 2005;17:288–90. doi: 10.1038/sj.ijir.3901311. [DOI] [PubMed] [Google Scholar]
- 105.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–51. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 106.Gur S, Kadowitz PJ, Hellstrom WJ. A critical appraisal of erectile function in animal models of diabetes mellitus. Int J Androl. 2009;32:93–114. doi: 10.1111/j.1365-2605.2008.00928.x. [DOI] [PubMed] [Google Scholar]
- 107.Winiarska K, Fraczyk T, Malinska D, Drozak J, Bryla J. Melatonin attenuates diabetes-induced oxidative stress in rabbits. J Pineal Res. 2006;40:168–76. doi: 10.1111/j.1600-079X.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 108.Qiu XF, Li XX, Chen Y, Lin HC, Yu W, et al. Mobilisation of endothelial progenitor cells: one of the possible mechanisms involved in the chronic administration of melatonin preventing erectile dysfunction in diabetic rats. Asian J Androl. 2012;14:481–6. doi: 10.1038/aja.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]


