Abstract
Recent studies have shown that infertility affects estimated 15% of all couples. Male infertility is the primary or contributory cause in 60% of these cases. Consequently, the application of assisted reproduction is increasing. These methods could benefit from an extended evaluation of sperm quality. For this reason, we analyzed sperm proteins from 30 men with normal spermiograms and 30 men with asthenozoospermia. Ejaculates of both groups were tested by flow cytometry (FCM) and fluorescence with a set of well-characterized anti-human sperm Hs-monoclonal antibodies (MoAbs), which were generated in our laboratory. No statistically significant differences were found between normospermics and asthenospermics in the expression of the sperm surface protein clusterin, evaluated with Hs-3 MoAb, and semenogelin, evaluated with Hs-9 MoAb. However, FCM revealed quantitative differences in the acrosomal proteins between normozoospermic and asthenozoospermic men, namely, in glyceraldehyde-3-phosphate dehydrogenase, evaluated with Hs-8 MoAb, valosin-containing protein, evaluated with Hs-14 MoAb, and ATP synthase (cAMP-dependent protein kinase II, PRKAR2A), evaluated with MoAb Hs-36. Asthenozoospermic men displayed a highly reduced expression of intra-acrosomal proteins, with a likely decrease in sperm quality, and thus a negative impact on successful reproduction. Asthenozoospermia seems to be a complex disorder involving intra-acrosomal proteins.
Keywords: asthenozoospermia, flow cytometry, fluorescence microscopy, monoclonal antibodies, sperm proteins
INTRODUCTION
Lifestyle changes and exposure to various detrimental factors in the environment result in increased incidences of male reproductive dysfunctions. To reveal the causes of infertility in a man, classical semen analysis is carried out according to the World Health Organization (WHO) guidelines.1 Semen parameters: sperm concentration, motility, viability, and morphology are assessed and the ejaculates are classified into four basic categories: normozoospermia (>15 × 106 spermatozoa ml−1, >40% motile spermatozoa and >32% spermatozoa with progressive motility, >4% spermatozoa with normal morphology), oligozoospermia (<15 × 106 spermatozoa ml−1, motility and morphology the same as normospermics), asthenozoospermia (<40% motile spermatozoa and <32% spermatozoa with progressive motility), and/or teratozoospermia (<4% spermatozoa with normal morphology), and their combinations, e.g., oligoasthenozoospermia, oligoasthenoteratozoospermia.
This evaluation provides only rough data and does not allow a more precise determination of the causes underlying infertility, especially in subfertile men and in men with idiopathic infertility.
Recently, new diagnostic tools - analysis of semen using antibodies to sperm proteins and proteomic analysis2,3,4 - have been introduced, and sperm assessment has definitely advanced to a molecular level. A number of antibodies have been prepared and proved to be useful in monitoring sperm processes and the role of individual proteins.5,6,7,8,9,10,11
We generated monoclonal antibodies (MoAbs)12,13,14,15,16 and used them to test the expression of relevant proteins on spermatozoa. This approach can reveal changes in protein expression in men whose spermatozoa are not able to fertilize the egg in a natural way.
For sperm evaluation using antibodies, the method of choice is flow cytometry (FCM). FCM is a reliable, objective technique allowing evaluation of a large number of cells and a variety of parameters and functions.17,18,19,20 In previous experiments, we used FCM for sorting the cell stages of spermatogenesis in infertile mice with chromosomal translocation21 and for the study of boar sperm capacitation.22 In this study, we applied MoAbs against human sperm proteins for the evaluation and comparison of the expression of these proteins in normospermic and asthenospermic men. Asthenozoospermia, the reduction of sperm motility, represents common sperm pathology in men. The concentration of sperm in the ejaculate and their morphology corresponds to normozoospermia, but the movement of sperm is changed, and their speed is reduced. The fertilizing capacity of asthenozoospermic men is restricted, and they seek help in centers for assisted reproduction.
Our objective was to determine by our MoAbs various sperm proteins and their differences between normozoospermia and asthenozoospermia to assess changes in protein detection in pathological sperm, and thus determine their importance in the reproduction process.
MATERIALS AND METHODS
Human ejaculates
Ejaculates were obtained with the donors’ consent from the Clinical Center ISCARE IVF (Prague, Czech Republic). The Institutional Review Board gave their consent to the proposed experiments. The average age of the men was 38 years old in the normospermic group (range 30–45 years) and 35 years old in the asthenospermic group (range 25–42 years). Semen samples were collected from men after 48–72 h of sexual abstinence and assessed according to WHO rules.1 Ejaculates of thirty normozoospermic (N) and thirty asthenozoospermic (A) men were used for the examination. The experiments included only men whose spermiograms that repeatedly (in three consecutive tests) demonstrated normospermic or asthenospermic characteristics and whose ejaculate(s) contained <1 × 106 ml−1 lymphocytes.
Viability of sperm was assessed twice: after ejaculation, and before cytometric and immunofluorescence analysis. Average values before cytometric, and immunofluorescence measurement were: 78% live cells in normospermics and 75% live cells in asthenospermics. Sperm of the donors was used for in vitro fertilization (IVF). According to the physicians’ decision, IVF was carried out with the sperm of asthenospermics and normospermics by intracytoplasmic sperm injection (ICSI).
Antibodies
Monoclonal antibodies of the Hs-series, which were established in our laboratory against human sperm proteins, were used. Briefly, BALB/c mice were immunized with human spermatozoa or their extract. After immunization, fusion of immune spleen cells with myeloma cells followed. Positive clones were selected by enzyme-linked immunosorbent assay with human sperm extract. Specificity of the antibody was tested by immunofluorescence and immunodetection after electrophoresis and Western blotting of the human sperm extract. Preparation of MoAbs and their characterization are described in Capkovα et al. 2002,13 Peknicova et al. 2005,23 Capkova et al. 2009.15
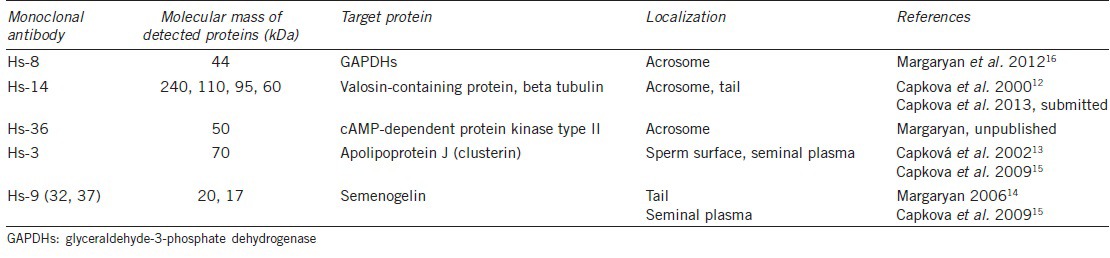
The Hs-8, Hs-14, and Hs-36 antibodies detect sperm proteins localized intra-acrosomally. The Hs-3 and Hs-9 antibodies detect seminal plasma proteins that secondarily bind to the sperm surface. The characteristics of MoAbs are summarized in Table 1.
Table 1.
Monoclonal antibodies to human sperm proteins
As secondary antibodies, we used Alexa Fluor 555 goat anti-mouse IgM (μ chain specific) and Alexa Fluor 488 goat anti-mouse IgG (H + L) (both Molecular Probes, Eugene, USA).
Procedures
The reaction of Hs-antibodies with human spermatozoa was visualized by indirect immunofluorescence (IIF) and evaluated under an IF microscope and by FCM. Before both procedures, the sperm samples were washed twice with phosphate-buffered saline (PBS, pH 7.4) and centrifuged at 200 g for 10 min.
Flow cytometry analysis
Each sperm sample was divided into parts A and B. Samples A were processed with a Fix and Perm Cell Permeabilization Kit (Grub Bio Research, Kaumberg, Austria) according to the manufacturer's instructions. Briefly, cells were incubated for 20 min with each reagent of the permeabilization kit. Between applications of individual reagents the sperm were centrifuged, twice washed with PBS and after the last washing, each sample was diluted with PBS to a final volume of 1 ml. Permeabilized cells were used for the detection and evaluation of intra-acrosomal sperm proteins. Samples B were not permeabilized. These samples were used for the diagnostics of sperm membrane integrity and surface proteins. The sperm concentration in both samples was determined by a hematocytometry chamber and suspensions were distributed by 5 × 106 per well into a 96-well plate, centrifuged at 200 g for 10 min and then the supernatant was removed. Two hundred microliter of MoAbs (diluted in PBS with 1% BSA to a final concentration of 5 μg Ig ml−1) was added per well and samples were incubated overnight at +4°C in an orbital shaker.
Sperm control samples were also diluted in PBS with 1% BSA to a final volume of 200 μl per well. After incubation, the samples were centrifuged (200 g, 10 min, +4°C), washed twice with 200 μl of PBS, and 200 μl of 1000× diluted secondary antibody Alexa Fluor 555 IgM (for Hs-8, Hs-14, Hs-36) or Alexa Fluor 488 IgG (for Hs-3, Hs-9) was added to each well. As a control, we used samples without primary and secondary antibodies (evaluation of autofluorescence) or without primary antibodies with secondary antibodies only (negative control). Re-suspended samples were incubated for 1 h at 37°C in the dark, washed twice with PBS and diluted to a final volume of 150 μl per well. FCM data acquisition was performed on a BD LSR II instrument (Becton Dickinson and Company, NJ, USA), excitation lasers 488 nm (Coherent Saphire 488-20 DPSS, filter 525/50, DM 505LP) and 561 nm (Melles Griot 85-YCA-25, filter 585/15, DM 565LP) to measure the fluorescent intensity in the Alexa Fluor 488 and Alexa Fluor 555 channels. Analysis was performed using FlowJo 7.5.4. software (TreeStar Inc., Ashland, OR, USA). The differences among individual samples in the percentage of cells (incidents) above the set threshold level of fluorescence intensity were assessed and statistically compared. Ten thousand cells were analyzed per well with a flow rate of 3000 cells.s−1.
Indirect immunofluorescence
Washed cells were diluted in PBS to a final concentration of 1 × 106 cells ml−1 and 10 μl drops were placed on glass slides. Drops were air-dried and permeabilized for 10 min at room temperature (RT) with acetone. Slides were rinsed in PBS, blocked in PBS-0.05% Tween + 1% BSA (Serva, Heidelberg, Germany) + 10% normal goat serum (Vector Laboratories, Burlingame, CA, USA) for 3 h at RT and incubated in a humid chamber with the primary antibody (hybridoma supernatant, immunoglobulin concentration <20 μg ml−1) overnight at +4°C. As a negative control, undiluted supernatant of Sp2/0 myeloma cells was used. After washing in PBS, the slides were incubated with Alexa Fluor 555 IgM or Alexa Fluor 488 IgG (see above) diluted 1:500 in PBS for 1 h at 37°C.
The slides were then washed with PBS, rinsed in deionized water, quickly air-dried, dropped with mounting medium Vectashield containing DAPI for DNA visualization (Vector Laboratories, Burlingame, CA, USA) and covered with a cover glass. Slides were stored at +4°C.
Samples were examined with a Nikon Eclipse E400 fluorescent microscope with a Nikon Plan Apo VC oil 60x objective, filters UV-2A (EX 330-380 nm, DM 400, BA 420) for DAPI, B-2A (EX 450-490 nm, DM 505, BA 520) for Alexa Fluor 488, and G-2A (EX 540-560, DM 585, BA 590) for Alexa Fluor 555, and photographed with a CCD VDS1300 camera (Vosskühler, Osnabrück, Germany) with the aid of the NIS elements AR imaging software (Laboratory Imaging, Prague, Czech Republic). Two hundred cells were analyzed per each sample.
Statistical analysis
Experimental data were analyzed using GraphPad Prism 5.04 (GraphPad Prism, La Jolla, CA, USA). The differences between the normospermic and the asthenospermic group in the number of Hs-antibody-positive cells were analyzed by the two-tailed Mann–Whitney test. The P ≤ 0.05 was considered to be significant.
Computed correlation coefficients (r) between the individual parameters and methods were tested for their significance (*P < 0.05, **P < 0.01, ***P < 0.001). The delta (r) test was performed with STATISTICA 6.0 (Statsoft, Prague, Czech Republic).
RESULTS
Thirty asthenozoospermics (A1-A30) and 30 normozoospermics (N1-N30) were examined and an identical A and N sample, respectively, was always used for testing with all five antibodies. Three MoAbs to intra-acrosomal proteins and two MoAbs against sperm adhesive proteins of seminal plasma were used to observe the expression of relevant proteins in/on the sperm. Target proteins and other characteristics of these antibodies are given in Table 1, and immunofluorescent labeling of normal sperm with individual antibodies is shown in Figure 1.
Figure 1.
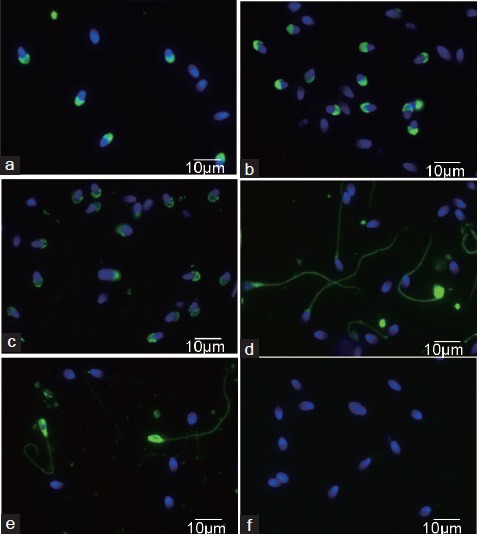
Immunofluorescent staining of normal human sperm with Hs-monoclonal antibodies: Hs-8 (a), Hs-14 (b), Hs-36 (c), Hs-3 (d), Hs-9 (e), Sp2/0 supernatant (f) - negative control; green color - FITC labeled, blue color - DNA labeled DAPI.
Flow cytometry detection of relevant proteins on fixed (permeabilized) and nonfixed cells
The fluorescent intensity of Alexa 555 (or Alexa 488)-conjugated secondary antibody in normospermic (N) and asthenospermic (A) sperm samples is shown in FCM histograms (Supplementary Figure 1 (766.2KB, tif) ). The percentage of the sperm stained by individual antibodies in N and A donors are shown in Figure 2.
Figure 2.
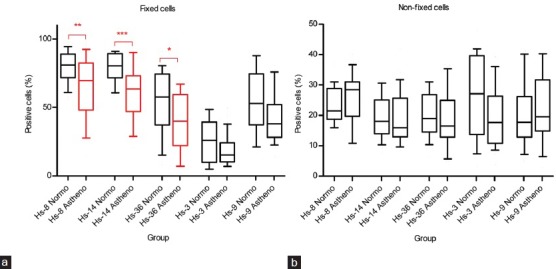
The differences in the number of stained cells (%) between the normozoospermic and asthenozoospermic sperm samples among different antibodies. Middle line indicates arithmetic mean, boxes indicate the 25th and 75th percentiles, whiskers indicate the 10th and 90th percentiles. The statistically significant differences are indicated by red color and asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). (a) fixed cells, (b) nonfixed cells.
FCM histograms: fluorescent intensity of Alexa 555 conjugated secondary antibody in normozoospermic (N) and asthenozoospermic (A) sperm samples. Hs 14 MoAb: Alexa 555 intensity is higher than 104 in 92% of N cells and 40% of A cells (a). Hs 3 MoAb: Alexa 488 intensity is higher than 104 in 12% of N cells and 18% of A cells (b). FCM: flow cytometry; MoAbs: monoclonal antibodies
The most significant differences in the percentage of labeled spermatozoa were found in fixed sperm cells with antibodies against acrosomal proteins. Statistical analysis showed a significantly reduced expression of proteins detected with Hs-8 (P < 0.01), Hs-14 (P < 0.001), and Hs-36 (P < 0.05) antibodies in asthenozoospermics compared to normozoospermics (Figure 2a).
Furthermore, Hs-3 and Hs-9 antibodies against sperm surface proteins labeled a lower percentage of fixed sperm in asthenospermics than in normospermics, but the differences were not statistically significant for any of these two antibodies (Figure 2a).
The expression of the relevant proteins was also investigated in nonfixed cells. No statistically significant differences were found in the percentage of cells labeled with Hs-anti-acrosomal or Hs-anti-sperm adhesive antibodies between N and A (Figure 2b). As expected, the Hs-8, Hs-14, Hs-36 antibodies labeled a lower percentage of nonfixed sperm (about 20%–30%) than the fixed ones. The Hs-3 antibody labeled an approximately equal percentage of fixed and nonfixed cells and Hs-9 labeled more fixed cells (roughly 40%–70%) than the nonfixed ones (roughly 15%–30%).
Correlation of Hs-8, Hs-14 and Hs-36 reactions with sperm of the same donor
The correlation between the percentage of sperm cells stained by individual anti-acrosomal antibodies was analyzed in the individual donors (Figure 3).
Figure 3.
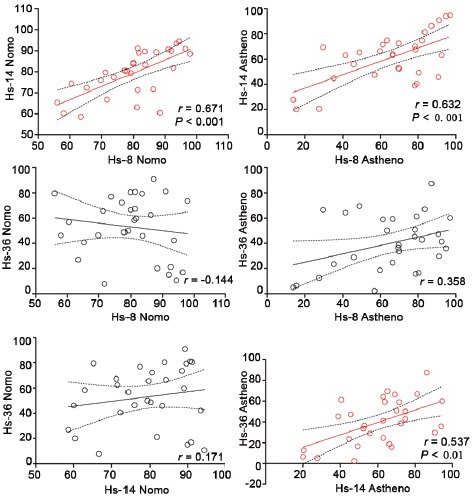
The correlation between the number of the cells stained by individual antibodies (%) in normozoospermic and asthenozoospermic sperm samples. r indicates the Pearson correlation coefficient. The correlation P ≤ 0.05 was considered to be significant, *P ≤ 0.05. The statistically significant correlation is indicated by red color.
A statistically significant correlation was found between the percentage of sperm labeled by Hs-8 and Hs-14 in the normospermics (r = 0.67, P < 0.001) and the asthenospermics (r = 0.63, (P < 0.001), but no significant correlation was found between the percentage of the cells stained by Hs-8 and Hs-36 in the normospermics and the asthenospermics, and between Hs-14 and Hs-36 in the normospermics. In these cases, no statistically significant differences between the individual Pearson correlation coefficients in normospermic and asthenospermic groups were detected (delta r test). The only difference between r close to the significant level was found in the correlation between Hs-14 and Hs-36 antibodies (P = 0.0614) in the asthenospermics (Figure 3 and Supplementary Table 1 (656.4KB, tif) ).
Correlation between the number of cells (%) stained by different antibodies in normozoospermic and asthenozoospermic sperm samples
Correlation of anti-acrosomal and anti-surface protein reactions with sperm of the same donor
The correlation between the percentages of sperm cells stained by individual anti-acrosomal and anti-surface protein antibodies was analyzed in the individual donors (Supplementary Table 1 (656.4KB, tif) ). A statistically significant correlation was found between the percentage of sperm labeled by Hs-8 and Hs-3 (r = −0.46, P < 0.01) and Hs-36 and Hs-3 (r = 0.61, P < 0.001) in the normospermics. In asthenospermics, the statistically significant correlation was found between the percentage of sperm labeled by Hs-8 and Hs-9 antibodies (r = −0.37, P < 0.05) and Hs-14 and Hs-9 antibodies (r = −0.38, P < 0.05).
Correlation of flow cytometry and indirect immunofluorescence results
Sperm labeling in individual donors was assessed by FCM. In the case of Hs-8 and Hs-14 antibodies, which displayed the largest differences in the number of labeled sperm cells between normospermics and asthenospermics, immunofluorescence microscopy was also used (Figure 4).
Figure 4.
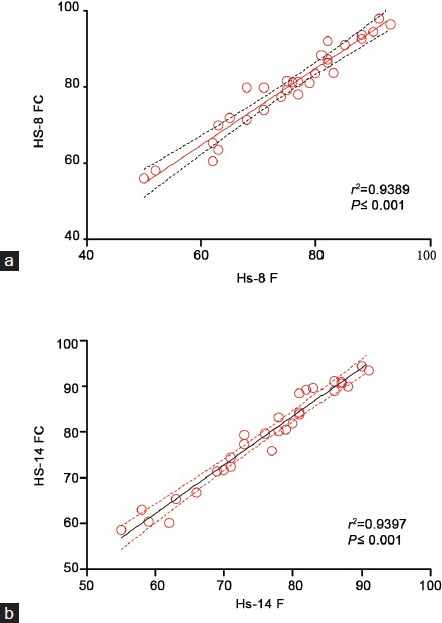
The correlation between the number of the cells stained by Hs-8 and/or Hs-14 in fluorescent microscopy analysis (%) and number of positive cells analyzed by flow cytometry (%). r indicates the Pearson correlation coefficient. The correlation P ≤ 0.05 was considered to be significant, *P ≤ 0.05. The statistically significant correlation is indicated by red color.
The percentage of cells stained by Hs-8 or Hs-14 obtained by fluorescence microscopy and the percentage of Hs-8 or Hs-14 positive cells analyzed by FCM correlated almost perfectly, as indicated by the squared Pearson correlation coefficient (Hs-8 r2 = 0.9389, correlation P < 0.0001; Hs-14 r2 = 0.9397, correlation P < 0.0001). The agreement of FCM and IIF results was also tested by Bland–Altman analysis (Supplementary Figure 2 (2.9MB, tif) ).
Bland-Altman plots of agreements between the results from fluorescence microscopy and flow cytometry analysis of cells stained by the Hs 8 antibody (a) and Hs 14 antibody (b).
Fertilization, pregnancy, and implantation rates (%) for normospermic and asthenospermic sperm and their correlation with flow cytometry results
Sperm of the donors was used for IVF. According to the physicians’ decision, IVF was carried out with asthenospermic and normospermic sperm by ICSI.
Data about fertility treatment are summarized in Supplementary Figure 3 (1.5MB, tif) . The fertilization and transfer rates were not different between the two groups. The pregnancy rate and the implantation rate were somewhat higher in healthy normozoospermic men, but without statistical significance.
The differences between normozoospermic and asthenozoospermic sperm samples in fertilization, transfer, pregnancy, and implantation rates. Definitions: fertilization rate – number of injected oocytes compared with fertilized oocytes; pregnancy rate – detected by serum HCG level at least 15 days after embryo replacement; implantation rate – number of gestational sacs observed at 6 weeks pregnancy divided by the number of embryos transferred; transfer rate – the number of embryos transferred to the total number of collected embryos. HCG: human chorionic gonadotropin.
DISCUSSION
The search for differences between the sperm capable of fertilizing eggs in a natural way and sperm that are not capable of fertilization is a long-lasting task. Recently, the possibilities of finding differences between normal and pathological sperm cells at a molecular level have greatly increased.
Two-dimensional electrophoresis of sperm proteins combined with computer analysis was used to find the differences between the sperm of fertile and infertile men.24,25,26 The advantage of this method is the rapid assessment of a large number of proteins and the identification of changes typical of a particular category of sperm.
Another approach is to find differences between fertile and infertile men using defined antibodies against sperm proteins.27,28 In the past, we applied immunofluorescence microscopy and described the changes in the protein expression in men with various sperm pathologies.23,29,30 Our set of MoAbs against human sperm (abbreviated Hs-antibodies) comprises of antibodies against sperm proteins and seminal plasma proteins that adhere to the sperm surface during their passage through the epididymis (Table 1).
Using FCM, various sperm features were measured such as sperm concentration,31 sperm viability and acrosomal integrity,32,33,34 mitochondrial function,35 and DNA integrity.36,37,38 Fluorescence-activated cell sorting applied in FCM is used for sorting sperm population with defined properties, e.g., with a reduced number of spermatozoa with fragmented DNA39 and a selection of spermatogenic cells from testicular biopsies.40
Using FCM, we evaluated the expression of sperm proteins and compared data between normozoospermic (N) and asthenozoospermic (A) men. Asthenozoospermia is characterized by reduced motility (<40% motile spermatozoa), and one would expect that the sperm tail would be affected in asthenospermics as it is the organ of the movement. However, experimental approach showed that asthenozoospermia is a complex, multifactorial damage, which interferes with cellular structures in different cell compartments. Low sperm motility seems to be accompanied by diminished sperm genomic integrity, abnormal DNA condensation and also by defects of the sperm midpiece.41 The complex nature of asthenozoospermia was also confirmed by our data obtained with anti-acrosomal antibodies. In comparison with the sperm of normal healthy men, the sperm of asthenospermics had a reduced amount of acrosomal proteins.
The largest differences in protein expression between normospermics (N) and asthenospermics (A) were found in the proteins detected by Hs-8 (P < 0.01) and Hs-14 (P < 0.001) antibodies. Therefore, we also assessed both of these groups under an immunofluorescence microscope and checked not only the expression of proteins, but also their localization on spermatozoa. A very high correlation of the results obtained by these two methods was confirmed by the regression curves and Bland–Altman analysis (Figure 4 and Supplementary Figure 2 (2.9MB, tif) ). We also observed differences in the expression of seminal plasma proteins on spermatozoa. Unlike our Hs-16 antibody, which more frequently detects SABP (sperm actin binding protein) on pathological sperm,42 we did not find a statistically significant difference in the expression of clusterin (detected by Hs-3 MoAb) and semenogelin (detected by Hs-9 MoAb) between the group of normal healthy men and the group of asthenospermics.
In addition, we correlated the number of cells stained by our anti-acrosomal antibodies in the groups of normospermics and asthenospermics. We found positive correlations between the number of stained cells using almost all antibodies, but only some of them were statistically significant. The Hs-8 and Hs-14 antibody staining patterns were very similar, but those of Hs-36 were different (Figure 3). This may reflect the fact that individual patients express the appropriate acrosomal proteins differentially, and the statistically significant differences between the normospermics and asthenospermics were caused not only by a lower amount of protein in the acrosome, but also by differential expression of individual proteins during spermatogenesis.
The most statistically significant differences in protein expression between N and A were found by the Hs-14 antibody. This MoAbs reacts with valosin-containing protein (VCP), and apparently nonspecifically binds to beta tubulin (unpublished results). However, the significant difference in the expression of Hs-14-detected protein(s) between N and A could not be influenced by an unspecific reaction with beta tubulin, because Hs-14 labeling of the sperm tail in both A and N groups was only exceptionally observed.
In general, we observed a decrease of the levels of proteins detected using our antibodies in the acrosomal area. This result is in agreement with our previously obtained results when we evaluated the fertilization rate of spermatozoa with various pathologies (oligoasthenoteratozoospermia, oligoasthenozoospermia, oligozoospermia) after ICSI. In this sperm, we found reduced levels of acrosome proteins in relation to the pathology and fertilization rate.30 Data about the fertilization treatment obtained after ICSI with sperm of the normospermics and asthenospermics tested in this study showed the same fertilization rate in N and A. This is probably due to the fertilization of eggs by the ICSI method, which eliminated the disadvantage of asthenospermic sperm cells with their reduced motility. Pregnancy and implantation rates were increased in normospermics compared with asthenospermics, but the differences were not statistically significant (Supplementary Figure 3 (1.5MB, tif) ).
As we also observed differences in the expression of individual epitopes detected by appropriate antibodies, we assume that the lower expression of individual proteins was caused by their impaired production during spermatogenesis. This idea is supported by experiments in which the experimentally-induced pathology in mice could influence the expression of genes having a role in spermatogenesis and presence of sperm proteins.43
We may also speculate that, conversely, the production of some other proteins may be up-regulated under pathological conditions, and the asthenospermics would consequently have higher levels of these other proteins in the acrosomes of their sperm population.
CONCLUSION
We found that intra-acrosomal sperm proteins VCP, glyceraldehyde-3-phosphate dehydrogenase, and PRKAR2A are differentially expressed in normal healthy men and asthenozoospermics, with a reduced expression in asthenozoospermics. These proteins are involved in energy metabolism and apoptosis of cells. Our results indicate the possibility of evaluating sperm quality in reproductive medicine by MoAbs against selected sperm proteins.
AUTHOR CONTRIBUTIONS
JC conceived the study, participated in its design and coordination and helped to draft the manuscript. AK carried out the immunoassays, LD participated in the design of the study and performed the statistical analysis, OT prepared and characterized the human sperm, JP prepared the MoAbs against human sperm and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Grant Agency of the Czech Republic, grant No. P503/12/1834, and P502-14-05547S and by BIOCEV project CZ.1.05/1.1.00/02.0109 from the European Regional Developmental Fund. The authors thank Timothy Hort for the English correction and Zdenek Cimburek for the technical assistance in FCM measuring.
Supplementary information is linked to the online version of the paper on the Asian journal of Andrology website.
REFERENCES
- 1.5th ed. Geneva, Switzerland: World Health Organization; 2010. World Health Organization. WHO Laboratory Manual for the Examination and Processing of human semen. [Google Scholar]
- 2.Oliva R, Martínez-Heredia J, Estanyol JM. Proteomics in the study of the sperm cell composition, differentiation and function. Syst Biol Reprod Med. 2008;54:23–36. doi: 10.1080/19396360701879595. [DOI] [PubMed] [Google Scholar]
- 3.Duplessis SS, Kashou AH, Benjamin DJ, Yadav SP, Agarval A. Proteomics: a subcellular look at spermatozoa. Reprod Biol Endocrinol. 2011;9:36–47. doi: 10.1186/1477-7827-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker MA, Nixon B, Naumovski N, Aitken RJ. Proteomic insights into the maturation and capacitation of mammalian spermatozoa. Syst Biol Reprod Med. 2012;58:211–7. doi: 10.3109/19396368.2011.639844. [DOI] [PubMed] [Google Scholar]
- 5.Yoshiki T, Lai BC, Dorjee S, Lee CY. Molecular characterizations of an intraacrosomal antigen defined by HS-33 monoclonal antibody. J Androl. 1996;17:666–73. [PubMed] [Google Scholar]
- 6.Dorjee S, Lai CL, Lee A, Lee CY. Monoclonal antibodies as direct probes for human sperm acrosome reaction. Am J Reprod Immunol. 1997;37:283–90. doi: 10.1111/j.1600-0897.1997.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamatani T, Tanabe K, Kamei K, Sakai N, Yamamoto Y, et al. A monoclonal antibody to human SP-10 inhibits in vitro the binding of human sperm to hamster oolemma but not to human Zona pellucida. Biol Reprod. 2000;62:1201–8. doi: 10.1095/biolreprod62.5.1201. [DOI] [PubMed] [Google Scholar]
- 8.Naz RK, Chauhan SC, Trivedi RN. Monoclonal antibody against human sperm-specific YLP12 peptide sequence involved in oocyte binding. Arch Androl. 2002;48:169–75. doi: 10.1080/01485010252869243. [DOI] [PubMed] [Google Scholar]
- 9.Reddy KV, Vijayalaxmi G, Rajeev KS, Aranha C. Inhibition of sperm-egg binding and fertilisation in mice by a monoclonal antibody reactive to 57-kDa human sperm surface antigen. Reprod Fertil Dev. 2006;18:875–84. doi: 10.1071/rd06028. [DOI] [PubMed] [Google Scholar]
- 10.Ulcova-Gallova Z, Gruberova J, Vrzalova J, Bibkova K, Peknicova J, et al. Sperm antibodies, intra-acrosomal sperm proteins, and cytokines in semen in men from infertile couples. Am J Reprod Immunol. 2009;61:236–45. doi: 10.1111/j.1600-0897.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- 11.Veaute C, Liu de Y, Furlong LI, Biancotti JC, Baker HW, et al. Anti-human proacrosin antibody inhibits the zona pellucida (ZP)-induced acrosome reaction of ZP-bound spermatozoa. Fertil Steril. 2010;93:2456–9. doi: 10.1016/j.fertnstert.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Capkova J, Geussova G, Peknicova J. Monoclonal antibody to human sperm acrosomal protein. Folia Biol (Praha) 2000;46:55–7. doi: 10.14712/fb2000046010055. [DOI] [PubMed] [Google Scholar]
- 13.Capková J, Geussová G, Peknicová J. New monoclonal antibody to human apolipoprotein J. Folia Biol (Praha) 2002;48:40–2. doi: 10.14712/fb2002048010040. [DOI] [PubMed] [Google Scholar]
- 14.Margaryan H. MAb Hs-9 against human semenogelin. Hybridoma. 2006;25:254. [Google Scholar]
- 15.Capkova J, Margaryan H, Elzeinova F, Koubek P, Peknicova J. Monoclonal antibodies to human seminal plasma proteins. In: Lagarda JL, Oliva R, editors. Proceedings of the 9th International Congress of Andrology. Barcelona (Spain): MEDIMOND; 2009. pp. 95–101. [Google Scholar]
- 16.Margaryan H, Peknicova J, Capkova J. Characterization of Human Sperm Protein Recognized by Monoclonal Antibody Hs-8. Proceedings of the 13th International Symposium for Immunology of Reproduction, Varna (Bulgaria) 2012:27. [Google Scholar]
- 17.Cordelli E, Eleuteri P, Leter G, Rescia M, Spanò M. Flow cytometry applications in the evaluation of sperm quality: semen analysis, sperm function and DNA integrity. Contraception. 2005;72:273–9. doi: 10.1016/j.contraception.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Mahfouz RZ, Tamer MS, Argaval A. The diagnostic and therapeutic applications of flow cytometry in male infertility. Arch Med Sci. 2009;5(1A):S99–108. [Google Scholar]
- 19.Robles V, Martínez-Pastor F. Flow cytometric methods for sperm assessment. Methods Mol Biol. 2013;927:175–86. doi: 10.1007/978-1-62703-038-0_16. [DOI] [PubMed] [Google Scholar]
- 20.Petrunkina AM, Harrison RA. Fluorescence technologies for evaluating male gamete (dys) function. Reprod Domest Anim. 2013;48(Suppl 1):11–24. doi: 10.1111/rda.12202. [DOI] [PubMed] [Google Scholar]
- 21.Homolka D, Ivanek R, Capkova J, Jansa P, Forejt J. Chromosomal rearrangement interferes with meiotic X chromosome inactivation. Genome Res. 2007;17:1431–7. doi: 10.1101/gr.6520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ded L, Dostalova P, Dorosh A, Dvorakova-Hortova K, Peknicova J. Effect of estrogens on boar sperm capacitation in vitro. Reprod Biol Endocrinol. 2010;8:87. doi: 10.1186/1477-7827-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peknicova J, Chladek D, Hozak P. Monoclonal antibodies against sperm intra-acrosomal antigens as markers for male infertility diagnostics and estimation of spermatogenesis. Am J Reprod Immunol. 2005;53:42–9. doi: 10.1111/j.1600-0897.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- 24.Pixton KL, Deeks ED, Flesch FM, Moseley FL, Björndahl L, et al. Sperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case report. Hum Reprod. 2004;19:1438–47. doi: 10.1093/humrep/deh224. [DOI] [PubMed] [Google Scholar]
- 25.Liao TT, Xiang Z, Zhu WB, Fan LQ. Proteome analysis of round-headed and normal spermatozoa by 2-D fluorescence difference gel electrophoresis and mass spectrometry. Asian J Androl. 2009;11:683–93. doi: 10.1038/aja.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thacker S, Yadav SP, Sharma RK, Kashou A, Willard B, et al. Evaluation of sperm proteins in infertile men: a proteomic approach. Fertil Steril. 2011;95:2745–8. doi: 10.1016/j.fertnstert.2011.03.112. [DOI] [PubMed] [Google Scholar]
- 27.Koide SS, Wang L, Kamada M. Antisperm antibodies associated with infertility: properties and encoding genes of target antigens. Proc Soc Exp Biol Med. 2000;224:123–32. doi: 10.1046/j.1525-1373.2000.22410.x. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa A, Fu Y, Tsubamoto H, Tsuji Y, Sawai H, et al. Epitope analysis for human sperm-immobilizing monoclonal antibodies, MAb H6-3C4, 1G12 and campath-1. Mol Hum Reprod. 2003;9:337–43. doi: 10.1093/molehr/gag045. [DOI] [PubMed] [Google Scholar]
- 29.Peknicova J, Pexidrova M, Kubatova A, Koubek P, Tepla O, et al. Expression of beta-tubulin epitope in human sperm with pathological spermiogram. Fertil Steril. 2007;88:1120–8. doi: 10.1016/j.fertnstert.2006.12.070. [DOI] [PubMed] [Google Scholar]
- 30.Tepla O, Peknicova J, Koci K, Mika J, Mrazek M, et al. Evaluation of reproductive potential after intracytoplasmic sperm injection of varied human semen tested by antiacrosomal antibodies. Fertil Steril. 2006;86:113–20. doi: 10.1016/j.fertnstert.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Eustache F, Jouannet P, Auger J. Evaluation of flow cytometric methods to measure human sperm concentration. J Androl. 2001;22:558–67. [PubMed] [Google Scholar]
- 32.Graham JK, Kunze E, Hammerstedt RH. Analysis of sperm cell viability, acrosomal integrity, and mitochondrial function using flow cytometry. Biol Reprod. 1990;43:55–64. doi: 10.1095/biolreprod43.1.55. [DOI] [PubMed] [Google Scholar]
- 33.Tao J, Du J, Critser ES, Critser JK. Assessment of the acrosomal status and viability of human spermatozoa simultaneously using flow cytometry. Hum Reprod. 1993;8:1879–85. doi: 10.1093/oxfordjournals.humrep.a137953. [DOI] [PubMed] [Google Scholar]
- 34.Zoppino FC, Halón ND, Bustos MA, Pavarotti MA, Mayorga LS. Recording and sorting live human sperm undergoing acrosome reaction. Fertil Steril. 2012;97:1309–15. doi: 10.1016/j.fertnstert.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Auger J, Leonce S, Jouannet P, Ronot X. Flow cytometric sorting of living, highly motile human spermatozoa based on evaluation of their mitochondrial activity. J Histochem Cytochem. 1993;41:1247–51. doi: 10.1177/41.8.8331289. [DOI] [PubMed] [Google Scholar]
- 36.Evenson D, Jost L. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci. 2000;22:169–89. doi: 10.1023/a:1009844109023. [DOI] [PubMed] [Google Scholar]
- 37.Zini A, Kamal K, Phang D, Willis J, Jarvi K. Biologic variability of sperm DNA denaturation in infertile men. Urology. 2001;58:258–61. doi: 10.1016/s0090-4295(01)01180-3. [DOI] [PubMed] [Google Scholar]
- 38.Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011;18:1005–13. doi: 10.1177/1933719111401662. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro SC, Sartorius G, Pletscher F, de Geyter M, Zhang H, et al. Isolation of spermatozoa with low levels of fragmented DNA with the use of flow cytometry and sorting. Fertil Steril. 2013;100:686–94. doi: 10.1016/j.fertnstert.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 40.Aslam I, Robins A, Dowell K, Fishel S. Isolation, purification and assessment of viability of spermatogenic cells from testicular biopsies of azoospermic men. Hum Reprod. 1998;13:639–45. doi: 10.1093/humrep/13.3.639. [DOI] [PubMed] [Google Scholar]
- 41.Piasecka M, Gaczarzewicz D, Laszczynska M, Starczewski A, Brodowska A. Flow cytometry application in the assessment of sperm DNA integrity of men with asthenozoospermia. Folia Histochem Cytobiol. 2007;45(Suppl 1):S127–36. [PubMed] [Google Scholar]
- 42.Capková J, Elzeinová F, Novák P. Increased expression of secretory actin-binding protein on human spermatozoa is associated with poor semen quality. Hum Reprod. 2007;22:1396–404. doi: 10.1093/humrep/del511. [DOI] [PubMed] [Google Scholar]
- 43.Zatecka E, Ded L, Elzeinova F, Kubatova A, Dorosh A, et al. Effect of tetrabrombisphenol A on induction of apoptosis in the testes and changes in expression of selected testicular genes in CD1 mice. Reprod Toxicol. 2013;35:32–9. doi: 10.1016/j.reprotox.2012.05.095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FCM histograms: fluorescent intensity of Alexa 555 conjugated secondary antibody in normozoospermic (N) and asthenozoospermic (A) sperm samples. Hs 14 MoAb: Alexa 555 intensity is higher than 104 in 92% of N cells and 40% of A cells (a). Hs 3 MoAb: Alexa 488 intensity is higher than 104 in 12% of N cells and 18% of A cells (b). FCM: flow cytometry; MoAbs: monoclonal antibodies
Correlation between the number of cells (%) stained by different antibodies in normozoospermic and asthenozoospermic sperm samples
Bland-Altman plots of agreements between the results from fluorescence microscopy and flow cytometry analysis of cells stained by the Hs 8 antibody (a) and Hs 14 antibody (b).
The differences between normozoospermic and asthenozoospermic sperm samples in fertilization, transfer, pregnancy, and implantation rates. Definitions: fertilization rate – number of injected oocytes compared with fertilized oocytes; pregnancy rate – detected by serum HCG level at least 15 days after embryo replacement; implantation rate – number of gestational sacs observed at 6 weeks pregnancy divided by the number of embryos transferred; transfer rate – the number of embryos transferred to the total number of collected embryos. HCG: human chorionic gonadotropin.


