Abstract
Implantation of an inflatable penile prosthesis (IPP) is a well-established definitive solution for erectile dysfunction when conservative treatments fail. Penile implants may shorten the penis. The AMS 700 LGX IPP is in common use but reports on its mechanical reliability, medium-term postsurgical patient satisfaction, and mean penile length preservation are lacking. We investigate the mean penile length, mechanical reliability, and patient satisfaction at 6 and 12 months after implantation of the AMS 700 LGX. This prospective study consecutively enrolled men undergoing first-time IPP implant surgery from February 2009 to April 2012. Stretched flaccid penile length, penile length at 50% and 100% of stiffness (P50 and P100) and International Index of Erectile Function (IIEF) and Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores, were measured at 6 and 12 months postsurgery. Of 45 patients who underwent AMS 700 LGX implantation (median age 61 years) and completed 6 months’ follow-up, 36 (80%) completed the study. A significant difference in stretched flaccid penile length was seen between 6 and 12 months (P = 0.033). P100 was also significantly increased at 6 and 12 months, with a mean 10% increase (1.3 ± 0.4 cm) from baseline to 12 months. Differences in mean IIEF scores at 6 and 12 months were significant for the desired domain (P = 0.0001) and for overall satisfaction (P = 0.002); however, mean EDITS scores at 6 and 12 months were not significantly improved. AMS 700 LGX is a powerful tool for preserving penile length in men undergoing penile prosthesis implantation.
Keywords: AMS 700 LGX, penile erection, penile implantation, penile prosthesis
INTRODUCTION
Implantation of an inflatable penile prosthesis (IPP) is a well-established definitive solution for erectile dysfunction when conservative treatments fail, with high satisfaction rates. Rajpurkar and Dhabuwala reported that 93% of patients were satisfied with their prosthesis, and 90% reported that treatment met their expectations.1 However, both patient and partner should be aware that the erection achieved with prosthesis is a compromise, and, to maximize satisfaction, preoperative counseling is mandatory to avoid unrealistic expectations. For example, the glans is not affected by any type of implant and will remain flaccid unless there is some residual erectile function in the corpus spongiosum. Also, although erection is not directly related to libido or orgasm, many patients have difficulty achieving orgasm when first using their prosthesis.2 One of the myths surrounding penile prosthesis surgery is that it lengthens the penis, when, in fact, the implant surgery may shorten it.3 Many patients undergoing penile prosthetic surgery desire increased penile length, so clinicians must guard against unrealistic expectations. Deveci et al.4 reported that 72% of patients complained of a loss of length after penile prosthesis insertion, negatively impacting on overall satisfaction. They demonstrated that despite the perception of length loss, there was no actual difference in penile length at baseline and at 1 and 6 months postsurgery.
In the 1990s, the Ultrex IPP device (American Medical Service [AMS], Minneapolis, MN, USA) was introduced to minimize penile shortening following prosthetic surgery. Unfortunately, mechanical failures led to its discontinuation.5,6 Recently, the AMS 700 LGX IPP (AMS, Minneapolis, MN, USA) was introduced with the same scope and with technical improvements to overcome the mechanical problems. Despite its wide use among erectile dysfunction surgeons, there are no reports regarding mechanical reliability, medium-term postsurgical patient satisfaction or mean length preservation obtained with the device.
The objective of this study was to investigate the preservation of penile length, mechanical reliability and patient satisfaction at 6 and 12 months postprosthetic surgery with the AMS 700 LGX inflatable penile implant.
METHODS
Patient selection
This prospective study was performed in consecutively enrolled men undergoing first-time IPP implantation from February 2009 to April 2012. Patients with Peyronie's disease (diagnosed as single palpable node or node seen on penile ultrasound, of <1 cm and without calcification), with no deformity or penile curvature ≤30° were considered eligible for the study. Patients with preoperative moderate-to-severe penile deformity (i.e., penile curvature >30°) or severe penile fibrosis/scarring were excluded.
All patients signed a specific informed consent form, and the investigation was conducted following the principles outlined in the Helsinki Declaration of 1975, revised in 1983.
Operative technique
Patients received appropriate preoperative antibiotics and surgery was performed by the same surgeon (M.P.) using regional anesthesia (e.g., spinal anesthesia with a 27G needle and hyperbaric marcaine usually injected in the sub-arachnoid space). Implantation was performed through a standard penoscrotal incision. Urethral catheter (14 Ch) was placed before suspension stitches were applied on corpora cavernosa. After corporotomies had been performed, both corpora cavernosa were dilated with Hegar dilators up to 12 mm. The length of the implanted AMS 700 LGX cylinder (American Medical Service [AMS], Minneapolis, MN, USA) corresponded exactly to intracorporeal measurements made by selecting a single reference point on the dilated corpora, measuring proximally and distally within the corpora with a Furlow inserter, and adding the two measurements. The measurement was repeated 2 times (two measurements) to ensure the correct sizing.
External inguinal ring was digitally probed in all cases when patent reservoir was placed in the paravesical space. When the ring was not patent (for example following inguinal hernia repair), the reservoir was placed in the peritoneum through an abdominal incision.
All patients were discharged home with the prosthesis inflated at 50% of the stiffness. Prosthesis was deflated as soon as the local conditions allowed such manoeuvre (i.e., no more pain when squeezing the pump and easy handling of the pump).
Main outcome measures
Patients had their penile length measured at baseline (i.e., prior to implantation surgery) and at 6 and 12 months after surgery. The following three penile length measurements were calculated for each patient: (1) stretched flaccid penile length, from the pubic bone to the meatus along the dorsum of the shaft using a tape measure; (2) P50: penile length with the prosthesis at 50% of its stiffness (half the number of squeezing cycles required to completely inflate the prosthesis), from the pubic bone to the meatus along the dorsum of the shaft using a tape measure; and (3) P100: penile length with the prosthesis at 100% of its stiffness with the prosthesis completely inflated, from the pubic bone to the meatus along the dorsum of the shaft using a tape measure. All patients received a 65-ml reservoir. These parameters were chosen because cylinder elongation occurs once the device stiffness is achieved.
Patients completed the International Index of Erectile Function (IIEF), and Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) questionnaires at 6 and 12 months after surgery. In addition, they answered a specific question about satisfaction with erect penile length (“Are you satisfied with the length of your penis when it is erect?”).
The IIEF is a validated instrument to assess erectile function, libido, and satisfaction after sexual relations.7 EDITS, a validated instrument to determine patient satisfaction in response to treatments, is an 11-item inventory with the score presented on a 100-point scale using the satisfaction domain (questions 6, 7, 8, 13 and 14 with a maximum score of 20), the orgasmic domain (questions 9 and 10 with a maximum score of 10); and the desire domain (questions 11 and 12 with a maximum score of 10).8
Statistical analysis
Mean penile length, IIEF and EDITS scores were compared using the two-sided paired Student t-test. All data were presented as the mean and standard deviation (s.d.) with P < 0.05 considered as statistically significant.
RESULTS
Among the 82 patients undergoing penile prosthesis implantation, 45 (median age 61 years) were suitable for an AMS 700 LGX (Table 1). Indications for implantation were Peyronie's disease in 15 patients (33%), vascular impotence in 12 patients (27%, 8 of whom were diabetic) and pelvic surgery in 18 patients (40%), of whom two had radical cystectomy plus orthotropic diversion for muscular invasive transitional cell carcinoma and 16 had radical prostatectomy for prostate cancer. There were no perioperative complications; one patient had the prosthesis removed due to an infection at 3 months following the implantation. Patients had their prosthesis deflated and were taught how to activated and deactivated at a median of 2 weeks following surgery. They were encouraged to cycle it every day 15–20 min per day for at least 8 months out of their sexual activity.
Table 1.
Clinical parameters of the study population
At median follow-up time of 19 months (range 6–38 months), no apical erosion occurred, and no device malfunctions, S-shaped or cylinder aneurysms were noted. All patients completed 6 months’ follow-up, and 36 patients (80%) completed 12 months’ follow-up. The mean and median prosthesis lengths used were 19.8 ± 1.5 cm and 20 cm, respectively.
Figure 1 demonstrates penile length in cm at baseline, 6 months and 12 months after surgery. The mean stretched flaccid penile length was 13.1 ± 1.2 cm at baseline, and was greater at 6 months (13.7 ± 1.1 cm [P = 0.018]), and at 12 months (14.2 ± 1.2 cm [P = 0.0001]); a mean difference of 1.1 ± 0.3 cm at 12 months versus baseline. A significant difference in stretched flaccid penile length was also seen between 6 and 12 months (P = 0.033).
Figure 1.
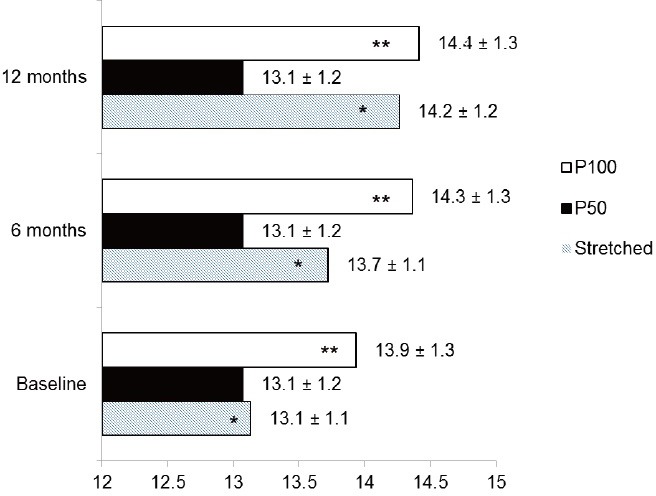
Penile length (stretched, P50 and P100) at baseline and at 6 and 12 months after surgery. *and **statistically significant differences (P < 0.05).
Mean P50 and P100 lengths were 13.1 ± 1.2 cm and 13.9 ± 1.3 cm (P = 0.002) at baseline; 13.1 ± 1.2 cm and 14.3 ± 1.3 cm (P = 0.0001) at 6 months; and 13.1 ± 1.2 cm and 14.4 ± 1.3 cm at 12 months (P = 0.0001). There was a significant difference in P100 from baseline to 6 and 12 months, with a 10% increase (1.2 ± 0.4 cm) from baseline at 12 months. No difference in P100 length was noted between 6 and 12 months. P50 remained the same throughout the study period. In three patients, a dorsal curvature of about 30° was observed when the prosthesis was completely inflated during surgery. All had ED associated with Peyronies disease but without a significant preoperative curvature (i.e., dorsal curvature ≤30°). Modeling was performed (as described by Wilson and Delk in 19949) with only one patient having a recurrence of the curvature, but without complaining of significant discomfort during sexual intercourse.
At 6 months, 36 patients (80%) answered “Yes” to the question “Are you satisfied with your penile length?” Among those who were satisfied with the length of their penis, 4/36 (11%) reported an increase in length while the others did not notice any change. Those replying “No” reported that the size of their penis was shorter than before surgery. Mean IIEF scores at 6 and 12 months as shown in Figure 2 were 10.5 ± 2.7 and 10.7 ± 2.1, (P = 0.709), respectively, for intercourse satisfaction (Q6–8); 4.0 ± 1 and 4.2 ± 1.1 (P = 0.414) for the orgasmic function (Q10); 7.6 ± 1.3 and 8.6 ± 1.1 (P = 0.0001) for the desired domain (Q11–12); and 7.3 ± 1.2 and 8.3 ± 1.5 (P = 0.002) for overall satisfaction (Q13–14). Mean EDITS scores at 6 and 12 months were 76.9 ± 12.8 and 77.8 ± 13.5 (P = 0.76).
Figure 2.
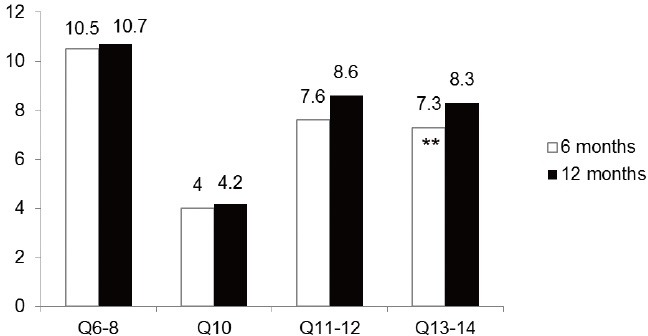
Variation in International Index of Erectile Function (IIEF) scores at 6 and 12 months. **Statistically significant differences (P < 0.05).
DISCUSSION
Penile prosthesis is a highly satisfactory treatment for erectile dysfunction, allowing patients with severe erectile dysfunction and end-organ disease to continue to enjoy the pleasure of sexual activity. It is a definitive and irreversible treatment with satisfaction rates as high as 93.8%.1 Mechanical reliability is also high, with many studies investigating the issue and all reporting high fidelity of the implants for up to 15 years.10,11 Wilson et al.11 reported a rate of freedom from mechanical breakage at 10 years of 79.4% and at 15 years of 71.2%; the addition of a parylene coating to the cylinders in the recent AMS CX models has further increased 3-year mechanical survival from 88.4% to 97.9% (P = 0.0002). In a long-term retrospective study on longevity, morbidity and patient satisfaction, mean mechanical reliability was 86.2% after 5 years, with 87.1% of patients with the prosthesis having an erection suitable for coitus 5 years after the implantation, and 88.2% said they would recommend an implant to a relative or a friend.12
Despite being a highly satisfactory treatment option with excellent mechanical reliability on long-term follow-up, reduced penile length after surgery is a major issue. “Short penis syndrome” is a well-known entity in patients undergoing penile prosthesis implantation. Although some studies have shown that perceived penile length reduction can be a misperception, such complaints have a negative impact on a reduction in the EDITS and IIEF satisfaction domain score (63.7 vs 60.8 and 6.5 vs 3.5 respectively).4 However, Wang et al.13 reported an objective (measurable) decrease in the erect penile length (from 0.2 to 3.0 cm) after IPP compared to the erect penis, secondary to intracavernosal injection of vasoactive agents. In total, 45% of patients reported subjective penile shortening after IPP, and none of the patients believed that their erect penile lengths were longer after IPP implantation.13 To minimize the problem, in the 1990s, AMS introduced the Ultrex cylinders. These were an expanding device, with the aim of increasing either the length or the girth of an implanted penis. Initial results in terms of length were promising; the difference in intraoperative mean penile length with the device deflated and inflated was 1.9 cm (range 1–4 cm). Postoperatively, more than 50% of patients maintained their penile length compared with presurgery while 12% of patients increased their penile length by at least 1 cm.5 However, early experience revealed reduced cylinder life5 and occurrence of S-shaped penile deformities.6
In 2006, the AMS 700 LGX prosthesis was introduced. It combined improvements already applied to the Ultrex cylinder after 1993, and new AMS 700 series features resolved the problem of premature wear.14 Our study showed that following implantation using the AMS 700 LGX, the in vivo mean length preservation with the prosthesis completely inflated compared with the prosthesis at 50% of its stiffness, was 1.3 cm (0.4 cm), corresponding to a 10% length restoration. This is comparable to the difference measured between ICI erection and IPP erection in the study by Wang et al.13 suggesting that the AMS 700 LGX provides an overall penile length very close to the one obtained with a “natural” erection.
Wilson et al.15 reported a mean increase in length of 2.2 cm following replacement of penile prosthesis in patients with scarred corpora cavernosa. This was achieved by inserting properly sized implant in a scarred corporal body (in which a normal dilatation could not be achieved) and using it as a tissue expander for 1 year, encouraging patients to inflate their implant for up to 3 h daily. When the prosthesis was replaced, they were able to insert wider and sometimes longer cylinders, and concluded that prolonged inflation over 8 to 12 months period resulted in expansion of the cylinder cavity, permitting standard-sized cylinders in all patients.15
In our study, the stretched penile length was at least 1 cm longer at 12 months than preoperative and 6 months measurements in all patients, confirming the elastic properties of the penis, irrespective of the normality or abnormality of the corpora cavernosa. In our series, overall patient satisfaction and satisfaction regarding penile length after surgery was high, with 80% of patients satisfied with the final length. Among these, 11% reported a length gain. Our data confirm results obtained by Mulhall et al.16 who reported IPP to be a highly satisfying ED treatment with the highest satisfaction seen during the first year following implantation, particularly during the second part of the first year. We confirm that significant improvement was seen from 6 to 12 months postsurgery, as shown in the overall IIEF satisfaction domain score. We found an interesting difference in the desired domain of IIEF at 6 and 12 months. In our opinion, these data reflect the learning curve, not only in prosthesis activation, but also in how to have sex with the prosthesis. In addition, this result is not related only to the satisfaction regarding the length, as it was also present in the 22% of patients who expressed dissatisfaction with the final length.
Our study confirms that patients experience the highest satisfaction in the second half of the first year after surgery, suggesting a learning curve that does not just involve the mechanical aspects of prosthesis use, but also suggest that time is required for behavioral and psychosexual adaptation, which would be of great interest to investigate further. It should be noted that although we did not perform a correlation analysis of increased orgasmic function and overall satisfaction with increased penile length, 80% of patients were satisfied with the length and the IEFF overall satisfaction scores indicated high levels of satisfaction with the treatment. Such correlations are an area for further study. In this medium-term follow-up, good mechanical reliability was confirmed, as at a maximum follow-up time of 38 months, no mechanical complication (S-shaped deformity or cylinder aneurysm) occurred.
The technique of measurement of the corpora cavernosa used in this study (using a cylinder the same size as the corpora) suggests there is no need to undersize the cylinder as advocated by some authors who suggest a systematic cylinder under sizing of 2 cm,17 particularly when using the Ultrex or LGX prosthesis to avoid an S-shaped deformity.18 In addition, there is no need to perform downsizing to avoid apical perforation/erosion of the implant, because, with an inflatable prosthesis, the apical tension is not constant as with malleable rods. This is of paramount importance when diabetic patients are considered for a penile implant.
Limitations of this study include the subjective nature of the patient reported outcomes and potential inaccuracy of the stretched penile length measurement. In addition, none of the questionnaire used has been specifically developed for assessing erection and satisfaction in patients with penile prosthesis (IIEF and EDITS). Unfortunately, no specific questionnaire has been developed yet for the specific purpose, thus we used IIEF as a useful for all the domain but erection, and we used EDITS to evaluate treatment satisfaction, knowing that EDITS too was developed mainly for pharmacological treatment.
Overall our study shows that the AMS 700 LGX provides a reliable solution to short penis syndrome post-IPP implantation, providing a penile length comparable to the natural erection. The AMS 700 LGX is a powerful tool to preserve penile length in patients undergoing penile prosthesis implantation as demonstrated by the high satisfaction rate, with 80% of patients satisfied with their final penile length. Therefore, the AMS 700 LGX should be considered in all patients (except those with penile fibrosis or scarring) in order to preserve penile length, allowing time for mechanical, behavioral and psychosexual adaptation.
AUTHOR CONTRIBUTIONS
CLAN and MP conceived of the study, collected data, participated in its design and coordination and was involved in the manuscript drafting. AR was involved in the manuscript drafting. FB conceived of the study and revised it critically. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.Rajpurkar A, Dhabuwala CB. Comparison of satisfaction rates and erectile function in patients treated with sildenafil, intracavernous prostaglandin E1 and penile implant surgery for erectile dysfunction in urology practice. J Urol. 2003;170:159–63. doi: 10.1097/01.ju.0000072524.82345.6d. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Bhojwani A, Terry TR. The role of penile prosthetic surgery in the modern management of erectile dysfunction. Postgrad Med J. 2000;76:22–5. doi: 10.1136/pmj.76.891.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furlow WL, Goldwasser B, Gundian JC. Implantation of model AMS 700 penile prosthesis: long-term results. J Urol. 1988;139:741–2. doi: 10.1016/s0022-5347(17)42618-8. [DOI] [PubMed] [Google Scholar]
- 4.Deveci S, Martin D, Parker M, Mulhall JP. Penile length alterations following penile prosthesis surgery. Eur Urol. 2007;51:1128–31. doi: 10.1016/j.eururo.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Montague DK, Lakin MM. Early experience with the controlled girth and length expanding cylinder of the American Medical Systems Ultrex penile prosthesis. J Urol. 1992;148:1444–6. doi: 10.1016/s0022-5347(17)36933-1. [DOI] [PubMed] [Google Scholar]
- 6.Wilson SK, Cleves MA, Delk JR., 2nd Ultrex cylinders: problems with uncontrolled lengthening (the S-shaped deformity) J Urol. 1996;155:135–7. doi: 10.1016/s0022-5347(01)66571-6. [DOI] [PubMed] [Google Scholar]
- 7.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 8.Althof SE, Corty EW, Levine SB, Levine F, Burnett AL, et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology. 1999;53:793–9. doi: 10.1016/s0090-4295(98)00582-2. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SK, Delk JR 2 nd. A new treatment for Peyronie's disease: modeling the penis over an inflatable penile prosthesis. J Urol. 1994;152:1121–3. doi: 10.1016/s0022-5347(17)32519-3. [DOI] [PubMed] [Google Scholar]
- 10.Dhar NB, Angermeier KW, Montague DK. Long-term mechanical reliability of AMS 700CX/CXM inflatable penile prosthesis. J Urol. 2006;176(6 Pt 1):2599–601. doi: 10.1016/j.juro.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SK, Delk JR, Salem EA, Cleves MA. Long-term survival of inflatable penile prostheses: single surgical group experience with 2,384 first-time implants spanning two decades. J Sex Med. 2007;4(4 Pt 1):1074–9. doi: 10.1111/j.1743-6109.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 12.Carson CC, Mulcahy JJ, Govier FE. Efficacy, safety and patient satisfaction outcomes of the AMS 700CX inflatable penile prosthesis: results of a long-term multicenter study. AMS 700CX Study Group. J Urol. 2000;164:376–80. [PubMed] [Google Scholar]
- 13.Wang R, Howard GE, Hoang A, Yuan JH, Lin HC, et al. Prospective and long-term evaluation of erect penile length obtained with inflatable penile prosthesis to that induced by intracavernosal injection. Asian J Androl. 2009;11:411–5. doi: 10.1038/aja.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milbank, AJ, Montague, DK, Angermeier KW, Lakin MM, Worley SE. Mechanical failure of the American Medical Systems Ultrex inflatable penile prosthesis: before and after 1993 structural modification. J Urol. 2002;167:2502–6. [PubMed] [Google Scholar]
- 15.Wilson SK, Delk JR, 2nd, Mulcahy JJ, Cleves M, Salem EA. Upsizing of inflatable penile implant cylinders in patients with corporal fibrosis. J Sex Med. 2006;3:736–42. doi: 10.1111/j.1743-6109.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 16.Mulhall JP, Ahmed A, Branch J, Parker M. Serial assessment of efficacy and satisfaction profiles following penile prosthesis surgery. J Urol. 2003;169:1429–33. doi: 10.1097/01.ju.0000056047.74268.9c. [DOI] [PubMed] [Google Scholar]
- 17.Montague DK, Angermeier KW. Cylinder sizing: less is more. Int J Impot Res. 2003;15(Suppl 5):S132–3. doi: 10.1038/sj.ijir.3901088. [DOI] [PubMed] [Google Scholar]
- 18.Montague DK, Angermeier KW. Increasing size with penile implants. Curr Urol Rep. 2008;9:483–6. doi: 10.1007/s11934-008-0082-4. [DOI] [PubMed] [Google Scholar]


