Abstract
The epididymis is a single convoluted tubule lined by a pseudostratified epithelium. Specialized epididymal epithelial cells, the so-called principal, basal, narrow, and clear cells, establish a unique luminal environment for the maturation and storage of spermatozoa. The epididymis is functionally and structurally divided into several segments and sub-segments that create regionally distinct luminal environments. This organ is immature at birth, and epithelial cells acquire their fully differentiated phenotype during an extended postnatal period, but the factors involved in this complex process remain incompletely characterized. In the adult epididymis, the establishment of an acidic luminal pH and low bicarbonate concentration in the epididymis contributes to preventing premature activation of spermatozoa during their maturation and storage. Clear cells are proton-secreting cells throughout the epididymis, but principal cells have distinct acid/base transport properties, depending on their localization within the epididymis. Basal cells are located in all epididymal segments, but they have a distinct morphology depending on the segment and species examined. How this structural plasticity of basal cells is regulated is discussed here. Also, the role of luminal factors and androgens in the regulation of epithelial cells is reviewed in relation to their respective localization in the proximal versus distal regions of the epididymis. Finally, we describe a novel role for CFTR in tubulogenesis and epithelial cell differentiation.
Keywords: basal cells, clear cells, principal cells, pseudostratified epithelia, transepithelial transport
INTRODUCTION
After being produced by the testis, spermatozoa acquire their capacity to reach and fertilize an oocyte while in the epididymis, a small organ located downstream of the testis.1,2,3,4,5 Epididymal sperm maturation is, therefore, essential for the establishment of male fertility. However, this process is often overlooked, and between 40% and 50% of male infertility cases diagnosed in the clinic are still labeled “idiopathic.”6 The epididymis is a long and single tubule that is organized into four major segments, the initial segment (IS), the caput, the corpus, and the cauda epididymidis, which are further divided into intra-segmental regions that are delineated by connective tissue septa and express a distinct set of genes.5,7,8,9 Epithelial cells with specific functions and morphological characteristics are located in these regions; they form the so-called blood-epididymis barrier,10,11 and establish a unique luminal microenvironment for the concentration, maturation, and storage of spermatozoa.2,12,13
The pseudostratified epithelium that lines the epididymal tubule includes principal, narrow, clear, and basal cells.1,2,3,4,5 Principal and basal cells are present throughout the epididymis, but narrow cells are located exclusively in the initial segment while clear cells are present in the caput, corpus, and cauda epididymidis. An elaborate communication network between these cells contributes to the regulation of various transport mechanisms in the epididymis.4,14 This review focuses on some aspects of epithelial cell function in the epididymis, including luminal acidification and regulation of the proximal epididymis by luminal factors, and describes a novel role for CFTR in the regulation of epithelial cell proliferation and differentiation.
LUMINAL ACIDIFICATION IN THE EPIDIDYMIS
Early micropuncture studies revealed that the luminal environment of the epididymis was remarkably distinct from blood.13,15,16 In particular, an acidic luminal pH and low bicarbonate concentration contribute to maintaining spermatozoa in a dormant state during their maturation and storage in the epididymis.4,17,18,19,20,21 During ejaculation and transit to the female reproductive tract, elevations of pH and bicarbonate concentration induce sperm capacitation, which involves modulation of key ion channels, activation of the sAC/cAMP-signaling pathway, and protein phosphorylation events in spermatozoa.22,23
Defective luminal acidification in the epididymis results in male infertility owing to the inability of spermatozoa to move through the female reproductive tract and fertilize an oocyte.24,25,26 Luminal acidification depends on several processes, including bicarbonate reabsorption and proton-secretion that occur in different cell types, depending on their location in the epididymis. Bicarbonate reabsorption is achieved by principal cells in the initial segment,20,27 and proton-secretion occurs in clear cells, which become progressively more abundant in the more distal regions, especially the cauda epididymidis and the proximal vas deferens.4,28 Clear cells express the proton pump V-ATPase in their apical membrane and sub-apical recycling vesicles28 (Figure 1). The V-ATPase is a complex enzyme that is composed of several subunits. A particular set of V-ATPase subunit isoforms, including the transmembrane a4 and the cytoplasmic subunits B1, A and E2, is highly enriched in clear cells.29,30 Foxi1 is a master regulator of V-ATPase subunit expression, and Foxi1 KO male mice have abnormally elevated epididymal luminal pH and are infertile, illustrating the importance of the V-ATPase in male fertility.24,25
Figure 1.
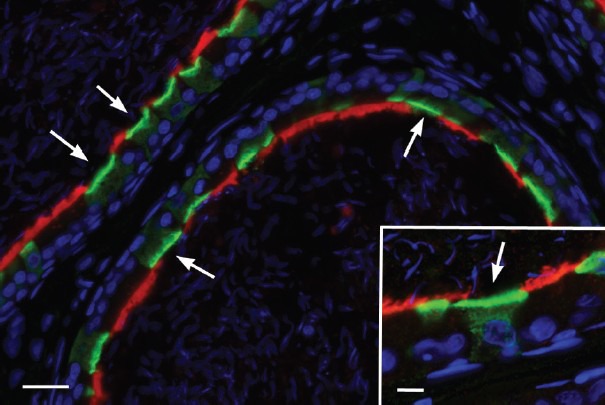
Expression of the water and solute channel aquaporin 9 (red) in principal cells, and the proton pumping V-ATPase (green) in clear cells of the cauda epididymidis. In this segment, numerous clear cells express the V-ATPase in their apical membrane and sub-apical vesicles (arrows). Nuclear and spermatozoa are visualized in blue with DAPI. Scale bar: 20 μm, Inset: 5 μm.
REGULATION OF LUMINAL ACIDIFICATION BY LUMINAL FACTORS
Proton-secretion by clear cells is regulated via recycling mechanisms.4 The activation of the cAMP and cGMP pathways in clear cells induces the redistribution of V-ATPase from sub-apical vesicles to the apical membrane, leading to an increase in proton-secretion.4,20,31 This process is accompanied by the formation and extension of numerous V-ATPase-rich apical membrane extensions. From their appearance revealed by transmission electron microscopy (TEM) and immunofluorescence, these membrane protrusions were initially named “microvilli,” but recent advances in high-resolution helium ion microscopy techniques have revealed the presence of membrane “ruffles” or “microplicae” that increase in number and become more numerous when clear cells are activated32 (Figure 2). These protrusions are analogous to the “leaf-like” structures previously described by the Hamilton group's scanning electron microscopy (SEM) study.33
Figure 2.
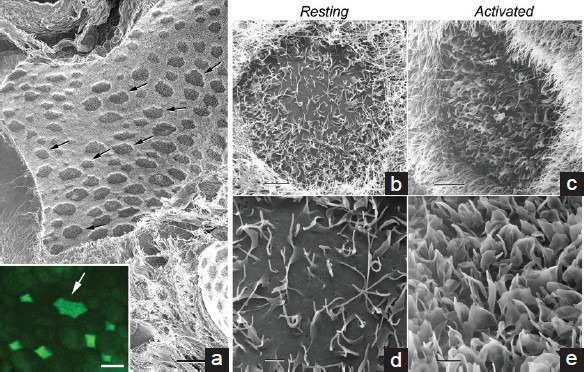
Helium Ion Microscope (HIM) imaging of the apical surface of the epididymal tubule. (a) Low-magnification image of a control rat cauda epididymidis, after removal of spermatozoa by in vivo luminal perfusion. Darker clear cells of various sizes (arrows) are interspersed among principal cells, which appear brighter owing to their apical stereocilia. The inset shows the apical surface of a rat cauda epididymidis immunolabeled for the V-ATPase (green). The arrow indicates a large clear cell. (b–e) High-magnification images of the apical surface of rat cauda epididymidal clear cells perfused under control conditions (b and d), and in the presence of the permeant cAMP analog, cpt-cAMP (c and e). Under control conditions, the clear cell shows a few microvilli and relatively short microplicae, and a large part of the membrane appears smooth and devoid of protrusions. cAMP induced the formation of numerous microplicae and the apical membrane did not show any smooth surface. In b and c, brighter principal cell stereocilia surrounding clear cells are evident. Scale bars: (a) 50 μm, Inset: 15 μm; (b and c): 4 μm; (d and e) 1 μm. Modified from Paunescu et al. Mol Hum Reprod 2014.
Clear cells respond to changes in their luminal environment, such as an increase from the physiological pH of 6.6 to the alkaline 7.8, by accumulating V-ATPase in their apical membrane, which increases proton-secretion.34,35 Depolymerization of the cortical actin cytoskeleton, via inhibition of RhoA and its effector ROCKII, favors the recruitment of V-ATPase from sub-apical vesicles to the apical membrane in clear cells.36 V-ATPase apical accumulation also requires intracellular calcium and is phospholipase C-dependent.34
While clear cells are involved in the maintenance of an acidic luminal pH in the steady state cauda epididymidis, principal cells have the ability to secrete bicarbonate upon basolateral stimulation.37,38,39,40,41,42 The subsequent increase in luminal bicarbonate concentration is proposed to “prime” spermatozoa before ejaculation.39,41 Bicarbonate secretion by principal cells is mediated by CFTR, and it induces an alkalinization of the luminal environment.39,43,44 However, a sustained elevation of luminal pH and bicarbonate concentration may be considered detrimental to spermatozoa, by maintaining them in a preactivated state during storage. This should be prevented by subsequent stimulation by luminal bicarbonate of proton-secretion in clear cells, via the bicarbonate-activated soluble adenylyl cyclase (sAC) and activation of the cAMP/PKA pathway.35,45
In addition to mediating bicarbonate secretion, CFTR participates in ATP secretion in principal cells.43 Luminal ATP and its hydrolysis product adenosine act as extracellular messengers that activate proton-secretion in clear cells via principal cell/clear cell crosstalk.46 Several purinergic receptors are expressed in epididymal epithelial cells isolated by laser cut micro-dissection (LCM).46 These include the P1 A2B receptor, which activates the cAMP pathway upon adenosine stimulation47 and the P2×4 receptor, which is activated by alkaline pH and triggers an elevation in intracellular calcium.48 Whether these receptors are involved in the activation of clear cells will require additional studies.
Taken together, these results indicate that activation of proton-secretion in clear cells occurs via crosstalk with principal cells and involves a dual mechanism that depends on ATP and bicarbonate secretion by principal cells. We are currently investigating the potential role of principal cells as direct modulators of luminal pH in the cauda epididymidis, in addition to their indirect role in the modulation of clear cells.
ROLE OF LUMINAL FACTORS IN THE MAINTENANCE AND REMODELING OF EPITHELIAL CELLS IN THE INITIAL SEGMENT
The epididymis is an immature organ at birth, and epithelial cells continue to develop and differentiate over an extended postnatal period.49,50,51,52,53,54 Failure of IS epithelial cells to differentiate results in male infertility.26,54,55,56,57,58,59,60 The IS epithelium receives luminal testicular factors from its junction with the efferent ducts. The establishment and maintenance of the IS epithelium require both androgen and lumicrine factors,8,61,62,63,64,65 which include several hormones, ligands, and unknown factors that contribute to maintaining the MAPK pathway in an activated state in this segment58 (Kim et al. unpublished). Another protein that is potentially activated by luminal factors is the orphan receptor tyrosine kinase ROS1, which was originally described as an oncogene receptor, but was later identified as a key player in the initiation of epithelial cell differentiation.66 In the epididymis, ROS1 expression is very low at birth, but it becomes activated in the IS at the onset of epithelial cell differentiation.67,68 Male mice KO for the c-ROS1 oncogene 1 (c-ros1 KO), or ROS1KM/KM mice carrying a kinase-dead allele of ROS1, the protein that is encoded by the c-ros1 gene, have an undifferentiated IS and are infertile, but have normal spermatogenesis.26,67 Impaired differentiation of IS epithelial cells in the ROS1KM/KM mice was demonstrated by a reduction of expression of AQP9, a “terminal differentiation” protein (Figure 3), and a reduction in epithelial height, a hallmark of immature cells in this segment.67
Figure 3.
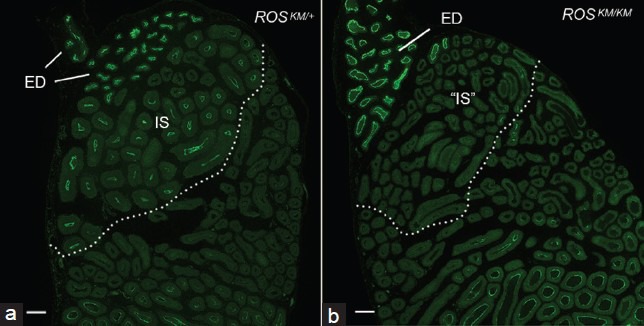
Impairment of terminal differentiation of principal cells in the initial segment of ROS1KM/KM mice. (a) Strong immunofluorescence labeling for AQP9 is detected in the initial segment (IS) and efferent ducts (ED) of an ROSKM/+ normal mouse. (b) AQP9 is not detectable in the corresponding segment “IS,” while AQP9 expression is not affected, in an ROSKM/KM mouse. Scale bars: 100 μm. Modified from Jun et al. Endocrinology 2014.
The ligand for ROS1 remains unknown, but an unbiased phosphoproteomics approach (Bio-Plex) has shown that the lack of ROS1 kinase activity in ROS1KM/KM mice induces a decrease in the phosphorylation of MAPK targets, including phosphoMEK1/2 (Ser217, Ser221), phosphoERK1/2 (Thr202/Tyr204), phosphoCREB (Ser133), phosphop90RSK (Ser380), and phosphoSTAT-3 (Ser727), but does not affect the AKT, p38 MAPK, or Src pathways.67 As the MAPK pathway is constitutively activated in the adult IS59,69 (Kim et al. unpublished), these data indicate that ROS1 participates, with other effectors, in maintaining MAPK activation. Spermatozoa from both c-ros1 KO mice and ROS1KM/KM mice display hairpin morphology, and CASA showed significantly reduced progressive and total sperm motility in ROS1KM/KM males (Figure 4).26,67 A pharmacological approach with Crizotinib during the prepubertal period (postnatal days 17–28) has shown that transient inhibition of ROS1 kinase activity delays, but does not permanently inhibit IS differentiation.67 However, when administered to adult animals for 7 or 14 days, Crizotinib has no effect on the architecture of the IS epithelium and fertility, and it was concluded that sustained ROS1 inhibition in the ROS1KM/KM mice is required to induce a permanent undifferentiated IS infertility phenotype.
Figure 4.
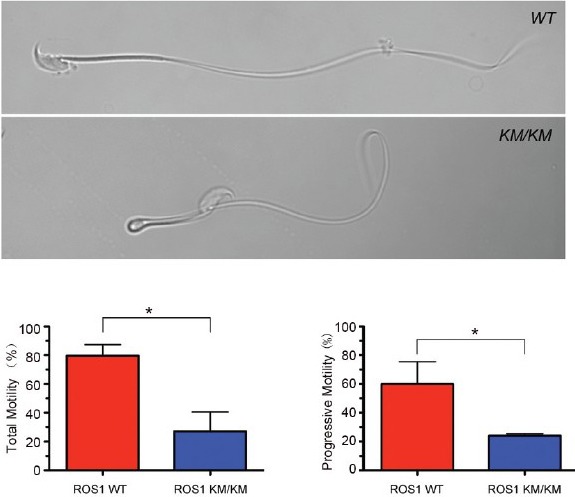
ROS1 kinase mutant spermatozoa exhibit abnormal morphology and reduced motility. Top panels: Spermatozoon isolated from the cauda epididymidis of a WT mouse or an ROSKM/KM mouse and suspended in Biggers, Whitten and Whittingham (BWW) medium containing polyvinyl alcohol (PVA). The ROSKM/KM spermatozoon shows flagellar angulation compared with the WT spermatozoon. Bottom panels: Computer-aided sperm analysis (CASA) showed decreases in the percentage of spermatozoa with total motility (left) and progressive motility (right) in ROSKM/KM mice compared with WT mice. Experiments were performed on three independent animals/genotype. Bars represent mean ± s.e.m. (*P < 0.05 by t-test), n = 3 mice per group. Scale bar: 10 μm. Modified from Jun et al. Endocrinology 2014.
In the IS epithelium, principal cells are the more-studied and better-characterized cell type in the IS, and they require lumicrine factors for their survival and differentiation.8,58,62,63,64,65 However, very little is known of the regulation of narrow cells and basal cells (BCs) by luminal factors in this segment. In the mouse IS, BCs send a narrow body projection between adjacent epithelial cells in the direction of the lumen.70,71 Labeling with the tight-junction protein ZO1 shows that BC projections, identified by their positive labeling for keratin 5, have the ability to cross the blood-epididymis barrier, so that the tip of the projection is in contact with the luminal content (Figure 5). The presence of BCs with intercellular projections in the mouse IS is compatible with their luminal sensory role, which has been shown in the rat epididymis.31 The formation of BC projections is segment-specific and species-dependent. In the rat, BC projections are present in the distal corpus and proximal cauda regions but are absent from the IS.31,72 In the mouse, they are exclusively present in the IS.70,71 Factors that regulate this segmental regulation of BC plasticity are for the most part unknown. Flutamide treatment reduces the number of BCs with projections in the rat epididymis,52 but not in the mouse epididymis.70 In the mouse IS, BC projections start to appear relatively soon after birth, at postnatal week 3,70 but in the rat epididymis BC projection formation is coincident with puberty.52 These results suggest a role for androgens in the formation and elongation of BC projections in the rat but not in the mouse. Mouse epididymal BCs progressively lose their projections after efferent duct ligation (EDL), a procedure that blocks luminal fluid entry to the epididymis without affecting blood flow (Figure 6).70 These results indicate the role of luminal factors in the maintenance of BC projections in the mouse IS.
Figure 5.
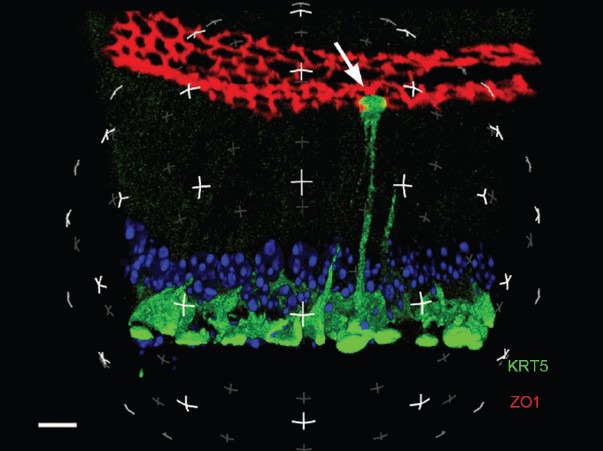
Three-dimensional reconstruction of a mouse initial segment by laser scanning confocal microscopy. BCs are labeled for keratin 5 (KRT5; green) and TJs are labeled for ZO1 (red). A BC with an intercellular projection that has crossed the TJs and is in contact with the luminal content is visible (arrow). Nuclei and spermatozoa are labeled with DAPI (blue). Scale bar: 5 μm. Modified from Kim et al. Biol Reprod 2015.
Figure 6.
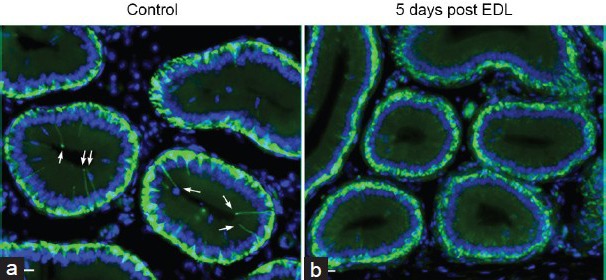
Effect of EDL on the number of BCs with intercellular projections in the mouse initial segment. BCs were labeled for KRT5 (green). (a) Numerous BCs with a long intercellular projection are visible in a control mouse. (b) No BC projections are detectable in a mouse initial segment 5 days after EDL. Scale bars: 15 μm. Modified from Kim et al. Biol Repro 2015.
Epithelial cell proliferation and apoptosis are very low in the control epididymis. However, EDL triggers a wave of apoptosis in BCs and principal cells with a maximum effect observed 1 day after EDL, followed by a progressive decrease in the number of apoptotic cells, 2 and 5 days after EDL.62,70,73 Treatment with the anti-androgen flutamide (without EDL) does not induce apoptosis but it further reduces the low proliferative activity of BCs.70 A subset of the BCs that survive EDL in the mouse epididymis show an increase in proliferation 2 days after EDL, followed by a return to a low proliferating state after 5 days. Flutamide treatment prevents the increase in BC proliferation induced 2 days after EDL, indicating the involvement of androgens in the maintenance and survival of this cell type. In contrast, EDL (Kim et al. data not shown) or castration74 does not affect narrow cell morphology, proliferation, and apoptosis. Thus, androgens may have a pro-proliferative effect on mouse epididymal BCs, similar to their well-recognized role in principal cell proliferation.75 We observed similar proliferative indices of IS epithelial cells in ROS1KM/KM and WT mice, indicating that ROS1 is not directly involved in the control of cell proliferation.67
REGULATION OF PRE- AND POST-NATAL EPIDIDYMAL DEVELOPMENT – AN UNEXPECTED ROLE OF CFTR
During tubulogenesis, epithelial cells receive signals that induce a switch between proliferation and differentiation. This switch depends on cell density and is partially initiated at the cell-cell junctions. Upon tight junction (TJ) formation, the TJ-associated protein ZO1 participates in the retention of a transcription factor (ZO1 nucleic acid binding protein; ZONAB) outside of the nucleus in TJs.76 ZONAB controls the expression of a subset of genes involved in cell growth and differentiation,77,78,79 and its relocalization to TJs decreases cell proliferation and promotes differentiation. We recently made the unexpected observation that CFTR was located in epididymal TJs, in addition to its being expressed in the apical membrane of principal cells (Figure 7).80 CFTR is a cAMP-activated anion channel previously implicated in anion secretion, followed by water transport, in several organs, including the epididymis.81,82,83,84,85,86 CFTR contains a PDZ-binding domain in its C-terminus,86 and we have shown that this motif participates in the interaction between CFTR and ZO1, which contains three PDZ domains.80 Using the epididymal cell culture DC2, developed in the laboratory of Marie-Claire Orgebin-Crist,87 we have shown that the absence of, or mutations in, CFTR reduces the stability of ZO1, leading to TJ disassembly and the release of ZONAB from TJs to nuclei.80 This promotes the transcription of the proliferation-associated gene cyclin D1 (CCND1)79 and represses the transcription of the differentiation-associated gene ErbB2.77 When DC2 cells are cultured on a matrigel layer, they form well-established tubular structures (Figure 8 Left). In contrast, a disorganized cell growth is induced after CFTR knockdown (KD) (Figure 8 Right) or inhibition,80 supporting the notion that CFTR plays an important role in tubulogenesis.
Figure 7.
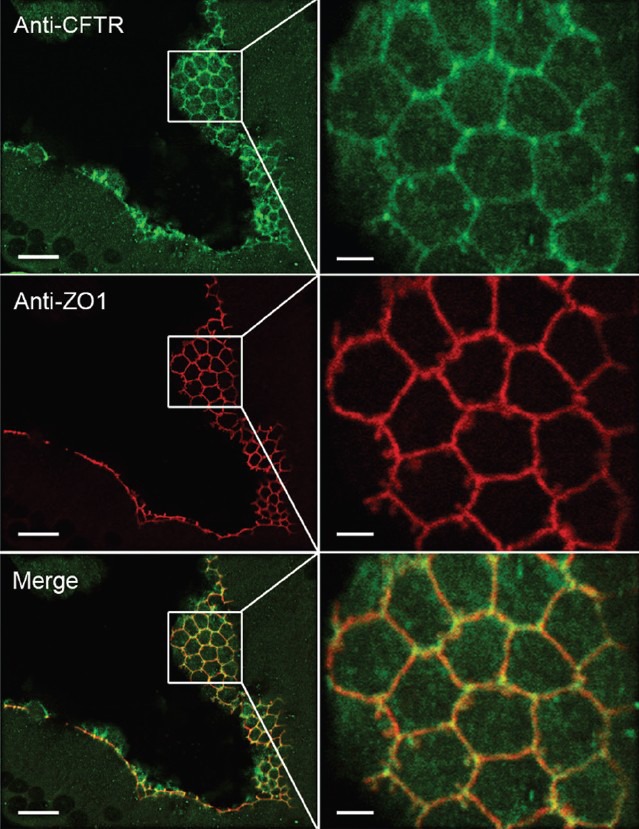
Co-localization of CFTR with ZO1 in TJs of the mouse initial segment. (Left panels) Confocal laser scanning microscope images showing CFTR (green) and ZO1 (red) labeling in TJs. (Right panels) Higher magnification images of the regions delineated in the boxes in the left panels. Co-localization of CFTR with ZO1 is indicated by the yellow/orange labeling in the merged panels. Scale bars: Left – 10 μm, Right – 2.5 μm. Modified from Ruan et al. J Cell Sci 2014.
Figure 8.
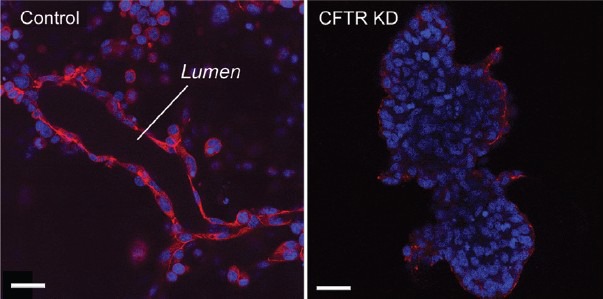
CFTR knockdown prevents tubulogenesis in DC2 cells. (Left) Laser scanning confocal imaging of control DC2 cells cultured on a matrigel layer and labeled for ZO1 (red). DC2 cells formed well-defined tubules with a clear luminal compartment. (Right) In contrast, after CFTR knockdown, DC2 showed a disorganized growth with the formation of cell masses lacking a lumen. Scale bars: 20 μm. Modified from Ruan et al. J Cell Sci 2014.
These results revealed a novel connection between CFTR and epithelial morphogenesis and differentiation. Mutations in CFTR cause dysfunction of tubular organs, resulting in cystic fibrosis (CF), the most common autosomal recessive disease in the Caucasian population. Almost all male CF patients have congenital bilateral absence of the vas deferens (CBAVD) or absence or atrophy of the epididymis.81,82,83,84,85,86,88 Defects in chloride, bicarbonate and water secretion, leading to increased viscosity of luminal fluid and blockage of Wolffian duct elongation, have been proposed as the primary cause of CF and CBAVD. However, this concept is challenged by the absence of luminal obstruction in the vas deferens and epididymis of human CF fetuses; they have normal microscopical morphology, with obvious lumen formation.89,90 CFTR expression precedes ZO1 in the TJs of the Wolffian duct,80 consistent with the participation of CFTR during embryonic development and tubulogenesis. We have, therefore, proposed that impairment of the ZO1/ZONAB interaction, secondary to the absence of or mutations in CFTR, affects the switch between proliferation and differentiation that occurs during development (Figure 9). The most common CF-associated mutation - deletion of phenylalanine at position 508 (DF508) - induces the retention of CFTR in the endoplasmic reticulum. According to our model, this would prevent its interaction with ZO1 in TJs and induce the nuclear translocation of ZONAB. Indeed, we have shown an increased level of ZONAB in nuclear extracts from mice expressing DF508-CFTR.80 We have also shown that the epididymis of adult CFTR KO mice has a lower expression of ZO1, AQP9 (in principal cells) and V-ATPase (in clear cells), indicating impaired epithelial cell differentiation in the absence of CFTR.80
Figure 9.
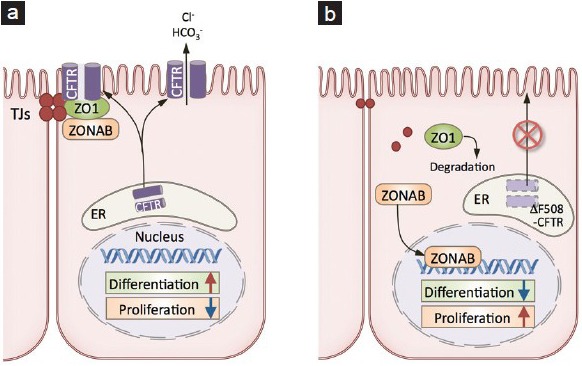
Proposed model for CFTR/ZO1 interaction in epithelial cells. (a) Through its interaction with ZO1, CFTR participates in the retention of ZONAB in TJs, which leads to a decrease in proliferation and activation of differentiation. (b) Retention of ΔF508-CFTR in the endoplasmic reticulum (ER) induces the redistribution of ZONAB from TJs to the nucleus. This increases proliferation and decreases differentiation.
At 26 weeks of gestation (which corresponds to the initiation of epithelial differentiation and apical localization of CFTR), destruction of the epithelial wall and infiltration of inflammatory cells was detected in a human CF fetus.90 In another CF-affected organ, the trachea, reduced airway caliber and reduced cross-sectional area were observed in newborn CFTR KO pigs and young CF patients.91 Of note, CFTR has recently been shown to regulate TJ-associated epithelial functions in a variety of organ systems, including the small intestine,92 colon,93 airway,94,95 and testis.96 Taken together, these results are consistent with the hypothesis that CFTR mutations affect TJs and restrict epithelial development and differentiation, leading to progressive deterioration of tissues that become more susceptible to stress and injury.
CONCLUSION
The epididymis, located downstream of the testis, is a complex organ that plays a crucial role in the maturation and storage of spermatozoa. However, only a few laboratories in the world study the posttesticular reproductive tract. Male infertility is often associated with dysfunctional spermatozoa that are produced in normal numbers, indicating impairment of the male excurrent duct, including the epididymis. The generation of transgenic mice and the development of novel imaging microscope modalities have allowed a better understanding of how the different cell types that line the epididymal tubule establish the unique environment, in which spermatozoa mature and are stored, and how their differentiated characteristics are regulated. However, much remains to be elucidated on how the epididymis operates in health and how it is affected in disease states.
COMPETING FINANCIAL INTERESTS
The author declared no competing interest.
ACKNOWLEDGMENTS
This work was supported by NIH grants RO1HD040793 and RO1DK097124 (to S.B.) and by postdoctoral fellowships from the Lalor Foundation (to Y.C.R and Y.J.P.). The Microscopy Core facility of the MGH Program in Membrane Biology receives support from the Boston Area Diabetes and Endocrinology Research Center (DK57521) and the Center for the Study of Inflammatory Bowel Disease (DK43351). S.B. is a recipient of the Charles and Ann Sanders Research Scholar Award at MGH.
REFERENCES
- 1.Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–27. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robaire B, Hinton BT, Orgebin-Crist MC. The epididymis. In: Neill JD, editor. Physiology of Reproduction. New York: Elsevier; 2006. pp. 1071–148. [Google Scholar]
- 3.Hermo L, Robaire B. Epididymal cell types and their functions. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecular to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Kluwer Academic/Plenum; 2002. pp. 81–102. [Google Scholar]
- 4.Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell cross talk in the epididymis: control of luminal acidification. J Androl. 2011;32:576–86. doi: 10.2164/jandrol.111.012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belleannee C, Thimon V, Sullivan R. Region-specific gene expression in the epididymis. Cell Tissue Res. 2012;349:717–31. doi: 10.1007/s00441-012-1381-0. [DOI] [PubMed] [Google Scholar]
- 6.Jose-Miller AB, Boyden JW, Frey KA. Infertility. Am Fam Physician. 2007;75:849–56. [PubMed] [Google Scholar]
- 7.Johnston DS, Turner TT, Finger JN, Owtscharuk TL, Kopf GS, et al. Identification of epididymis-specific transcripts in the mouse and rat by transcriptional profiling. Asian J Androl. 2007;9:522–7. doi: 10.1111/j.1745-7262.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- 8.Turner TT, Johnston DS, Finger JN, Jelinsky SA. Differential gene expression among the proximal segments of the rat epididymis is lost after efferent duct ligation. Biol Reprod. 2007;77:165–71. doi: 10.1095/biolreprod.106.059493. [DOI] [PubMed] [Google Scholar]
- 9.Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, et al. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–70. doi: 10.1095/biolreprod.106.057323. [DOI] [PubMed] [Google Scholar]
- 10.Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–59. [PubMed] [Google Scholar]
- 11.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–8. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong PY, Gong XD, Leung GP, Cheuk BL. Formation of the epididymal fluid microenvironment. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecular to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Kluwer Academic/Plenum; 2002. pp. 119–30. [Google Scholar]
- 13.Turner TT. Necessity's potion: Inorganic ions and small organic molecules in the epididymal lumen. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecular to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Kluwer Academic/Plenum; 2002. pp. 131–50. [Google Scholar]
- 14.Cheung KH, Leung GP, Leung MC, Shum WW, Zhou WL, et al. Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J Gen Physiol. 2005;125:443–54. doi: 10.1085/jgp.200409205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine N, Kelly H. Measurement of pH in the rat epididymis in vivo. J Reprod Fertil. 1978;52:333–5. doi: 10.1530/jrf.0.0520333. [DOI] [PubMed] [Google Scholar]
- 16.Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971;213:557–70. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acott TS, Carr DW. Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol Reprod. 1984;30:926–35. doi: 10.1095/biolreprod30.4.926. [DOI] [PubMed] [Google Scholar]
- 18.Carr DW, Acott TS. Intracellular pH regulates bovine sperm motility and protein phosphorylation. Biol Reprod. 1989;41:907–20. doi: 10.1095/biolreprod41.5.907. [DOI] [PubMed] [Google Scholar]
- 19.Carr DW, Usselman MC, Acott TS. Effects of pH, lactate, and viscoelastic drag on sperm motility: a species comparison. Biol Reprod. 1985;33:588–95. doi: 10.1095/biolreprod33.3.588. [DOI] [PubMed] [Google Scholar]
- 20.Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology (Bethesda) 2005;20:417–28. doi: 10.1152/physiol.00036.2005. [DOI] [PubMed] [Google Scholar]
- 21.Da Silva N, Shum WW, El-Annan J, Paunescu TG, McKee M, et al. Relocalization of the V-ATPase B2 subunit to the apical membrane of epididymal clear cells of mice deficient in the B1 subunit. Am J Physiol Cell Physiol. 2007;293:C199–210. doi: 10.1152/ajpcell.00596.2006. [DOI] [PubMed] [Google Scholar]
- 22.Signorelli J, Diaz ES, Morales P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res. 2012;349:765–82. doi: 10.1007/s00441-012-1370-3. [DOI] [PubMed] [Google Scholar]
- 23.Ickowicz D, Finkelstein M, Breitbart H. Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl. 2012;14:816–21. doi: 10.1038/aja.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J. 2006;25:4131–41. doi: 10.1038/sj.emboj.7601272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, et al. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS One. 2009;4:e4471. doi: 10.1371/journal.pone.0004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeung CH, Breton S, Stetiawan I, Xu Y, Lang F, et al. Increased luminal pH in the epididymis of infertile c-ros knockout mice and the expression of sodium-hydrogen exchangers and vacuolar proton pump H+-ATPase. Mol Reprod Dev. 2004;68:159–68. doi: 10.1002/mrd.20067. [DOI] [PubMed] [Google Scholar]
- 27.Breton S. Luminal acidification in the epididymis and vas deferens. In: Hinton BT, Turner TT, editors. Epididymis III. Charlottesville: The Van Doren Company; 2003. pp. 60–72. [Google Scholar]
- 28.Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H +)-ATPase. Nat Med. 1996;2:470–2. doi: 10.1038/nm0496-470. [DOI] [PubMed] [Google Scholar]
- 29.Pietrement C, Sun-Wada GH, Da Silva N, McKee M, Marshansky V, et al. Distinct Expression Patterns of Different Subunit Isoforms of the V-ATPase in the rat epididymis. Biol Reprod. 2006;74:185–94. doi: 10.1095/biolreprod.105.043752. [DOI] [PubMed] [Google Scholar]
- 30.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, et al. The V-ATPase B1 subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol. 2005;288:C1134–44. doi: 10.1152/ajpcell.00084.2004. [DOI] [PubMed] [Google Scholar]
- 31.Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, et al. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–17. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paunescu TG, Shum WW, Huynh C, Lechner L, Goetze B, et al. High-resolution helium ion microscopy of epididymal epithelial cells and their interaction with spermatozoa. Mol Hum Reprod. 2014;20:929–37. doi: 10.1093/molehr/gau052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton DW, Olson GE, Cooper TG. Regional variation in the surface morphology of the epithelium of the rat ductuli efferentes, ductus epididymidis and vas deferens. Anat Rec. 1977;188:13–27. doi: 10.1002/ar.1091880103. [DOI] [PubMed] [Google Scholar]
- 34.Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, et al. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H + ATPase (V-ATPase) recycling. J Biol Chem. 2005;280:8452–63. doi: 10.1074/jbc.M412750200. [DOI] [PubMed] [Google Scholar]
- 35.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, et al. Bicarbonate regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–9. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shum WW, Da Silva N, Belleannée C, McKee M, Brown D, et al. Regulation of V-ATPase recycling via a RhoA- and ROCKII-dependent pathway in epididymal clear cells. Am J Physiol Cell Physiol. 2011;301:C31–43. doi: 10.1152/ajpcell.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung AY, Yip WK, Wong PY. Characterization of adrenoceptors involved in the electrogenic chloride secretion by cultured rat epididymal epithelium. Br J Pharmacol. 1992;107:146–51. doi: 10.1111/j.1476-5381.1992.tb14477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedlacek RL, Carlin RW, Singh AK, Schultz BD. Neurotransmitter-stimulated ion transport by cultured porcine vas deferens epithelium. Am J Physiol Ren Physiol. 2001;281:F557–70. doi: 10.1152/ajprenal.2001.281.3.F557. [DOI] [PubMed] [Google Scholar]
- 39.Carlin RW, Lee JH, Marcus DC, Schultz BD. Adenosine stimulates anion secretion across cultured and native adult human vas deferens epithelia. Biol Reprod. 2003;68:1027–34. doi: 10.1095/biolreprod.102.009381. [DOI] [PubMed] [Google Scholar]
- 40.Hagedorn TM, Carlin RW, Schultz BD. Oxytocin and vasopressin stimulate anion secretion by human and porcine vas deferens epithelia. Biol Reprod. 2007;77:416–24. doi: 10.1095/biolreprod.106.056762. [DOI] [PubMed] [Google Scholar]
- 41.Pierucci-Alves F, Duncan CL, Lillich JD, Schultz BD. Porcine vas deferens luminal pH is acutely increased by systemic xylazine administration. Biol Reprod. 2010;82:132–5. doi: 10.1095/biolreprod.109.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierucci-Alves F, Schultz BD. Bradykinin-stimulated cyclooxygenase activity stimulates vas deferens epithelial anion secretion in vitro in swine and humans. Biol Reprod. 2008;79:501–9. doi: 10.1095/biolreprod.107.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruan YC, Shum WW, Belleannée C, Da Silva N, Breton S. ATP secretion in the male reproductive tract: essential role of CFTR. J Physiol. 2012;590:4209–22. doi: 10.1113/jphysiol.2012.230581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung GP, Wong PY. Activation of cystic fibrosis transmembrane conductance regulator in rat epididymal epithelium by genistein. Biol Reprod. 2000;62:143–9. doi: 10.1095/biolreprod62.1.143. [DOI] [PubMed] [Google Scholar]
- 45.Pastor-Soler N, Hallows KR, Smolak C, Gong F, Brown D, et al. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol. 2008;294:C488–94. doi: 10.1152/ajpcell.00537.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belleannee C, Da Silva N, Shum WW, Brown D, Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to the apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol. 2010;298:C817–30. doi: 10.1152/ajpcell.00460.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal. 2002;14:99–108. doi: 10.1016/s0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- 48.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–32. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 49.Da Silva N, Silberstein C, Beaulieu V, Pietrement C, Van Hoek AN, et al. Postnatal expression of aquaporins in epithelial cells of the rat epididymis. Biol Reprod. 2006;74:427–38. doi: 10.1095/biolreprod.105.044735. [DOI] [PubMed] [Google Scholar]
- 50.Nilnophakoon N. Histological studies on the regional postnatal differentiation of the epididymis in the ram. Anat Histol Embryol. 1978;7:253–72. doi: 10.1111/j.1439-0264.1978.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez CM, Kirby JL, Hinton BT. The development of the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecular to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Kluwer Academic/Plenum; 2002. pp. 251–67. [Google Scholar]
- 52.Shum WW, Hill E, Brown D, Breton S. Plasticity of basal cells during postnatal development in the rat epididymis. Reproduction. 2013;146:455–69. doi: 10.1530/REP-12-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun EL, Flickinger CJ. Development of cell types and of regional differences in the postnatal rat epididymis. Am J Anat. 1979;154:27–55. doi: 10.1002/aja.1001540104. [DOI] [PubMed] [Google Scholar]
- 54.Murashima A, Xu B, Hinton BT. Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J Androl. 2015;17:749–55. doi: 10.4103/1008-682X.155540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M, et al. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology. 2011;152:689–96. doi: 10.1210/en.2010-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bjorkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M, et al. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS One. 2012;7:e38457. doi: 10.1371/journal.pone.0038457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sipilä P, Cooper TG, Yeung CH, Mustonen M, Penttinen J, et al. Epididymal dysfunction initiated by the expression of simian virus 40 T-antigen leads to angulated sperm flagella and infertility in transgenic mice. Mol Endocrinol. 2002;16:2603–17. doi: 10.1210/me.2002-0100. [DOI] [PubMed] [Google Scholar]
- 58.Xu B, Abdel-Fattah R, Yang L, Crenshaw SA, Black MB, et al. Testicular lumicrine factors regulate ERK, STAT, and NFKB pathways in the initial segment of the rat epididymis to prevent apoptosis. Biol Reprod. 2011;84:1282–91. doi: 10.1095/biolreprod.110.090324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu B, Yang L, Hinton BT. The Role of fibroblast growth factor receptor substrate 2 (FRS2) in the regulation of two activity levels of the components of the extracellular signal-regulated kinase (ERK) pathway in the mouse epididymis. Biol Reprod. 2013;89:48. doi: 10.1095/biolreprod.112.107185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu B, Yang L, Lye RJ, Hinton BT. p-MAPK1/3 and DUSP6 regulate epididymal cell proliferation and survival in a region-specific manner in mice. Biol Reprod. 2010;83:807–17. doi: 10.1095/biolreprod.110.085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robaire B, Hamzeh M. Androgen action in the epididymis. J Androl. 2011;32:592–9. doi: 10.2164/jandrol.111.014266. [DOI] [PubMed] [Google Scholar]
- 62.Abe K, Takano H. Early degeneration of the epithelial cells in the initial segment of the epididymal duct in mice after efferent duct cutting. Arch Histol Cytol. 1989;52:299–310. doi: 10.1679/aohc.52.299. [DOI] [PubMed] [Google Scholar]
- 63.Fan X, Robaire B. Orchidectomy induces a wave of apoptotic cell death in the epididymis. Endocrinology. 1998;139:2128–36. doi: 10.1210/endo.139.4.5888. [DOI] [PubMed] [Google Scholar]
- 64.Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl. 1998;53:47–57. [PubMed] [Google Scholar]
- 65.Nicander L, Osman DI, Ploen L, Bugge HP, Kvisgaard KN. Early effects of efferent ductule ligation on the proximal segment of the rat epididymis. Int J Androl. 1983;6:91–102. doi: 10.1111/j.1365-2605.1983.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 66.Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta. 2009;1795:37–52. doi: 10.1016/j.bbcan.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Jun HJ, Roy J, Smith TB, Wood LB, Lane K, et al. ROS1 signaling regulates epithelial differentiation in the epididymis. Endocrinology. 2014;155:3661–73. doi: 10.1210/en.2014-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonnenberg-Riethmacher E, Walter B, Riethmacher D, Godecke S, Birchmeier C. The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev. 1996;10:1184–93. doi: 10.1101/gad.10.10.1184. [DOI] [PubMed] [Google Scholar]
- 69.Xu B, Washington AM, Hinton BT. PTEN signaling through RAF1 proto-oncogene serine/threonine kinase (RAF1)/ERK in the epididymis is essential for male fertility. Proc Natl Acad Sci U S A. 2014;111:18643–8. doi: 10.1073/pnas.1413186112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim B, Roy J, Shum WW, Da Silva N, Breton S. Role of testicular luminal factors on basal cell elongation and proliferation in the mouse epididymis. Biol Reprod. 2015;92:9. doi: 10.1095/biolreprod.114.123943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, et al. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod. 2014;90:1–10. doi: 10.1095/biolreprod.113.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Veri JP, Hermo L, Robaire B. Immunocytochemical localization of the Yf subunit of glutathione S-transferase P shows regional variation in the staining of epithelial cells of the testis, efferent ducts, and epididymis of the male rat. J Androl. 1993;14:23–44. [PubMed] [Google Scholar]
- 73.Turner T, Riley T. p53 independent, region-specific epithelial apoptosis is induced in the rat epididymis by deprivation of luminal factors. Mol Reprod Dev. 1999;53:188–97. doi: 10.1002/(SICI)1098-2795(199906)53:2<188::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 74.Moore H, Bedford J. Short-term effects of androgen withdrawal on the structure of different epithelial cells in the rat epididymis. Anat Rec. 1979;193:293–311. doi: 10.1002/ar.1091930209. [DOI] [PubMed] [Google Scholar]
- 75.Hamzeh M, Robaire B. Effect of testosterone on epithelial cell proliferation in the regressed rat epididymis. J Androl. 2009;30:200–12. doi: 10.2164/jandrol.108.006171. [DOI] [PubMed] [Google Scholar]
- 76.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–7. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 77.Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. Embo J. 2000;19:2024–33. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lima WR, Parreira KS, Devuyst O, Caplanusi A, N’Kuli F, et al. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J Am Soc Nephrol. 2010;21:478–88. doi: 10.1681/ASN.2009070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387–98. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruan YC, Wang Y, Da Silva N, Kim B, Diao RY, et al. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci. 2014;127:4396–408. doi: 10.1242/jcs.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anguiano A, Oates RD, Amos JA, Dean M, Gerrard B, et al. Congenital bilateral absence of the vas deferens. A primarily genital form of cystic fibrosis. JAMA. 1992;267:1794–7. [PubMed] [Google Scholar]
- 82.Cuppens H, Cassiman JJ. CFTR mutations and polymorphisms in male infertility. Int J Androl. 2004;27:251–6. doi: 10.1111/j.1365-2605.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 83.Oates RD, Amos JA. The genetic basis of congenital bilateral absence of the vas deferens and cystic fibrosis. J Androl. 1994;15:1–8. [PubMed] [Google Scholar]
- 84.Patrizio P, Zielenski J. Congenital absence of the vas deferens: a mild form of cystic fibrosis. Mol Med Today. 1996;2:24–31. doi: 10.1016/1357-4310(96)88755-7. [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Chen Z, Ni Y, Li Z. CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): a systemic [sic] review and meta-analysis. Hum Reprod. 2012;27:25–35. doi: 10.1093/humrep/der377. [DOI] [PubMed] [Google Scholar]
- 86.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–36. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 87.Araki Y, Suzuki K, Matusik RJ, Obinata M, Orgebin-Crist MC. Immortalized epididymal cell lines from transgenic mice overexpressing temperature-sensitive simian virus 40 large T-antigen gene. J Androl. 2002;23:854–69. [PubMed] [Google Scholar]
- 88.Joseph A, Yao H, Hinton BT. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev Biol. 2009;325:6–14. doi: 10.1016/j.ydbio.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaillard DA, Carre-Pigeon F, Lallemand A. Normal vas deferens in fetuses with cystic fibrosis. J Urol. 1997;158:1549–52. [PubMed] [Google Scholar]
- 90.Marcorelles P, Gillet D, Friocourt G, Lede F, Samaison L, et al. Cystic fibrosis transmembrane conductance regulator protein expression in the male excretory duct system during development. Hum Pathol. 2012;43:390–7. doi: 10.1016/j.humpath.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 91.Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182:1251–61. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Lisle RC. Disrupted tight junctions in the small intestine of cystic fibrosis mice. Cell Tissue Res. 2014;355:131–42. doi: 10.1007/s00441-013-1734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun TT, Wang Y, Cheng H, Xiao HZ, Xiang JJ, et al. Disrupted interaction between CFTR and AF-6/afadin aggravates malignant phenotypes of colon cancer. Biochim Biophys Acta. 2014;1843:618–28. doi: 10.1016/j.bbamcr.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 94.Castellani S, Guerra L, Favia M, Di Gioia S, Casavola V, et al. NHERF1 and CFTR restore tight junction organisation and function in cystic fibrosis airway epithelial cells: role of ezrin and the RhoA/ROCK pathway. Lab Invest. 2012;92:1527–40. doi: 10.1038/labinvest.2012.123. [DOI] [PubMed] [Google Scholar]
- 95.Molina SA, Stauffer B, Moriarty HK, Kim AH, McCarty NA, et al. Junctional abnormalities in human airway epithelial cells expressing F508del CFTR. Am J Physiol Lung Cell Mol Physiol. 2015;309:L475–87. doi: 10.1152/ajplung.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J, Fok KL, Chen H, Zhang XH, Xu WM, et al. Cryptorchidism-induced CFTR down-regulation results in disruption of testicular tight junctions through up-regulation of NF-kappaB/COX-2/PGE2. Hum Reprod. 2012;27:2585–97. doi: 10.1093/humrep/des254. [DOI] [PubMed] [Google Scholar]


