Abstract
The present study determined the relationship of male circumcision (MC) prevalence with prostatic carcinoma mortality rate in the 85 countries globally for which data on each were available. MC prevalence in different countries were obtained from a WHO report and allocated to WHO categories of 81%–100%, 20%–80%, and 0%–19%. Prostatic carcinoma mortality data were from Globoscan, gross national income per capita as well as male life expectancy were from a World Bank report, and percentages of Jews and Muslims by country were from the Pew Research Institute and the North American Jewish Data Bank. Negative binomial regression was used to estimate prostatic carcinoma mortality rate ratios. Compared to countries with 81%–100% MC prevalence, prostatic carcinoma mortality rate was higher in those with MC prevalence of 0%–19% (adjusted OR [adjOR] =1.82; 95% CI 1.14, 2.91) and 20%–80% (adjOR = 1.80; 95% CI, 1.16, 2.78). Higher Muslim percentage (adjOR = 0.92 [95% CI 0.87, 0.98] for each 10% increase) and longer life expectancy (adjOR = 0.82 [95% CI 0.72, 0.93] for each 5 additional years) were associated with lower prostatic carcinoma mortality. Higher gross national income per capita (adjOR = 1.10 [95% CI 1.01, 1.20] for double this parameter) correlated with higher mortality. Compared with American countries, prostatic carcinoma mortality rate was similar in Eastern Mediterranean countries (adjOR = 1.02; 95% CI 0.58, 1.76), but was lower in European (adjOR = 0.60; 95% CI 0.50, 0.74) and Western Pacific countries (adjOR = 0.54, 95% CI 0.37, 0.78). Thus, prostate cancer mortality is significantly lower in countries in which MC prevalence exceeds 80%.
Keywords: circumcision, country comparisons, epidemiology, global, prostate cancer, public health, urology
INTRODUCTION
Prostate cancer is the second most common cancer in men after lung cancer.1 Previous studies of the relationship of male circumcision (MC) and prostatic carcinoma incidence have either used small sample sizes2,3,4,5 and/or a single locality.4,5,6,7 In a previous evaluation in the current journal of 178 countries, the last author and a colleague found an association between prostatic carcinoma incidence and MC prevalence, but that study did not adjust for potential confounding variables.8 To date, an association study of MC prevalence with death arising from prostatic carcinoma globally has not been performed.
The aim of the present study was to determine the association of prostatic carcinoma mortality rate with the prevalence of MC in the 85 countries in the world for which data on each were available. The study used adjustments for potential confounding factors such as the percentages of countries that were predominantly Muslim or Jewish, gross national income, life expectancy, and geographical region.
MATERIALS AND METHODS
Prevalence data for MC for various countries were obtained from a report in 2006 by the World Health Organization (WHO) and were grouped into WHO categories of <20%, 20%–80%, and >80% MC prevalence (see Figure 4 of the WHO document).9 Prostate cancer deaths per 100 000 man-years (age-standardized mortality rate), and characterizations of data sources were obtained from a report in 2012 by the International Agency for Research on Cancer10 and logarithms of quotients of deaths served as offsets for mortality rates in regression analyses. The 2012 gross national income per capita and data on male life expectancy at birth were obtained from a report by the World Bank,11 with logarithms of gross national income per capita entered into regression analyses. Estimates of prevalence in 2010 of Jews in different countries were obtained from the North American Jewish Data Bank and for Muslims from the Pew Research Center.12
Negative binomial regression13 was used to calculate estimates and standard errors of the regression coefficients. The outcome, or Y, variable was the number of prostate cancer deaths, adjusted by means of an offset for the population of each country. The predictor, or X, variables were (1) male circumcision prevalence, (2) percentages of countries that were Muslim, (3) percentages of countries that were Jewish, (4) gross national income, and (5) WHO region. Analyses were performed with the MASS package14 on R 3.1.2.15 Null hypotheses were rejected for P < 0.05.
RESULTS
We evaluated the distributions of 197 434 prostatic carcinoma deaths among 85 countries with known gross national income per capita, male life expectancies, incidence of prostatic carcinoma death obtained from complete cancer registry data reported by the International Agency for Research on Cancer,10 and MC prevalence data reported by the WHO.9
Figure 1 shows mortality by MC and religious orientation prevalence. Countries with >81% MC prevalence had lower prostatic carcinoma mortality rate than countries with lower MC prevalence. The incidence of death appeared to decline as the percentage of Muslims increased. This did not appear to be the case for the percentage of Jews, although it is important to note that no country apart from Israel has even a modest percentage of Jews.
Figure 1.
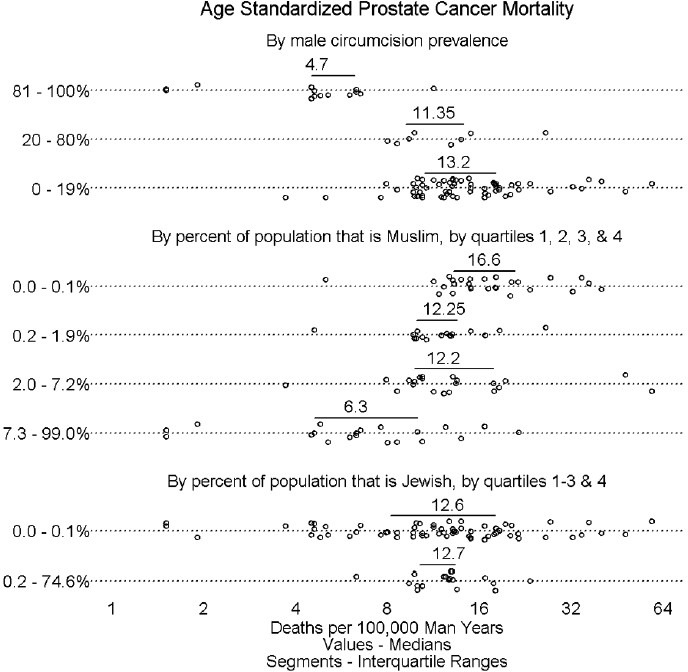
Prostate cancer mortality by male circumcision and religious preference. Each circle represents the age-standardized mortality for a country. Horizontal bars are interquartile ranges. Values above horizontal bars are medians. The upper panel shows prostate cancer mortality for high (>80%), medium (20%–80%), and low (<20%) MC prevalence countries. The middle panel shows prostate cancer mortality in relation to the population prevalence of Muslims, a religious group that requires MC, divided by quartiles of 0%–0.1%, 0.1%–1.9%. 2%–7.2% and 7.3%–99% of the population that is Muslim in the 85 countries assessed. The lower panel shows quartiles for percentage of Jews in the 58 countries; because of lower n values data for the lower 3 quartiles were combined.
Figure 2 shows mortality by gross national income per capita, life expectancy, and geographical region. A very minimal increment in mortality is seen with greater gross national income per capita. A slight trend of lower mortality appears with higher life expectancy. North, Central, and South American (hereafter, “American”) countries appeared to have higher mortality than did European, Eastern Mediterranean, and Western Pacific countries.
Figure 2.
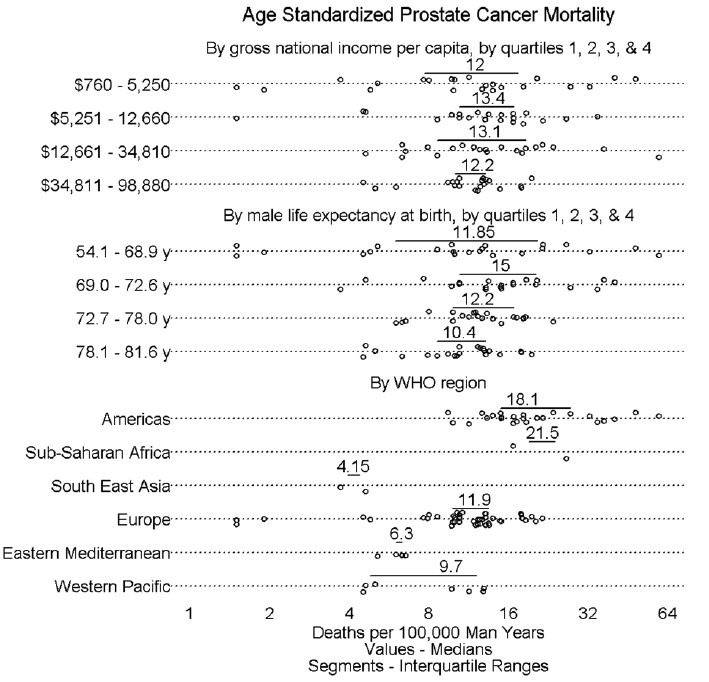
Prostate cancer mortality incidence by factors other than MC, namely gross national income, male life expectancy, and WHO region.
Figure 3 shows results of negative binomial regression. Values in the diagram are adjusted odds ratios (adjOR), with 95% confidence intervals (CI) shown as horizontal lines. As is the convention, when a CI line does not cross the vertical dotted line, the corresponding OR was deemed statistically significant (P < 0.05, Wald's χ2 test). The incidence of prostatic carcinoma death for countries in which MC prevalence was 0%–19% was 82% higher than that of countries in which MC prevalence was >80% (adjOR = 1.82; 95% CI 1.14, 2.91). Similar results were obtained when comparing high prevalence MC countries with countries having an MC prevalence of 20%–80% (adjOR = 1.80; 95% CI, 1.16, 2.78). The higher the percentage of Muslims in a country the lower the prostatic carcinoma mortality (adjOR = 0.92 [95% CI 0.87, 0.98] for each 10% increase in percentage of Muslims). Failure to find a similar association for Jews might have been due to lower population prevalence and thus power of the analysis (P > 0.05). A barely statistically significant association was found for higher gross national income (adjOR = 1.10 [95% CI 1.01, 1.20] for double the gross national income). Longer life expectancy was associated with lower incidence of prostatic carcinoma death (adjOR = 0.82 [95% CI 0.72, 0.93] for 5 years increase in lifespan). Owing to the availability of data from only two countries in each of Africa and South East Asia, no conclusion could be made about prostatic carcinoma, gross national income per capita, and life expectancy specifically in these regions. Eastern Mediterranean countries had similar prostatic carcinoma mortality incidence as American countries (adjOR = 1.02; 95% CI 0.58, 1.76), while for European (adjusted OR = 0.60; 95% CI 0.50, 0.74) and Western Pacific (adjOR = 0.54, 95% CI 0.37, 0.78) countries prostatic carcinoma mortality was lower. Because the analysis was multivariate, each odds ratio took into account the findings of the other analyses. Differences in mortality with respect to MC prevalence were adjusted for percentages of Muslims and Jews, for gross national income per capita, for male life expectancy at birth, and for WHO region.
Figure 3.
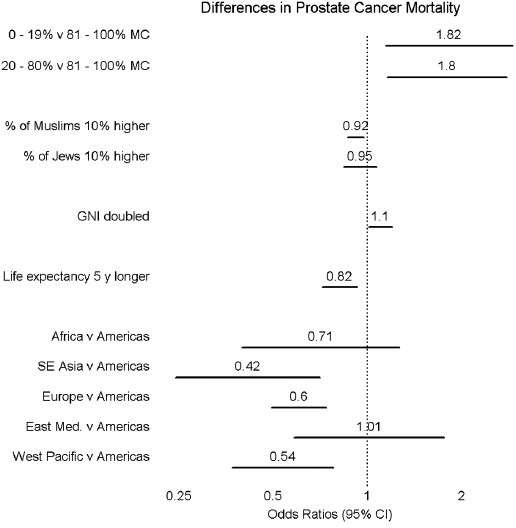
Results of negative binomial regression.
Neither violations of assumptions of generalized linear model regression nor important outliers were detected.
DISCUSSION
The present study found that prostatic carcinoma mortality rate in countries in which MC prevalence was >80% was approximately 45% lower than in countries in which MC prevalence was <80%. Living in a country with a higher percentage of Muslims, a lower gross national income per capita, longer life expectancy, and elsewhere than the Americas or Western Europe was associated with lower prevalence of prostatic carcinoma death. Our findings are consistent with, but do not prove, the hypothesis that MC affords a protective effect against prostatic carcinoma mortality.
Besides prostatic carcinoma incidence,8 inter-country comparisons have shown MC prevalence to be associated with a reduction in penile carcinoma.16 The various case-control studies that have reported an association between MC and reduced prevalence of prostatic carcinoma include the following. The first, in 1951, found that in men operated on for prostatic obstruction, only 1.8% of obstructions were cancerous in Jews, a group in which virtually all males are circumcised, compared with 19% in gentiles.2 Subsequent studies in Sweden,3 Southern California,4 and the UK5 found prostatic carcinoma prevalence was 50%–62% lower in circumcised men compared with uncircumcised men. These earlier studies were, however, small, comprising 100–300 subjects per group. A study in Seattle in 2012 comprising 1754 cases and 1645 controls found that MC was associated with an 18% reduction in aggressive prostatic carcinoma and a 12% reduction in less aggressive prostatic carcinoma, but only if the circumcision had been performed prior to sexual debut.6 The study found, moreover that the protective effect of MC was not affected by socioeconomic status. In 2014 a study in Montreal involving 1590 cases and 1618 controls found prostatic carcinoma was 11% lower in circumcised men, prevalence being 14% lower in those circumcised during infancy, 45% lower in those circumcised after age 35 years, and 60% lower in circumcised Black men.7
Although MC prevalence is high in many sub-Saharan African countries, relevant data needed for our study were available for only two countries in that region. Moreover, we deliberately excluded sub-Saharan African countries that have recently adopted voluntary medical MC as part of large-scale programs to reduce HIV prevalence. This decision was made on the basis of the slow time-course of prostatic carcinoma onset, meaning any effect of recent MC would be unlikely to be apparent.
Prostate-specific antigen screening, available in some, but not all, high gross national income countries, by detecting more prostate tumors, generates higher prevalence data for prostatic carcinoma.17 Because the present study concerned incidence of prostatic carcinoma mortality, not incidence of prostatic carcinoma itself, this potential confounding variable was not an issue in our analyses.
All epidemiology studies, including this one, suffer from the modifiable areal unit problem (MAUP),18 sometimes referred to as the “ecological” problem. The MAUP exists because results taken at different area levels can reveal different results. Thus, an analysis of all the countries of Western Europe might produce different patterns than analyses of all the provinces of European countries. No epidemiological study can draw definite conclusions at the individual level. On the other hand, when policy considerations are made at national levels, country comparisons are precisely what matters. National comparisons such as the landmark one by Doll on the relationship between cigarette smoking and lung cancer19 retain their utility, even though they do not themselves prove a relationship at the individual level. The present study, while not proving that men who are circumcised bear a lesser risk of developing prostate cancer, does add to the evidence that policy changes at the national level to encourage MC will likely be associated in the long run with decreasing rates of death from prostate cancer.
Although the mechanism of the protective effect of MC against prostatic carcinoma is yet to be defined, an infectious pathway has been considered. Since MC confers varying degrees of protection against certain common sexually transmitted infections (STIs), the reduced prostatic carcinoma incidence in men circumcised early in life6 lends support to findings that history of STIs increases risk of prostate cancer by 48%.20 In the US Physicians Health Study prevalence of the most common STI, Trichomonas vaginalis, was associated with risk of prostatic carcinoma later in life.21 Risk was two-fold higher for advanced prostatic carcinoma and three-fold higher for terminal cases. T. vaginalis is symptomless in most men carrying these infectious organisms, and a randomized controlled trial found MC reduced risk of T. vaginalis infection by 59%.22 Although there is no convincing evidence for a role of oncogenic types of HPV in prostate cancer etiology, a recent study noted that the presence of HPV16 in prostate tumor biopsies was associated with earlier death. A meta-analysis of 46 studies published between 1971 and 2011 found ever having had an STI to be associated with a 49% higher relative risk of prostatic carcinoma, relative risk of gonorrhea being 20% higher, but data for 3 studies of T. vaginalis (presumably included within the “any STI” group) were not reported.7 In potential contrast, the group in Montreal found that, in their cohort, those men having had more than 20 lifetime female sexual partners had a 28% lower risk of prostatic carcinoma, whereas having had several male sexual partners increased the risk;23 there was no association with STIs, although STI prevalence in the population was low.
Our additional finding of decreased risk of prostatic carcinoma with increased percentage of Muslims in a population could alternatively reflect any one of a number of cultural or genetic factors such as, for example, dietary customs or the Islamic prohibition of alcohol.24 Another dietary factor is high red meat consumption by more affluent populations. This increases risk of several cancers,25 in part involving metabolites of methionine,26 an amino acid that is much lower in vegan diets.26 In the case of prostatic carcinoma some studies,27 but not others,28 have implicated red meat consumption as a factor. Attempting to adjust for country-specific differences in dietary factors was, however, deemed too complicated and beyond the limit of the number of variables that could be incorporated into our multivariable analysis.
The association of decrease in prostatic carcinoma mortality with increased life expectancy may reflect the major impact that prostatic carcinoma has on male mortality. Prostate cancer is the second most common cancer in men after lung cancer.1 Because prostatic carcinoma is common in older men a decrease in prostatic carcinoma mortality should increase male lifespan. Although this notion, as well as the benefit of prostate-specific antigen (PSA) screening, has been questioned,29 more recent analyses found that PSA screening reduced the rate of prostatic carcinoma death30 and that discontinuation of PSA screening would likely increase the number of deaths due to prostatic carcinoma.31 The increased (competing) risk of dying from other age-related diseases might, however, partially explain the reduced rate of prostatic carcinoma death in screened subjects.
Our geographical findings may reflect any number of differences. A factor contributing to higher risk of prostatic carcinoma death in Middle Eastern and American countries could be racial since Black men have an elevated risk of prostatic carcinoma compared with white men.32
The negative impact we found of gross national income per capita on lower prostatic carcinoma mortality might reflect factors associated with higher living standards. Obesity appears to be a major factor,33 most likely contributed by factors such as Western sedentary lifestyle and diet.34
Future directions for research on risk factors for prostatic carcinoma include (i) better analyses of specific effects of alcohol to ascertain with greater certainty whether or not this is indeed a risk factor, (ii) dietary analyses that examine the quantity as well as the specific types of meats and other protein sources consumed, (iii) molecular studies of the relationship of prostate carcinogenesis to those STIs which MC is known to protect against and (iv) determination of the specific factors that might explain why an increase in gross national income per capita results in a modest increment in death from prostatic carcinoma.
CONCLUSION
By use of negative binomial regression analyses adjusting for potential confounding factors, the present study has shown for the first time that prostatic carcinoma mortality rate is lower in countries that have an MC prevalence of >80%. Lower prostatic cancer death was found in countries with a higher percentage of Muslims, a lower gross national income per capita, longer life expectancy, and elsewhere than the Americas or Western Europe. Our findings are consistent with, but do not prove, the hypothesis that MC affords a protective effect against prostatic carcinoma mortality.
AUTHOR CONTRIBUTIONS
MSW conceived the project and provided statistical evaluation, drafting of the manuscript and intellectual input; SY performed statistical analyses, intellectual input and critical review of drafts; BJM provided intellectual input and prepared the final draft. All authors have read and approved the final manuscript and agree with the order of appearance of the authors.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ravich A, Ravich RA. Prophylaxis of cancer of the prostate, penis, and cervix by circumcision. N Y State J Med. 1951;51:1519–1520. [PubMed] [Google Scholar]
- 3.Apt A. Circumcision and prostatic cancer. Acta Med Scand. 1965;178:493–504. doi: 10.1111/j.0954-6820.1965.tb04294.x. [DOI] [PubMed] [Google Scholar]
- 4.Ross RK, Shimizu H, Paganini-Hill A, Honda G, Henderson BE. Case-control studies of prostate cancer in blacks and whites in Southern California. J Natl Cancer Inst. 1987;78:869–74. [PubMed] [Google Scholar]
- 5.Ewings P, Bowie C. A case-control study of cancer of the prostate in Somerset and East Devon. Br J Cancer. 1996;74:661–6. doi: 10.1038/bjc.1996.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright JL, Lin DW, Stanford JL. Circumcision and the risk of prostate cancer. Cancer. 2012;118:4437–43. doi: 10.1002/cncr.26653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spence AR, Rousseau MC, Karakiewicz PI, Parent MÉ. Circumcision and prostate cancer: a population-based case-control study in Montréal, Canada. BJU Int. 2014;114:E90–8. doi: 10.1111/bju.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris BJ, Waskett JH. Circumcision reduces prostate cancer risk. Asian J Androl. 2012;14:661–2. doi: 10.1038/aja.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geneva: World Health Organization; 2007. [Last accessed on 2015 Jul 15]. WHO and UNAIDS. Male Circumcision: Global Trends and Determinants of Prevalence, Safety and Acceptability. (191 refs) p. 42. Available from: http://www.whqlibdoc.who.int/publications/2007/9789241596169_eng.pdf . [Google Scholar]
- 10.International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012. [Last accessed on 2014 Dec 29]. Available from: http://www.globocan.iarc.fr/Pages/fact_sheets_cancer.aspx .
- 11.The World Bank Open Data. 2014. [Last accessed on Jan 02, 2014]. Available from: http://www.data.worldbank.org/
- 12.Della Pergola S. Connecticut: University of Connecticut; 2010. World Jewish Population, 2010. [Google Scholar]
- 13.Hardin JW, Hilbe J. College Station, Texas, USA: Stata Press; 2001. Generalized Linear Models and Extensions. [Google Scholar]
- 14.Venables WN, Ripley BD. 4th ed. New York, USA: Springer; 2002. Modern Applied Statistics with S. [Google Scholar]
- 15.Vienna, Austria: R Foundation for Statistical Computing; 2014. R Core Team. R: A Language and Environment for Statistical Computing. R 3.1.2. [Google Scholar]
- 16.Chaux A, Cubilla AL. Advances in the pathology of penile carcinomas. Hum Pathol. 2012;43:771–89. doi: 10.1016/j.humpath.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA. 1995;273:548–52. [PubMed] [Google Scholar]
- 18.Dark SJ, Bram D. The modifiable areal unit problem (MAUP) in physical geography. Prog Phys Geogr. 2007;31:471–9. [Google Scholar]
- 19.Doll R, Hill AB, Gray PG, Parr EA. Lung cancer mortality and the length of cigarette ends; an international comparison. Br Med J. 1959;1:322–5. doi: 10.1136/bmj.1.5118.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor ML, Mainous AG, 3rd, Wells BJ. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam Med. 2005;37:506–12. [PubMed] [Google Scholar]
- 21.Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: physicians’ health study. J Natl Cancer Inst. 2009;101:1406–11. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobngwi-Tambekou J, Taljaard D, Nieuwoudt M, Lissouba P, Puren A, et al. Male circumcision and Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis: observations after a randomised controlled trial for HIV prevention. Sex Transm Infect. 2009;85:116–20. doi: 10.1136/sti.2008.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spence AR, Rousseau MC, Parent ME. Sexual partners, sexually transmitted infections, and prostate cancer risk. Cancer Epidemiol. 2014;38:700–7. doi: 10.1016/j.canep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 24.McGregor SE, Courneya KS, Kopciuk KA, Tosevski C, Friedenreich CM. Case-control study of lifetime alcohol intake and prostate cancer risk. Cancer Causes Control. 2013;24:451–61. doi: 10.1007/s10552-012-0131-7. [DOI] [PubMed] [Google Scholar]
- 25.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–17. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavuoto P, Fenech MF. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat Rev. 2012;38:726–36. doi: 10.1016/j.ctrv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 27.John EM, Stern MC, Sinha R, Koo J. Meat consumption, cooking practices, meat mutagens, and risk of prostate cancer. Nutr Cancer. 2011;63:525–37. doi: 10.1080/01635581.2011.539311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenfield SA, DuPre N, Richman EL, Stampfer MJ, Chan JM, et al. Mediterranean diet and prostate cancer risk and mortality in the Health Professionals Follow-up Study. Eur Urol. 2014;65:887–94. doi: 10.1016/j.eururo.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 30.Wachtel MS, Nelius T, Haynes AL, Dahlbeck S, de Riese W. PSA screening and deaths from prostate cancer after diagnosis – A population based analysis. Prostate. 2013;73:1365–9. doi: 10.1002/pros.22680. [DOI] [PubMed] [Google Scholar]
- 31.Gulati R, Tsodikov A, Etzioni R, Hunter-Merrill RA, Gore JL, et al. Expected population impacts of discontinued prostate-specific antigen screening. Cancer. 2014;120:3519–26. doi: 10.1002/cncr.28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Shlomo Y, Evans S, Ibrahim F, Patel B, Anson K, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53:99–105. doi: 10.1016/j.eururo.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 33.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–9. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]


