Abstract
MiR-200a was shown to be upregulated in the corpus cavernosum (CC) of rats with aging-related erectile dysfunction (A-ED) in our previous study. Among its target genes, SIRT1 was also reported as a protective factor in erectile function by our groups previously. Thus, miR-200a might attenuate the erectile function in A-ED via SIRT1 inhibition. In the present study, three animal groups were included: aged rats with ED (group AE, n = 8), aged rats with normal erectile function (group AN, n = 8), and young rats as normal controls (group YN, n = 8). CCs from each group were collected for histological and molecular measurements to validate the dysregulation of miR-200a and SIRT1. After that, the cavernous endothelial cells (CECs) from CC of aged rats with normal erectile function were transfected with miR-200a in vitro. Then the expression of SIRT1 and molecules within the eNOS/NO/PKG pathway were measured to investigate whether the transfection could imitate the attenuated process of erectile function in the aged. As a result, miR-200a was upregulated while the SIRT1, the levels of eNOS and cGMP were all downregulated in the CCs from AE group. After transfection in vitro, the miR-200a was upregulated while the SIRT1 and levels of eNOS and cGMP were obviously downregulated. Finally, based on the results of our previous study, we further verify that up-regulation of miR-200a could participate in the mechanisms of A-ED via SIRT1 inhibition, and mainly attenuate endothelial function via influencing the eNOS/NO/PKGpathway.
Keywords: aging, endothelial nitric oxide synthase, erectile dysfunction, mir-200a, SIRT1
INTRODUCTION
Erectile dysfunction (ED) is defined as a persistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance. Epidemiologic studies show that aging is an independent predictor of ED, irrespective of comorbid diseases such as cardiovascular diseases and diabetes.1,2,3 In addition, prevalence of ED increases progressively with aging.4,5,6 Since human life expectancy increased enormously in the last century, aging-related ED (A-ED) gained in importance.7,8 The underlying pathogenetic mechanisms of A-ED, however, are poorly understood. As a result, shortages could be seen in several treatments of A-ED despite many treatment strategies have been developed.9 Thus, research on the mechanism of A-ED is urgently needed.
MicroRNAs (miRNAs), which generally exist in the eukaryote, are strongly conservative noncoding small RNAs with 19–23nt length. They can regulate gene expression by binding completely or partially to the 3’UTR of target gene related mRNA for posttranscriptional inhibition.10 It has been proven that miRNAs modulate a variety of functions including proliferation, differentiation, apoptosis, and senescence.11,12 Importantly, a series of aging-related genes that could be regulated by many miRNAs have been demonstrated to be involved in the pathogenesis of A-ED.13,14,15 Thus, miRNAs may be involved in the mechanisms of A-ED via regulating various protein functions at the upstream level. In our previous report,16 we have identified the miRNAs profile in the corpus cavernosum (CC) of rats with A-ED. As a result, up-regulation of miR-200a was identified in the CC of rats with A-ED and its main target pathway was eNOS/NO/PKG as the bioinformatic analysis predicted, which had been considered to be one of the important pathways of the physiology of normal erection.
Among the predicted target genes of miR-200a, silent information regulator 1 (SIRT1) is a highly conserved nicotinamide adenine dinucleotide (NAD)+-dependent protein deacetylase and plays important roles in aging, obesity, and cancer.17,18 It could be deduced that SIRT1 might act as a protected factor in maintaining erectile function because it could up-regulate the endothelial nitric oxide synthase (eNOS) activity, increase the NO production, and promote the endothelium-dependent relaxation.19,20,21 Our group has previously found that SIRT1 was decreased in the CC of rats with some types of ED. Administration of its activator could ameliorate the erectile function probably by improving endothelial function.14 Similar to our previous findings,16 recently miR-200 has been reported by another research to regulate SIRT1 through targeting SIRT1 3’- UTR.22 Therefore, it could be hypothesized that miR-200a might be involved in the mechanism of A-ED via regulating the expression of SIRT1, and the most probable target tissue might be endothelial cells.
Based on the above findings, the present study was designed, firstly to testify whether the up-regulated miR-200a was associated with the down-regulated SIRT1, and influenced the endothelial function in the CC of rats with A-ED; secondly, to investigate whether up-regulation of miR-200a in the endothelial cells of CC of aged rats with normal erectile function could lead to the down-regulation of SIRT1 and attenuate the endothelial function, and thus to demonstrate that the miR-200a could contribute to the decrease of erectile function of aged rats to some extent via SIRT1 inhibition and the eNOS/NO/PKG pathway influencing. To our knowledge, this is the first study designed to explore the potential role of miR-200 in the development of A-ED, which will provide new perspectives for the researches regarding the underlying mechanism of A-ED.
MATERIALS AND METHODS
Animal groups and study design
Forty 18-month old male Sprague–Dawley (SD) rats defined as aged rats and eight 3-month SD rats defined as young normal controls (group YN) were used in the present study. All rats underwent apomorphine-induced penile erection test to evaluate the erectile function according to a previously described protocol.16 Finally, aged rats were divided into two groups: aged rats with normal erectile function (group AN) and aged rats with erectile dysfunction (group AE). Intracavernous pressure (ICP) investigation and histological measurement were applied to further confirm the grouping. After the functional test, all rats were sacrificed, and CCs from different groups were collected for molecular measurements. To testify the potential role of miR-200 in the development of A-ED, the cavernous endothelial cells (CECs) from AN groups were isolated and transfected with miR-200 mimics or negative control. SIRT1 expression and eNOS/NO/PKG pathway were detected after transfection. All procedures were approved by the Institutional Animal Care and Use Committee at Nanjing University.
In vivo penile erection
The apomorphine-induced penile erection test was performed according to the protocol previously described.16,23 Briefly, apomorphine (Sigma-Aldrich, St. Louis, MO, USA) dissolved in sodium chloride with 0.1% ascorbic acid was injected into the napes of rats at a dosage of 80 μg kg−1. Each rat was observed in a transparent cage for 30 min. The latency of the first erection and the number of erections per animal were documented.
Electrostimulation for penile erection
ICP response to electrostimulation of the cavernous nerve (CN) was performed as our groups once described.24 Briefly, the CNs on both sides were exposed. A 25-gauge needle connected to a PE-50 tube was inserted into the left crura for measurement of ICP. The left carotid artery was subsequently exposed and cannulated with a PE-50 tube to record the mean arterial pressure (MAP). The MAP and ICP were reported by RM6042B/C multichannel signal collection processing system (Chengdu Implement Company, Chengdu, China). Stimulation of CN was performed at a frequency of 15 Hz. with a pulse width of 5 ms, and at 5 V for 60 s with resting periods of 5 min between subsequent stimulations. The highest ICP was chosen for statistical analysis. The ratio of peak ICP/MAP was calculated to evaluate the erectile function.
Immunohistochemistry staining
The histological measurement was performed as reported.16,24,25 Briefly, the middle penile shafts were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) at 4°C overnight, after which the tissue was transferred to 30% sucrose in PBS at 4°C overnight. The tissue was then embedded and cut transversely at a thickness of 5 μm. The sections were incubated with 3% bovine serum albumin for 30 min at room temperature and then incubated with mouse anti-alpha smooth muscleactin (anti-α-SMA, 1:200, Abcam Inc, Cambridge, Massachusetts) or anti-von Willebrand factor (anti-vWF, 1:200, Abcam Inc, Cambridge, MA, USA) at 4°C overnight, followed by Alexa-488- or Alexa-594-conjugated secondary antibodies (1:500, Invitrogen, Carlsbad, CA, USA). The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Image analysis was performed by computerized densitometry using Image-Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA). To quantify smooth muscle, the percentage of α-SMA indicating positive area within the CC was analyzed. Similarly, the percentage of vWF indicating positive area was analyzed to quantify the endothelium.
Isolation of CECs
Isolation of CECs was performed according to the protocol as previously described.26 Briefly, each tissue sample was minced into pieces of about 1 mm3, digested in a solution containing 9 ml of 0.1% collagenase II (Invitrogen, Carlsbad, CA, USA) and 1 ml of dispase (Invitrogen) for 30 min at 37°C with vigorous shaking. A volume of 75 μl of 1 mg ml−1 DNase I (Sigma-Aldrich, St. Louis, MO, USA) was added for further digestion for 30 min, followed by a 5-min incubation period on ice, and a subsequent addition of 5 ml of Endothelial Growth Medium-2 (EGM2, Lonza Biologics, Portsmouth, NH, USA). The mixture was then filtered through a 100-μm cell strainer (BD Biosciences, Bedford, MA, USA), followed by centrifugation at 400 g for 5 min. The cell pellet was washed with PBS, resuspended in 0.5 ml of EGM2, and incubated with anti-CD31 antibody (Abcam, Cambridge, MA, USA) for 45 min at 4°C. The cells were then washed with EGM2 and resuspended in 80 μl of isolation buffer containing 2 mmol l-1 EDTA and 0.5% bovine serum albumin in PBS, followed by the addition of 20 μl anti-mouse IgG microbeads (Miltenyi Biotec, Auburn, CA, USA) and incubation at 4°C for 15 min. The cell mixture was then washed and resuspended in 500 μl of isolation buffer, and loaded onto the magnetic column (Miltenyi Biotec) preequilibrated with 3 ml isolation buffer. After washing the column 3 times with 3 ml isolation buffer each time, the target endothelial cells were eluted with 5 ml of isolation buffer. Finally, the target cells were centrifuged and cultured in EGM2 supplemented with 20% fetal bovine serum at 37°C in an atmosphere containing 5% CO2.
Cell transfection
As reported,27,28 the cultured CECs were seeded on six-well plates and were transfected with miR-200a mimics (100 pmol, Genepharma) or mimics negative control RNA (mimics N.C., 100 pmol, Genepharma) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were harvested 24 h after transfection for Quantitative Real-time PCR (qRT-PCR) and 48 h for protein analysis.
Western blotting
The protein measurement was performed according to our previously described protocol.14,27 In brief, the tissues stored in liquid nitrogen were powdered and lysed. Then the samples were homogenized in extraction buffer (10.0 mmol l−1 Tris pH 7.4, 150.0 mmol l−1 sodium chloride, 0.4% TritonX-100 and 1/100 complete protease inhibitors), and centrifuged at 17 000 g for 20 min. Thereafter, the protein samples were quantified using a BCA kit (Thermo Scientific, Rockford, IL, USA). Prepared protein samples were separated on 10% SDS-PAGE and transferred onto PVDF Western Blotting Membranes (Roche Diagnostics). The membranes were blocked and then incubated with primary antibodies overnight at 4°C. The final dilutions for primary antibodies were anti-SIRT1 (Abcam, 1:500) and anti-GAPDH (Abcam, 1:1000). Horseradish peroxidase anti-mouse and anti-rabbit (Santa Cruz Biotechnology) were used as secondary antibodies. The signal was detected by the super-signal-enhanced chemiluminescence system (Pierce, Rockford, IL, USA).
RNA isolation and miRNA qRT-PCR assay
As previously reported,16,27 total RNA of penile tissue samples without urethra and other adventitial tissues was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instructions. Glycogen (Invitrogen) was added to the RNA precipitation step. The concentration and quality of the extracted RNA were measured with a spectrophotometer (Eppendorf) at 260 nm and 280 nm (i.e., the A260/A280 ratio). qRT-PCR was performed using a TaqMan PCR kit and the Applied Biosystems 7300 Sequence Detection System. The reactions were incubated in a 96-well optical plate at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. All reactions, including no-template controls, were run in triplicate. After the reactions, the cycle threshold (CT) data were determined using default threshold settings, and the mean CT was determined from the duplicate PCRs. A comparative ΔCT method29 was used to compare each condition with controls, and values are expressed as 2−ΔCT. The relative levels of miRNAs in cells and tissues were normalized to U6, a ubiquitously expressed small nuclear RNA.
Enzyme-linked immunosorbent assay
To determine the level of eNOS and cyclic guanosine monophosphate (cGMP) in different groups, we performed the enzyme-linked immunosorbent assay (ELISA) quantitative assay using the rat eNOS and cGMP ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The experimental conditions were maintained according to the manufacturer's protocol.
Data analysis
All data were presented as means ± standard deviation (s.d.). Statistical analysis was determined by the independent Student's t-test and one-way ANOVA followed by the Turkey-Kramer test for post hoc comparisons using SPSS 16.0 software (SPSS, Chicago, IL, USA). A P value cut-off 0.05 was considered as statistically significant.
RESULTS
The erectile function of rats decrease in the AE group
According to the apomorphine test, the experimental animals were divided into different groups. As the results showed, 32 rats in the old group were defined as rats with A-ED. Eight of them were randomly selected for the further experiments and were named as the group of aged rats with ED (group AE). The left 8 aged rats with normal erectile function were included in the group of aged rats with normal erectile function (group AN). Eight 3-month old young rats were used as young normal controls (group YN, n = 8). The erectile function test and histological measurements further confirmed the grouping. As illustrated in Figure 1, the ratios of ICP/MAP decreased obviously in AE group compared with those in both AN and YN groups while the AN and YN groups were similar in this aspect. Accompanied with the functional data, the smooth muscle and endothelium content within the CC of AE group decreased significantly compared with other two groups. Also, the AN and YN groups were similar in these aspects.
Figure 1.
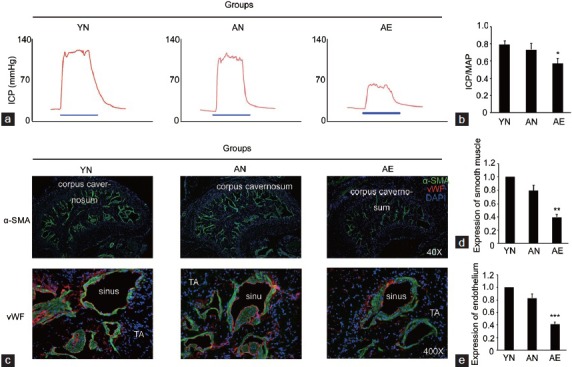
The erectile function and penile histology of three experimental groups. (a) Representative ICP recording of each experimental group. The blue bar represents 60s electrical stimulation of the CN. The red curve represents ICP values in response to CN stimulation. (b) Erectile function parameters (ICP/MAP ratio) of each experimental group. (c) Representative images of the smooth muscle (indicated by α-SMA) and endothelium (indicated by vWF) in corpus cavernosum of each group. Percentage of smooth muscle (d) and endothelium (e) within corpus cavernosum as relative expression as compared with YN group. Original magnifications are ×40 when showed α-SMA, and ×400 when showed vWF. TA: tunica albuginea. *P = 0.013, **P = 0.003, ***P = 0.001 compared with other groups.
The expression of miR-200a is upregulated while the SIRT1 protein, the levels of eNOS and cGMP are all downregulated in AE group
According to the qRT-PCR results, the expression of miR-200a was up-regulated in AE group at a high level with the average threshold criterion CT ≤25.0, and more than two-fold compared with that in both AN and YN groups. On the contrary, the expression of SIRT1 and eNOS and cGMP levels which reflected the endothelial function was all notably downregulated in AE group according to the Western Blotting and ELISA results (Figure 2).
Figure 2.
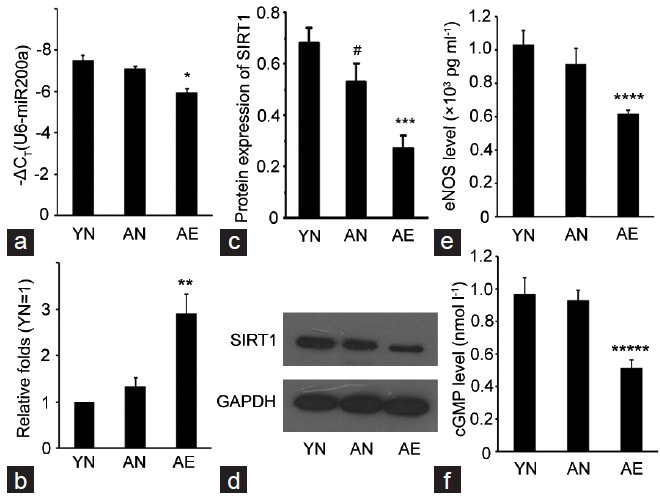
Real-time quantification of miR-200a, Western Blotting results of SIRT1 and ELISA results of eNOS and cGMP levels in three groups. (a) The expression levels of miR-200a in each group (analyzed by qRT-PCR). For comparison, the CT value of sno RNA U6, whose mean value in each group was 18.415, 18.635, and 17.909, respectively, was subtracted from the CT value of miR-200a (ΔCT). The y-axis showed the –ΔCT and represents relative miR-200a expression levels. (b) The relative expression of miR-200a in each group as compared with YN group. (c) Protein expression of SIRT1 in each group as compared with the expression of GAPDH (analyzed by Western Blotting). (d) Representative images of the Western Blotting results of SIRT1 and GAPDH. (e and f) The eNOS and cGMP levels in each group (analyzed by ELISA). The experiments were repeated at least 3 times. *P = 0.01, **P = 0.001, ***P = 0.001, ****P = 0.021, *****P = 0.034, when compared with YN and AN groups. #P = 0.038 compared with YN group.
Upregulated miR-200a in CECs results in the downregulation of SIRT1 protein and eNOS and cGMP levels in vitro
To study whether up-regulation of miR-200a in the CECs of aged rats with normal erectile function could lead to the downregulation of SIRT1 protein and eNOS and cGMP levels, the CECs from the CCs of group AN were collected, cultured and grouped. The grouping of CECs included CECs from AN group (group E-AN), CECs from AN group transfected with mimics N.C. (group E-AN + NC), CECs from AN group transfected with miR-200a mimics (group E-AN + M). After transfection with miR-200a mimics, the expression of miR-200a was upregulated (P = 0.015) and the expression of SIRT1 and eNOS and cGMP levels were all downregulated (P = 0.012, 0.004, and 0.024). These regulated relationships manifested that the up-regulation of miR-200a could partially contribute to the decrease of the erectile function of aged rats via SIRT1 inhibition and the eNOS/NO/PKG pathway. Meanwhile, there were no significant differences in the expression of miR-200a, SIRT1, eNOS and cGMP levels between the E-AN and E-AN + NC groups (Figure 3).
Figure 3.
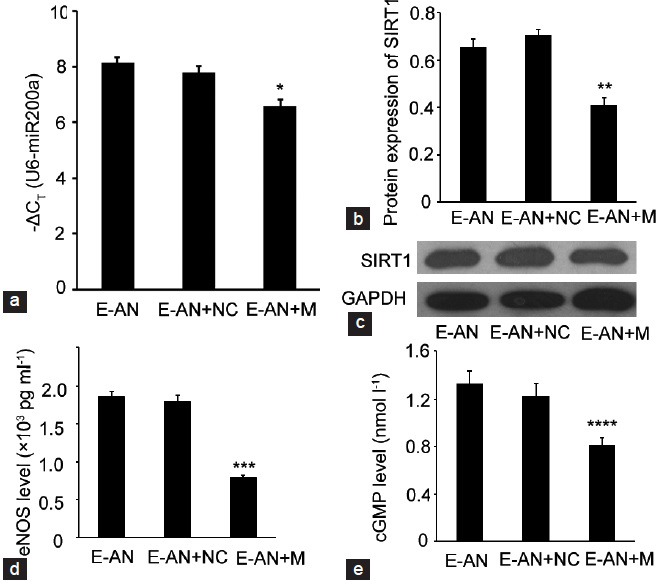
Alteration of miR-200a, SIRT1, eNOS and cGMP in different cellular groups in vitro. (a) The expression levels of miR-200a in each group (analyzed by qRT-PCR). The CT value of sno RNA U6, whose mean value in each group was 18.321, 18.346, and 17.849 respectively, was subtracted from the CT value of miR-200a (ΔCT). The y-axis showed the −ΔCT and represents relative miR-200a expression levels. (b) Protein expression of SIRT1 in each group as compared with the expression of GAPDH (analyzed by Western Blotting). (c) Representative images of the Western Blotting results of SIRT1 and GAPDH. (d and e) The eNOS and cGMP levels in each group (analyzed by ELISA). The experiments were repeated at least 3 times. *P = 0.015, **P = 0.012, ***P = 0.004, ****P = 0.024, when compared with E-AN and E-AN + NC groups.
DISCUSSION
The genesis and development of A-ED are based on the functional change accomplished with aging. A-ED could be aggravated by exposure to a series of exogenous factors in different degrees or by experiencing several relative systemic diseases such as cardiovascular diseases and diabetes mellitus. Several factors including accelerated aging of the internal pudendal artery, endothelial dysfunction, smooth muscle decrease, deposition of collagen fibers, androgen deficiency, have been demonstrated to be pathophysiological characters of A-ED.9,30 Also, many signaling pathways and a large amount of related molecules are speculated to be involved in the development of A-ED.12,31
MiRNAs could modulate a variety of pathophysiological processes via network regulation to different signaling pathways and molecules. A large number of A-ED related genes, such as IGF1, TGFB2, END1, CAV1, SIRT1, BDNF, have been demonstrated to be regulated by miRNAs.13,14,15,32,33,34 Based on these findings, our group has explored the miRNAs profiling in aged rats with ED. As a result, four miRNAs were identified as upregulated, and were predicted mainly to regulate eNOS/NO/PKG and PGE1/PKA pathways, which have been considered to be important pathways in the physiology of normal erection. Therefore, it's reasonable to speculate that miRNAs might play a crucial role in the development of A-ED.
As one of the predicted miRNAs, miR-200a might be involved in the underlying mechanisms of A-ED through regulating SIRT1 expression and influencing the eNOS/NO/PKG pathway function consequently. MiR-200a belongs to the miR-200 family that is an important regulator of tumor progression and metastasis.35 Much of its predicted and identified targets had been reported, such as ZEB1 and SIP1.36 Its targets could constitute networks to participate into different pathophysiological processes via coordinated effects on many pathways, such as Rho-ROCK signaling, MMP activity, and focal adhesions, and thus could also involve in maintaining physiological homeostasis in the vascular system.37 Interestingly, one study about the function of miR-200a in regulating the epithelial to mesenchymal transition-like transformation manifested that miR-200a could regulate SIRT1.22 In this study, the researchers established the regulated relationship between the miR-200a and SIRT1 according to the results of luciferase reporter assays. And the 7-mer exact seed at the SIRT1 3’- UTR as a miR-200a target was consistent with our previous study predicted via bioinformatic analysis (Figure 4). SIRT1 is a highly conserved nicotinamide adenine dinucleotide (NAD)+-dependent protein deacetylase.17 It has been highlighted for its importance in several pathophysiological processes of a living organism, particularly in aging, and has been increasingly referenced as a longevity gene.17,18,22 SIRT1 can also act as a significant protein in maintaining erectile function in the penis. Research has pointed out that SIRT1 could regulate the endothelial homeostasis,38,39 up-regulate the eNOS activity, increase the NO production, and promote the endothelium-dependent relaxation.19,20,21 In our previous studies, we also found that the elevation of SIRT1 expression in penile tissue of diabetic ED rats could ameliorate erectile potency and probably ameliorate the endothelial function through suppressing apoptosis and oxidative stress.14 Based on a combination of these findings, we hypothesized that miR-200a might be involved in the mechanism of A-ED via regulating the expression of SIRT1, and the most probable target tissue might be endothelial cells.
Figure 4.
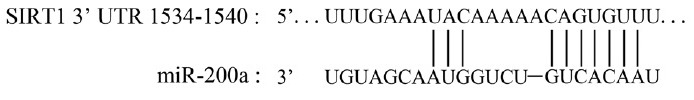
Predicted target region on the 3’UTR of SIRT1 as well as the seed sequence of miR-200a by the bioinformatic analysis (TargetScan 6.2, www. targetscan.org).
Our in vivo data showed that the expression of miR-200a was upregulated (P = 0.01) at a high level while the expression of SIRT1 and eNOS and cGMP were all downregulated (P = 0.001, 0.021, and 0.034) in the CCs from the aged rats with ED. This is consistent with our previous results that miR-200a could influence eNOS/NO/PKG pathway through regulating SIRT1, suggesting the protential role of miR-200a in the pathophysiology of A-ED. To further validate our hypothesis, we designed an in vitro study using endothelial cells isolated form rat CC. Accordingly, the expression of SIRT1 in the CECs reduced (P = 0.012) after transfection of miR-200a mimics. As the same binded site reported by other reseachers,22 we didn’t conduct the luciferase reporter assays repeatedly to identify the regulated relationship between the miR-200a and SIRT1. As two main molecules of the eNOS/NO/PKG pathway, the levels of eNOS and cGMP were both consistent with the expression of SIRT1 in different groups, which were similar to the results of many previous researches about the SIRT1 influencing the endothelial function,14,19,20,21,38,39 and could also verify that the miR-200a could regulate SIRT1, and mainly influence the eNOS/NO/PKG pathway to contribute to the pathological processes of A-ED.
The main limitation of this study was that, to avoid the degradation of the exogenous RNAs, we only conducted the experiments of transfection in vitro, which could only partially represent the situation in vivo. Thus, further experiments in vivo are needed.
CONCLUSIONS
Based on the results of our previous study, we further investigate the relationship between the miR-200a and SIRT1 in the A-ED using a rodent animal model. As a result, we verify that up-regulation of miR-200a could participate in the mechanism of A-ED, to some extent, via SIRT1 inhibition, and mainly attenuate endothelial function via influencing the eNOS/NO/PKG pathway. The results of this work could provide new perspectives for the basic researches in the study of A-ED despite lots of further in vivo experiments are still needed.
AUTHOR CONTRIBUTIONS
FP, XFQ, AXZ and YTD designed the study. FP, QPZ, and QC carried out the experiments of cytobiology and zoology. WY and YC participated in acquisition of data. FP, LJP, and YC analyzed the experimental data, and then drafted the article. CYZ, AXZ, and YTD revised the paper for intellectual content. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGMENT
This work was supported by grant from the National Natural Science Foundation of China (81170563), and funded by Key Project supported by Medical Science and Technology development Foundation, Nanjing Department of Health (YKK12096), and by Key Project supported by Science and Technology development Foundation, Nanjing Medical University (2014NJMUZD053).
REFERENCES
- 1.Corona G, Lee DM, Forti G, O’Connor DB, Maggi M, et al. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS) J Sex Med. 2010;7:1362–80. doi: 10.1111/j.1743-6109.2009.01601.x. [DOI] [PubMed] [Google Scholar]
- 2.Ponholzer A, Temml C, Mock K, Marszalek M, Obermayr R, et al. Prevalence and risk factors for erectile dysfunction in 2869 men using a validated questionnaire. Eur Urol. 2005;47:80–5. doi: 10.1016/j.eururo.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Gades NM, Jacobson DJ, McGree ME, St Sauver JL, Lieber MM, et al. Longitudinal evaluation of sexual function in a male cohort: the Olmsted county study of urinary symptoms and health status among men. J Sex Med. 2009;6:2455–66. doi: 10.1111/j.1743-6109.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 5.Shiri R, Koskimäki J, Hakama M, Häkkinen J, Tammela TL, et al. Prevalence and severity of erectile dysfunction in 50 to 75-year-old Finnish men. J Urol. 2003;170(6 Pt 1):2342–4. doi: 10.1097/01.ju.0000090963.88752.84. [DOI] [PubMed] [Google Scholar]
- 6.Marumo K, Nakashima J, Murai M. Age-related prevalence of erectile dysfunction in Japan: assessment by the International Index of Erectile Function. Int J Urol. 2001;8:53–9. doi: 10.1046/j.1442-2042.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- 7.Gott M, Hinchliff S. How important is sex in later life. The views of older people? Soc Sci Med. 2003;56:1617–28. doi: 10.1016/s0277-9536(02)00180-6. [DOI] [PubMed] [Google Scholar]
- 8.Nicolosi A, Laumann EO, Glasser DB, Moreira ED, Jr, Paik A, et al. Sexual behavior and sexual dysfunctions after age 40: the global study of sexual attitudes and behaviors. Urology. 2004;64:991–7. doi: 10.1016/j.urology.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 9.Albersen M, Orabi H, Lue TF. Evaluation and treatment of erectile dysfunction in the aging male: a mini-review. Gerontology. 2012;58:3–14. doi: 10.1159/000329598. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen LH, Chiou GY, Chen YW, Li HY, Chiou SH. MicroRNA and aging: a novel modulator in regulating the aging network. Ageing Res Rev. 2010;9(Suppl 1):S59–66. doi: 10.1016/j.arr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Tomada I, Tomada N, Almeida H, Neves D. Androgen depletion in humans leads to cavernous tissue reorganization and upregulation of Sirt1-eNOS axis. Age (Dordr) 2013;35:35–47. doi: 10.1007/s11357-011-9328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, Wan Z, Qiu XF, Chen Y, Dai YT. Resveratrol, an activator of SIRT1, restores erectile function in streptozotocin-induced diabetic rats. Asian J Androl. 2013;15:646–51. doi: 10.1038/aja.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castela Â, Soares R, Rocha F, Medeiros R, Ribeiro R, et al. Differentially expressed angiogenic genes in diabetic erectile tissue – Results from a microarray screening. Mol Genet Metab. 2012;105:255–62. doi: 10.1016/j.ymgme.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Pan F, Xu J, Zhang Q, Qiu X, Yu W, et al. Identification and characterization of the MicroRNA profile in aging rats with erectile dysfunction. J Sex Med. 2014;11:1646–56. doi: 10.1111/jsm.12500. [DOI] [PubMed] [Google Scholar]
- 17.Yamakuchi M. MicroRNA Regulation of SIRT1. Front Physiol. 2012;3:68. doi: 10.3389/fphys.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, et al. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010;2:415–31. doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 20.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuhara S, Tsujimura A, Okuda H, Yamamoto K, Takao T, et al. Vardenafil and resveratrol synergistically enhance the nitric oxide/cyclic guanosine monophosphate pathway in corpus cavernosal smooth muscle cells and its therapeutic potential for erectile dysfunction in the streptozotocin-induced diabetic rat: preliminary findings. J Sex Med. 2011;8:1061–71. doi: 10.1111/j.1743-6109.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- 22.Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, et al. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286:25992–6002. doi: 10.1074/jbc.M111.229401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan L, Xia X, Feng Y, Jiang C, Cui Y, et al. Exposure of juvenile rats to the phytoestrogen daidzein impairs erectile function in a dose-related manner in adulthood. J Androl. 2008;29:55–62. doi: 10.2164/jandrol.107.003392. [DOI] [PubMed] [Google Scholar]
- 24.Qiu X, Sun C, Yu W, Lin H, Sun Z, et al. Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J Androl. 2012;33:37–44. doi: 10.2164/jandrol.110.012666. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Zhou F, Li GY, Wang L, Li HX, et al. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci. 2013;14:10661–73. doi: 10.3390/ijms140510661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ning H, Qiu X, Baine L, Lin G, Lue TF, et al. Effects of high glucose on human cavernous endothelial cells. Urology. 2012;80:1162.e7–11. doi: 10.1016/j.urology.2012.04.071. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Chen Q, Zen K, Zhang C, Zhang Q. Synaptosomes secrete and uptake functionally active microRNAs via exocytosis and endocytosis pathways. J Neurochem. 2013;124:15–25. doi: 10.1111/jnc.12057. [DOI] [PubMed] [Google Scholar]
- 28.Sun Q, Mao S, Li H, Zen K, Zhang CY, et al. Role of miR-17 family in the negative feedback loop of bone morphogenetic protein signaling in neuron. PLoS One. 2013;8:e83067. doi: 10.1371/journal.pone.0083067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Hannan JL, Blaser MC, Oldfield L, Pang JJ, Adams SM, et al. Morphological and functional evidence for the contribution of the pudendal artery in aging-induced erectile dysfunction. J Sex Med. 2010;7:3373–84. doi: 10.1111/j.1743-6109.2010.01920.x. [DOI] [PubMed] [Google Scholar]
- 31.Lafferty-Whyte K, Cairney CJ, Jamieson NB, Oien KA, Keith WN. Pathway analysis of senescence-associated miRNA targets reveals common processes to different senescence induction mechanisms. Biochim Biophys Acta. 2009;1792:341–52. doi: 10.1016/j.bbadis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Cadavid NF, Rajfer J. Molecular pathophysiology and gene therapy of aging-related erectile dysfunction. Exp Gerontol. 2004;39:1705–12. doi: 10.1016/j.exger.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Bakircioglu ME, Sievert KD, Nunes L, Lau A, Lin CS, et al. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol. 2001;166:734–8. [PubMed] [Google Scholar]
- 34.Chen Y, Yang R, Yao L, Sun Z, Wang R, et al. Differential expression of neurotrophins in penises of streptozotocin-induced diabetic rats. J Androl. 2007;28:306–12. doi: 10.2164/jandrol.106.000794. [DOI] [PubMed] [Google Scholar]
- 35.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop – a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–7. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 37.Bracken CP, Li X, Wright JA, Lawrence DM, Pillman KA, et al. Genome-wide identification of miR-200 targets reveals a regulatory network controlling cell invasion. EMBO J. 2014;33:2040–56. doi: 10.15252/embj.201488641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, et al. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–9. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–58. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]


