Abstract
C-type natriuretic peptide (CNP) is a newly discovered type of local regulatory factor that mediates its biological effects through the specific, membrane-bound natriuretic peptide receptor-B (NPR-B). Recent studies have established that CNP is closely related to male reproductive function. The aims of this study were to determine the distribution of CNP/NPR-B in human ejaculated spermatozoa through different methods (such as immunolocalization, real time polymerase chain reaction and Western Blot), and then to evaluate the influence of CNP on sperm function in vitro, such as motility and acrosome reaction. Human semen samples were collected from consenting donors who met the criteria of the World Health Organization for normozoospermia. Our results show that the specific receptor NPR-B of CNP is localized in the acrosomal region of the head and the membrane of the front-end tail of the sperm, and there is no signal of CNP in human sperm. Compared with the control, CNP can induce a significant dose-dependent increase in spermatozoa motility and acrosome reaction. In summary, CNP/NPR-B can affect sperm motility and acrosome reaction, thus regulating the reproductive function of males. CNP may be a new key factor in regulating sperm function.
Keywords: acrosome reaction, C-type natriuretic peptide, natriuretic peptide receptor-B, sperm motility
INTRODUCTION
Male fertility requires the production of a sufficient number of normally functioning sperm that can move through the female reproductive tract and bind to and penetrate the zona pellucida of the oocyte, which subsequently forms a viable fetus. A defect in any aspect of sperm function, including sperm concentration, motility, acrosome reaction, binding and penetrating the zona pellucida, and chromatin integrity, could lead to male infertility.1,2
C-type natriuretic peptide (CNP) is a peptide 22 amino acids in length that acts as a local paracrine or autocrine regulator.3 CNP was first isolated from the porcine brain as a small protein by Sudoh et al. in 1990.4 By comparing the expression of proCNP and CNP in extracts from 32 different porcine tissues, Nielsen et al.5 found that the highest peptide concentrations were in male reproductive tissues (such as the epididymis, seminal vesicles and prostate); CNP mRNA in the seminal vesicles and epididymis was 125-fold higher than in other tissues. Moreover, Chrisman et al.6 reported that CNP concentrations in porcine seminal plasma and seminal vesicle fluid were 2000- and 100,000-fold greater than those found in the porcine brain, respectively. In addition, further studies showed that the concentration of CNP in human seminal plasma was >200-fold higher than that of blood plasma.7 The high expression level of CNP specifically in male reproductive organs indicates the importance of CNP in male reproduction.
However, the specific effect of CNP on sperm function is unclear. In this study, we detected the distribution of CNP/natriuretic peptide receptor-B (NPR-B) in human ejaculated spermatozoa and evaluated the influence of CNP on sperm function in vitro, including motility and acrosome reaction.
MATERIALS AND METHODS
Human semen and testes samples
Human semen samples were collected from consenting donors at the Hubei human sperm bank of China. All of the semen samples met the World Health Organization 1999 criterion8 for normozoospermia and were collected by masturbation after 3-5 days of abstinence. Ethical approval for human study was completed from the institutional review board. Semen samples were diluted 1:5 with Ham's F-10 medium (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged for 5 min at 800 g. The supernatant was discarded and the remaining pellet resuspended with fresh Ham's F-10 medium (wash). The washed human sperm suspension was used for analysis.
Human testes were from patients undergoing testicular biopsy in the Center for Reproductive Medicine of Tongji Medical College. After pathologic evaluation, testicular biopsy specimens showed normal spermatogenesis. The study required informed consent by patients and was permitted by the Ethics Committee.
Immunofluorescence of human sperm
Smears of human ejaculated spermatozoa were used to analyze the distribution of CNP/NPR-B by using indirect immunofluorescence, noting the presence or absence of staining on the sperm. The indirect immunofluorescence technique was performed as previously described.9 Briefly, the sperm were air-dried, fixed, permeabilized, treated with serum and probed with either anti-human CNP or NPR-B polyclonal antibodies (1:200) (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Fluorescein isothiocyanate-conjugated secondary antibodies (1:100) (Boster Biotechnology Co. Ltd., Wuhan, China) were used to detect CNP/NPR-B. For the negative control, 0.01 mol l−1 phosphate-buffered saline (PBS; pH 7.4) was used instead of the primary antibody. The immunohistochemical results were assessed using a confocal laser scanning microscope (Tokyo, Japan) equipped with a FV10-ASW 2.1 Viewer.
Real-time reverse transcription polymerase chain reaction
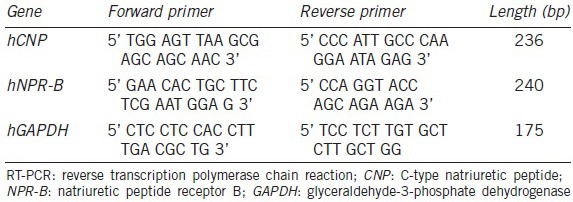
Total RNA was extracted from healthy human ejaculated spermatozoa. The experiment was repeated 3 times to estimate expression stability. Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed as previously described.10 Briefly, each sample was pipetted into 1 ml Trizol reagent. Total RNA was reverse transcribed into first-strand cDNA using a first strand cDNA Synthesis Kit (Toyobo, Tokyo, Japan). Each RT reaction mixture contained 3 μg of total RNA, 1× RT buffer, 0.5 mmol l−1 of each dNTP, 1 μg of oligo d(T)20, 400 IU Moloney murine leukemia virus reverse transcriptase, 40 IU RNasin and H2 O to a final volume of 40 μL. The reaction mixture was incubated at 42°C for 20 min, then at 99°C for 5 min. The primer pairs used in RT-PCR are listed in Table 1.
Table 1.
Real-time RT-PCR primers
Sodium dodecyl sulfate-polyacrylamide gelelectrophoresis and immunoblotting
Total protein of sperm samples and testes were obtained by using radio-immunoprecipitation assay lysis buffer according to the manufacturer's instructions. Concentration of protein was estimated by an Enhanced bicinchoninic acid assay kit. Equal amounts of protein samples were loaded to 10% sodium dodecyl sulfate-polyacrylamide gelelectrophoresis, and electrophoretic transfer of proteins to a nitrocellulose membrane was done by using a wet transfer. Immunodetection of NPR-B was carried out through incubating the membrane with NPR-B polyclonal antibody (dilution at 1:200) and developed with a secondary antibody conjugated with HRP (dilution at 1:7500). The reactive bands were detected by an enhanced chemiluminescence reagent kit according to the manufacturer's instructions.
Determination of human sperm motility
The washed human sperm suspension described above (n = 15) was divided into three aliquots. The sperm suspensions were incubated with either 10−6 or 10−7 mol l−1 CNP (Sigma-Aldrich) diluted with Ham's F-10 medium or with Ham's F-10 medium alone. Then, the sperm suspensions were incubated at 37°C in 5% CO2 incubator. The motility of sperm was analyzed at 5 min, 30 min, 60 min, 120 min, and 240 min for each group. The sperm motility was determined as the percentage of progressively motile sperm, in which over 200 sperm were counted.
Determination of human sperm acrosome status
Acrosome reaction was determined by using the Coomassie brilliant blue staining technique described by Feng et al.11 The washed human sperm suspension (n = 10) was incubated for 1 h to induce capacitation at 37°C in 5% CO2 incubator. Next, the sperm suspension was divided into three groups as described above. The acrosome reaction of sperm was analyzed at 0 h, 3 h, and 6 h for each group. Briefly, the sperm was air-dried, fixed with 4% paraformaldehyde in PBS (pH 7.4) for 10 min, and washed 3 times with PBS. Slides were stained for 20 min with aqueous 0.05% Coomassie brilliant blue R-250 (Boster Biotechnology Co. Ltd., Wuhan, China) in 3.5% perchloric acid; they were then rinsed with distilled water and covered with coverslips under neutral resin. The acrosomal status was viewed under a light microscope at ×1000 magnification. A minimum of 200 sperm cells was analyzed for the presence or absence of an acrosome reaction per slide.
Statistical analysis
The sperm motility and acrosome reaction were evaluated by one-way analysis of variance after checking for normal distributions by means of the Kolmogorov-Smirnov test. All statistical tests were two-tailed, and P < 0.05 were considered to be statistically significant. All analyses were performed using the SPSS software version 17 computer program (SPSS software, Chicago, IL, USA).
RESULTS
Protein and gene expression of CNP/natriuretic peptide receptor-B in human sperm
Under a confocal laser scanning microscope, we observed green fluorescence in the acrosomal region of the head and the membrane of the front-end tail of sperm when we detected the expression of NPR-B, but there was no signal of green fluorescence when we detected the expression of CNP (Figure 1a). The results indicated that NPR-B, the specific receptor of CNP, was localized in the acrosomal region of the head and the membrane of the front-end tail of the sperm but that CNP was not expressed in human sperm. Moreover, NPR-B protein can also be detected in human sperm and testes with a Western Blot (Figure 1b). The RT-PCR results showed that there was gene expression of NPR-B in human sperm, but not of CNP (Figure 2), which matched the confocal laser scanning microscope results described above.
Figure 1.
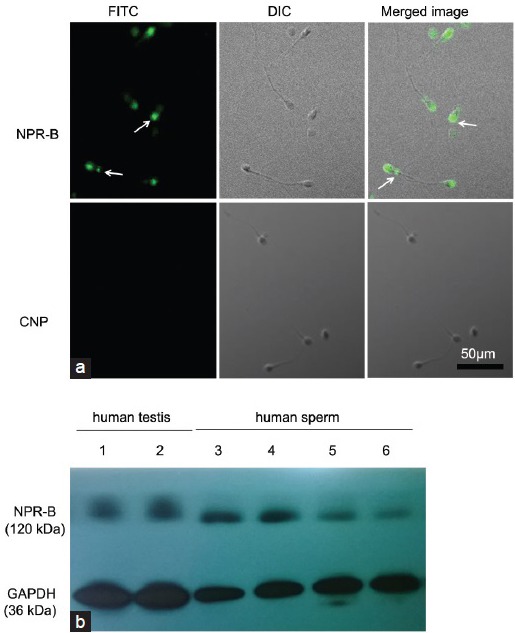
Protein location of CNP/NPR-B in human sperm. (a) Immunolocalization of CNP/NPR-B in human sperm. FITC-conjugated secondary antibodies were used for the detection of CNP/NPR-B. DIC images were acquired in parallel and served as the control for sperm morphology. The fluorescence image was merged with the DIC image to show the exact location of the fluorescence. Arrowheads indicate the location of CNP/NPR-B in human sperm. Indirect immunofluorescence ×600. (b) Western Blotting analysis of NPR-B in human testis and human sperm. CNP: C-type natriuretic peptide; NPR-B: natriuretic peptide receptor-B; FITC: fluorescein isothiocyanate; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Figure 2.
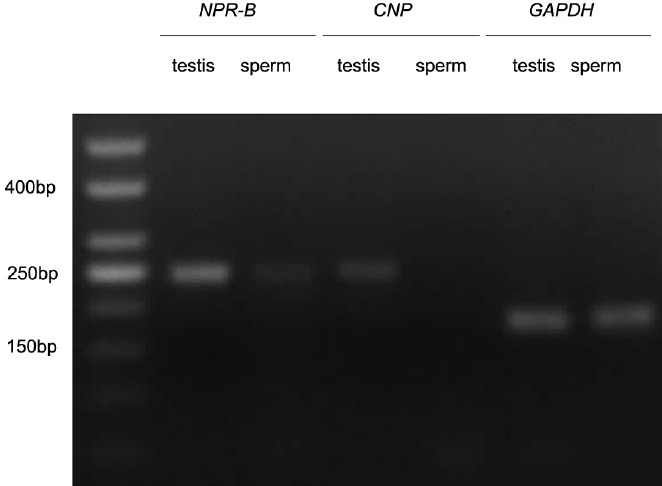
Semiquantitative RT-PCR analyses of CNP and NPR-B expression in sperm or testes coamplified with GAPDH. RT-PCR: reverse transcription polymerase chain reaction; CNP: C-type natriuretic peptide; NPR-B: natriuretic peptide receptor-B, GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
C-type natriuretic peptide can stimulate the motility of human sperm
Because the specific receptor (NPR-B) of CNP was present in the sperm, we hypothesized that CNP may be a key player in sperm function. Therefore, we assessed the motility of sperm after culture in the presence of different CNP concentrations (0, 10−7 and 10−6 mol l−1) at 5 min, 30 min, 60 min, 120 min, and 240 min. As shown in Figure 3, compared with the control, the motility of the 10−7 and 10−6 mol l−1 CNP-treated groups were higher 30 min after CNP was added to the sperm suspension. The changes were dose-dependent, as CNP 10−6 mol l−1 showed a more significant difference (P = 0.001) than did CNP 10−7 mol l−1 (P = 0.019).
Figure 3.
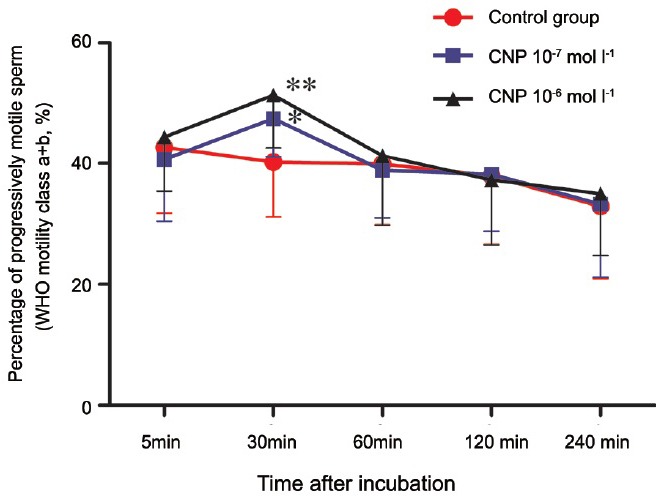
Influence of CNP on the motility of human sperm (X̄ ± standard deviation, n = 15, *P < 0.05, **P < 0.01, compared with the control group). As shown in the graph, compared with the control, percentage of progressively motile sperm (WHO motility class a + b) was increased at 30 min after CNP was added to the sperm suspension, and the changes were dose-dependent. CNP: C-type natriuretic peptide; WHO: World Health Organization.
C-type natriuretic peptide can promote the acrosome reaction of human sperm
Having established the expression of the specific receptor of CNP on the sperm surface, we hoped to demonstrate its role in the sperm acrosome reaction. Therefore, we monitored the sperm acrosome reaction using Coomassie brilliant blue staining techniques. As shown in Figure 4a, the percentage of sperm that underwent an acrosome reaction began to increase compared with the control after incubation for 3 h with different CNP concentrations (10−7 and 10−6 mol l−1), and it was markedly increased after 6 h of incubation with CNP at all concentrations. Moreover, the percentage of sperm undergoing an acrosome reaction was more significantly increased in the 10−6 mol l−1 CNP (P < 0.0001) than in the 10−7 mol l−1 CNP (P = 0.007) group, and the changes were dose-dependent. The acrosome region was stained blue in acrosome-intact sperm but remained unstained or stained light purple in acrosome-reacted sperm11 (Figure 4b).
Figure 4.
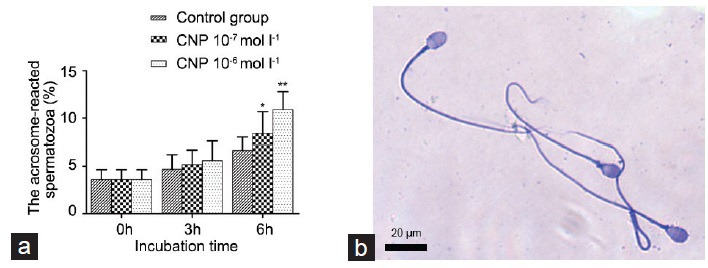
Effect of CNP on the human sperm acrosome reaction by the Coomassie brilliant blue staining method. (a) Data showing the percentage of acrosome-reacted cells at different incubation times under different concentrations of CNP (0, 10−7 and 10−6 mol l−1). (x̄ ± standard deviation, n = 10, *P < 0.05, **P < 0.01, compared with the control group). (b) The acrosome region is stained blue in the acrosome-intact sperm but unstained or stained light purple in the acrosome-reacted sperm, Original magnification ×1000. CNP: C-type natriuretic peptide.
DISCUSSION
Natriuretic peptides belong to a family of small proteins that play a major role in the modulation of natriuresis, diuresis, and vasodilatation.12 The natriuretic peptide family in mammals consists of three structurally related peptides: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and CNP. All three natriuretic peptide members exert their biological effects through two different membrane-bound natriuretic peptide receptors (NPR-A and NPR-B). ANP and BNP have been shown to bind preferentially to NPR-A, whereas CNP displays a greater affinity for NPR-B, which is considered to be the specific receptor of CNP.13 NPR-A binds natriuretic peptides at a stoichiometry of 2:1 with a rank natriuretic peptide preference of: ANP ≥ BNP >>CNP; NPR-B binds natriuretic peptides with a selectivity preference of CNP >>ANP ≥ BNP.14 CNP does not increase guanosine 3’,5’- monophosphate accumulation in cells expressing human NPR-A, the affinity of CNP for NPR-B is 50- or 500-fold higher than ANP or BNP, respectively.15
C-type natriuretic peptide has been shown to play a key role in female reproduction. NPR-B−/− female mice are sterile, which is caused by the failure to properly develop functional ovaries and uteruses.16 In rats, the expression of CNP in uterine tissue is modulated by the estrous cycle, with the highest expression during proestrus.17 An intraperitoneal infusion of estradiol increases uterine CNP in a dose-dependent manner in ovariectomized mice.18 Furthermore, CNP produced by mural granulosa cells and NPR-B produced by cumulus cells can prevent the precocious meiotic maturation of oocytes, which is critical for maturation and ovulation synchrony and normal female fertility.19 Moreover, CNP is capable of stimulating preantral and antral follicle growth; therefore, CNP treatment could provide an alternative therapy for female infertility in place of follicle-stimulating hormone.20
The key role of CNP in female reproduction has been demonstrated, but the effect of CNP on male reproduction has been reported in few studies, though there are high expression levels of CNP specifically in male reproductive organs. To determine the role of CNP/NPR-B signaling in male reproduction, Sogawa et al.21 investigated phenotype of Npr2-deficient short-limbed-dwarfism (Npr2slw/slw) mice, and found that the developmental onset and acquisition of spermatogenic function is delayed in Npr2slw/slw mutant mice. In the male reproductive system, both CNP and its receptor NPR-B are expressed in human testes, CNP is mainly expressed in Leydig cells,22 yet NPR-B is mainly in Sertoli cell.23 The occurrence of CNP, as well as its receptor, in human testes points to a local role of the peptide to regulate the function of Sertoli cells. In cultured rat Sertoli cells, CNP supplementation can stimulate the gene expression of androgen-binding protein/inhibin B/transferrin.24 Moreover, CNP secreted by rat Leydig and germ cells into the blood-testis barrier (BTB) microenvironment regulates BTB dynamics during spermatogenesis.23
In this study, we first investigated the expression of CNP/NPR-B in human ejaculated spermatozoa. The results showed that NPR-B was highly localized to the acrosomal region of the head and the membrane of the front-end tail of the sperm, but there was no CNP signal in human sperm. As we described above, CNP is a local paracrine or autocrine regulator that mediates its biological effects via the specific membrane-bound NPR-B; therefore, we hypothesized that CNP, which shows a high concentration in the seminal plasma, may affect sperm motor function either by binding to NPR-B located in the region of mitochondria or by the acrosome reaction through NPR-B localized in the head.
To prove the function of CNP/NPR-B in the sperm mentioned above, we used different concentrations of CNP to stimulate human sperm function. The concentrations of CNP chosen were referred to in an article that mentioned the effective concentrations of CNP in its studies.24 Our results showed that CNP could significantly increase the motility of sperm at 30 min and induce an acrosome reaction at 6 h. Furthermore, these effects were dose-dependent. This part of our study supported our hypothesis mentioned above. It has been reported that sperm motility and the acrosome action are the crucial parameters for fertility. CNP, which plays a role in sperm motility and the acrosome reaction, may also affect sperm fertility, but further studies are needed to confirm this hypothesis.
The mechanism of the biological effects of CNP/NPR-B may be generally associated with the intracellular accumulation of cGMP.3 cGMP is produced by different isoforms of the enzyme guanylate cyclase (GC). Natriuretic peptides (ANP and CNP) bind to and activate particulate GCs, whereas nitric oxide (NO) and carbon monoxide activate a soluble form of GC (sGC).25 The specific relevance of the cGMP system for reproductive functions has been recently demonstrated by the successful use of sildenafil (Viagra), an inhibitor of cGMP-specific phosphodiesterase type 5, for the treatment of erectile dysfunction.26 In the testes, cGMP signal transduction pathways are involved in a variety of local functions, based on autocrine or paracrine effects. In particular, cGMP has been suggested to influence the motility in spermatozoa,27 testosterone syntheses in Leydig cells,28 development of testicular germ cells,29 dilatation of testicular blood vessels30 and so on. Miraglia et al.31 reported that NO exerted a significant enhancing effect on progressive motility of human spermatozoa, which is medicated by activation of the sGC/cGMP pathway.
CONCLUSION
Our work has investigated the expression of CNP/NPR-B in human ejaculated spermatozoa and revealed that CNP/NPR-B can affect sperm motility and acrosome action, which are closely related to the reproductive function of males. The function of CNP/NPR-B is usually mediated by cGMP, but this pathway in spermatozoa needs to be proved. Our future studies will focus on the signal conduction of CNP/NPR-B in sperm.
AUTHOR CONTRIBUTIONS
HX performed most parts of experiments, analyzed data and wrote the manuscript. YC finished the experiments and analyzed the data with HX. HZ and KJW helped with some of the experiments, such as immunofluorescence of human sperm, data acquisition and revision of the manuscript. CLX helped with experimental design and revision of the manuscript. DHH experimental design, directed and performed experiments, wrote and revised the manuscript, and provided funding.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by a grant from the Doctoral Programs Foundation of the Ministry of Education of China (No. 20090142120040) and the Independent Innovation Foundation of the Medical College of Hua Zhong University of Science and Technology (No. 2011QN253 and No. 0118519013). We are grateful to the Hubei human sperm bank of China for kindly providing the semen samples for this study.
REFERENCES
- 1.Lu N, Sargent KM, Clopton DT, Pohlmeier WE, Brauer VM, et al. Loss of vascular endothelial growth factor A (VEGFA) isoforms in the testes of male mice causes subfertility, reduces sperm numbers, and alters expression of genes that regulate undifferentiated spermatogonia. Endocrinology. 2013;154:4790–802. doi: 10.1210/en.2013-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramonda R, Foresta C, Ortolan A, Bertoldo A, Oliviero F, et al. Influence of tumor necrosis factor a inhibitors on testicular function and semen in spondyloarthritis patients. Fertil Steril. 2014;101:359–65. doi: 10.1016/j.fertnstert.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 3.Xia C, Nguyen M, Garrison AK, Zhao Z, Wang Z, et al. CNP/cGMP signaling regulates axon branching and growth by modulating microtubule polymerization. Dev Neurobiol. 2013;73:673–87. doi: 10.1002/dneu.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–70. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen SJ, Gøtze JP, Jensen HL, Rehfeld JF. ProCNP and CNP are expressed primarily in male genital organs. Regul Pept. 2008;146:204–12. doi: 10.1016/j.regpep.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Chrisman TD, Schulz S, Potter LR, Garbers DL. Seminal plasma factors that cause large elevations in cellular cyclic GMP are C-type natriuretic peptides. J Biol Chem. 1993;268:3698–703. [PubMed] [Google Scholar]
- 7.Nielsen SJ, Rehfeld JF, Pedersen F, Kastrup J, Videbaek R, et al. Measurement of pro-C-type natriuretic peptide in plasma. Clin Chem. 2005;51:2173–6. doi: 10.1373/clinchem.2005.053488. [DOI] [PubMed] [Google Scholar]
- 8.Vol. 4. New York: Cambridge University Press; 1999. WHO. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interactions. [Google Scholar]
- 9.Zhang YQ, He XZ, Zhang JS, Wang RA, Zhou J, et al. Stage-specific localization of transforming growth factor beta1 and beta3 and their receptors during spermatogenesis in men. Asian J Androl. 2004;6:105–9. [PubMed] [Google Scholar]
- 10.Huang DH, Zhao H, Tian YH, Li HG, Ding XF, et al. Gene expression changes of urokinase plasminogen activator and urokinase receptor in rat testes at postnatal stages. Asian J Androl. 2007;9:679–83. doi: 10.1111/j.1745-7262.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 11.Feng HL, Han YB, Hershlag A, Zheng LJ. Impact of Ca2+ flux inhibitors on acrosome reaction of hamster spermatozoa. J Androl. 2007;28:561–4. doi: 10.2164/jandrol.106.001958. [DOI] [PubMed] [Google Scholar]
- 12.Volpe M, Rubattu S, Burnett J., Jr Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–25. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikimi T, Kuwahara K, Nakao K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J Cardiol. 2011;57:131–40. doi: 10.1016/j.jjcc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–66. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, et al. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP) Science. 1991;252:120–3. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 16.Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, et al. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci U S A. 2004;101:17300–5. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walther T, Stepan H. C-type natriuretic peptide in reproduction, pregnancy and fetal development. J Endocrinol. 2004;180:17–22. doi: 10.1677/joe.0.1800017. [DOI] [PubMed] [Google Scholar]
- 18.Acuff CG, Huang H, Steinhelper ME. Estradiol induces C-type natriuretic peptide gene expression in mouse uterus. Am J Physiol. 1997;273:H2672–7. doi: 10.1152/ajpheart.1997.273.6.H2672. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–9. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamura K, Cheng Y, Kawamura N, Takae S, Okada A, et al. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod. 2011;26:3094–101. doi: 10.1093/humrep/der282. [DOI] [PubMed] [Google Scholar]
- 21.Sogawa C, Fujiwara Y, Tsukamoto S, Ishida Y, Yoshii Y, et al. Mutant phenotype analysis suggests potential roles for C-type natriuretic peptide receptor (NPR-B) in male mouse fertility. Reprod Biol Endocrinol. 2014;12:64. doi: 10.1186/1477-7827-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collin O, Lissbrant E, Bergh A. Atrial natriuretic peptide, brain natriuretic peptide and c-type natriuretic peptide: effects on testicular microcirculation and immunohistochemical localization. Int J Androl. 1997;20:55–60. doi: 10.1046/j.1365-2605.1997.00108.x. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Mruk DD, Cheng CY. C-type natriuretic peptide regulates blood-testis barrier dynamics in adult rat testes. Proc Natl Acad Sci U S A. 2007;104:3841–6. doi: 10.1073/pnas.0610100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang DH, Zhang SW, Zhao H, Zhang L. The role of C-type natriuretic peptide in rat testes during spermatogenesis. Asian J Androl. 2011;13:275–80. doi: 10.1038/aja.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middendorff R, Davidoff MS, Behrends S, Mewe M, Miethens A, et al. Multiple roles of the messenger molecule cGMP in testicular function. Andrologia. 2000;32:55–9. [PubMed] [Google Scholar]
- 26.Carvalheira A, Forjaz V, Pereira NM. Adherence to phosphodiesterase type 5 inhibitors in the treatment of erectile dysfunction in long-term users: how do men use the inhibitors? Sex Med. 2014;2:96–102. doi: 10.1002/sm2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derkach KV, Shpakov AO, Griaznov AIu. Functional activity of adenylyl and guanylyl cyclases in human spermatozoa with different motility. Tsitologiia. 2013;55:123–30. [PubMed] [Google Scholar]
- 28.Stojkov NJ, Janjic MM, Kostic TS, Andric SA. Orally applied doxazosin disturbed testosterone homeostasis and changed the transcriptional profile of steroidogenic machinery, cAMP/cGMP signalling and adrenergic receptors in Leydig cells of adult rats. Andrology. 2013;1:332–47. doi: 10.1111/j.2047-2927.2012.00035.x. [DOI] [PubMed] [Google Scholar]
- 29.Shi F, Perez E, Wang T, Peitz B, Lapolt PS. Stage- and cell-specific expression of soluble guanylyl cyclase alpha and beta subunits, cGMP-dependent protein kinase I alpha and beta, and cyclic nucleotide-gated channel subunit 1 in the rat testis. J Androl. 2005;26:258–63. doi: 10.1002/j.1939-4640.2005.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 30.Welter H, Kampfer C, Lauf S, Feil R, Schwarzer JU, et al. Partial loss of contractile marker proteins in human testicular peritubular cells in infertility patients. Andrology. 2013;1:318–24. doi: 10.1111/j.2047-2927.2012.00030.x. [DOI] [PubMed] [Google Scholar]
- 31.Miraglia E, De Angelis F, Gazzano E, Hassanpour H, Bertagna A, et al. Nitric oxide stimulates human sperm motility via activation of the cyclic GMP/protein kinase G signaling pathway. Reproduction. 2011;141:47–54. doi: 10.1530/REP-10-0151. [DOI] [PubMed] [Google Scholar]


