Abstract
Testicular cancer (TC) is the most common solid cancer in men between the third and fourth decade of life. Due to successful treatment approaches, TC survivors (TCSs) have long life expectancy, but with numerous potential long-term sequelae, including sexual dysfunction. We investigated predictors of long-term normal sexual function (SF) recovery in TCSs. Sociodemographic, medical, and psychometric data were analyzed in 143 Caucasian-European TCSs, who underwent orchiectomy at a single institution. Health-significant comorbidities were scored with the Charlson Comorbidity Index (CCI). Patients completed the International Index of Erectile Function (IIEF). Statistical models tested the association between predictors (including age at surgery, body mass index, CCI, and adjuvant therapy: radiotherapy [RT], chemotherapy [CT], CT followed by retroperitoneal lymph node dissection [RPLND] and RPLND alone) and the long-term recovery of normal SF (defined as IIEF-erectile function [EF] ≥26, and sexual desire [SD], intercourse satisfaction [IS] orgasmic function [OF], and overall satisfaction [OS] domain scores in the upper tertiles). At a mean follow-up of 86 months, 35 (25.5%) TCSs had erectile dysfunction (ED), with 16 (11.2%) experiencing severe ED. Median time of EF recovery was 60, 60, and 70 months after CT, RT, and RPLND, respectively. Only adjuvant RT emerged as an independent predictor of nonrecovery of normal EF (HR: 0.55, P= 0.01). Neither adjuvant CT nor CT plus RPLND or RPLND alone significantly impaired the recovery of normal erections. Adjuvant therapy was not associated with impaired recovery of normal sexuality as a whole, considering the IIEF-SD, -OF, -IS, and OS domains.
Keywords: chemotherapy, erectile dysfunction, radiotherapy, retroperitoneal lymph node dissection, survivors, testicular cancer
INTRODUCTION
Testicular cancer (TC) is the most common solid cancer in men between the third and fourth decade of life.1 Since successful treatment approaches have resulted in an overall 10 years disease-specific survival >95%,2,3 and because of their young age at diagnosis, TC survivors (TCSs) are expected to have a long life expectancy, but also a number of potential long-term sequelae of treatments.4 Among these, it is certainly relevant to emphasize the importance of cardiovascular disease, nephrotoxicity, neurotoxicity and a variable impairment of the reproductive health, including both aspects of reproductive capacity as well as sexual health and quality-of-life as a whole – namely, chronic fatigue, hypogonadism, infertility, and sexual dysfunction.4,5 Carefully analyzing this picture, it is clear how each of these latter possible long-term treatment-related consequences may act as an etiological factor for the onset of sexual dysfunction, foremost, erectile dysfunction (ED).4,5,6,7,8 Though the findings are not unique, a number of studies have explored rates of sexual function (SF) impairment and primarily the influence of different treatment modalities on SF in TCSs.4,5,8,9 In this context, age at treatment and treatment modality (i.e., chemotherapy [CT] vs radiotherapy [RT] vs CT supplemented with retroperitoneal lymph node dissection [RPLND] or RPLND alone) have been reported to differently predispose TCSs to sexual dysfunction (namely, low sexual desire [SD], ED, ejaculatory disorders, symptoms of testosterone deficiency), and with differing severity.4,5,7,9,10 Nevertheless, together with the negative psychological impact of having cancer – namely, psychological distress8,11 and the possible sequelae of treatment, even orchiectomy per se may be associated with a change in self-perceived body image and a reduced self-perception of masculinity, with a likely further involvement of reproductive health as a whole.4,5,12,13 All this often occurs in people in the prime of their exuberant sexual and reproductive life, and in the midst of their perception of their own masculinity.
These observations prompted us to assess the predictors of long-term normal SF recovery in a cohort of Caucasian-European TCSs seen at a single academic department who had received either RT, or CT, or CT followed by RPLND.
MATERIALS AND METHODS
Population
The analyses were based on a cohort of 448 consecutive Caucasian-European patients who underwent either uni- or bilateral orchiectomy for testicular germ-cell cancer at a single tertiary academic center from December 1986 to October 2011. In early February 2012, all patients, who were alive, were invited to participate in a questionnaire survey.
A total of 305 men were excluded because they lacked one or more of the entry criteria: deceased patients (n = 6; 1.3%); contact details not otherwise recoverable (n = 35; 7.8%); missing or imprecise data about SF before cancer diagnosis (n = 57; 12.7%); incomplete sociodemographic information (n = 5; 1.1%); missing detailed medical history (n = 32; 7.1%); missing detailed postorchiectomy treatment data (n = 7; 1.6%); incomplete psychometric information at long-term follow-up (FU) (any reason) (n = 112; 25%); or refusal to participate in the survey (n = 46; 10.3%). A sample of 143 patients (31.9%) was included in the analysis.
The study was approved by IRCCS Ospedale San Raffaele's Ethics Committee, and all patients signed an informed consent agreeing to provide their own anonymous information for future studies.
Patients were invited to complete a self-administered questionnaire, sent by e-mail, which included sections assessing sociodemographic variables, detailed medical history, and long-term SF.
Sociodemographic variables included: current age and age at diagnosis, educational level – defined as low educational level group, which included patients with an elementary or secondary school education, a high school degree group, and a university/postgraduate degree – relationship status - defined as “stable sexual relationship” if the patients had the same partner for 12 or more consecutive months; otherwise “no stable relationship” or widowhood. Based on the reported height and weight, calculated body mass index (BMI), defined as weight in kilograms by height in square meters, was considered for each patient.
Along with a comprehensive revision of medical records to collect pathological data (namely, seminoma vs nonseminoma), patients were assessed with a thorough medical history, including data on health-significant comorbidities as scored with the Charlson Comorbidity Index (CCI).14 We used the International Classification of Diseases, 9th revision, because its coding algorithms were used to define the 17 comorbidities that constitute the most widely used CCI score. For the specific purpose of the analysis, CCI was categorized as 0, 1, or 2 or higher. Likewise, responders were classified into three groups according to the treatment received after diagnosis; RT, consisting patients who had undergone orchiectomy followed by subdiaphragmatic RT to a para-aortic (PA) field or to a hockeystick field (PA and ipsilateral iliac nodes), with moderate doses (total 20–24 Gy); CT, thus including patients who had received CT (any combination) at some stage of their treatment after orchiectomy; or CT followed by RPLND, consisting of men who had undergone orchiectomy followed by CT and RPLND consisting in a right-sided modified template resection including the precaval, paracaval, retrocaval, interaortocaval regions, and the area lateral to the common iliac vessels or in a left-sided modified template resection including the preaortic artery up to the inferior mesenteric artery, with the PA and retroaortic areas and the area lateral to the common iliac vessels, or radical template resection in all cases with contralateral spread, including the dissections fields of the right and the left-modified resection.
Main outcome measures
The sexual health assessment consisted of a brief semi-structured questionnaire including specific questions to recall SF before cancer diagnosis. Moreover, to provide a frame of reference for objectively interpreting SF over the long-term FU, patients were invited to complete a real-time (=targeting the 4 weeks prior to survey) International Index of Erectile Function (IIEF), the most widely used, internationally-validated 15-item psychometric tool assessing male SF through its 5 domains. To interpret ED severity, we used the IIEF-erectile function (EF) domain classification as proposed by Cappelleri et al.15 Conversely, normality for the other IIEF domains (namely, SD [IIEF-SD], intercourse satisfaction [IIEF-IS], orgasmic function [IIEF-OF], and overall satisfaction [IIEF-OS]) was arbitrarily defined for values in their upper tertiles.
Statistical analysis
Data are presented as means (median; range). The statistical significance of differences in means and proportions was tested with the one-way analysis of variance and the χ2 trend test, respectively. Exploratory analyses were initially applied to all variables in a preliminary analysis, and variables were then kept where appropriate as significant to the results. Logistic cox regression models tested the association between predictors (e.g. age at surgery; BMI; CCI; and, treatment received – i.e., RT vs CT vs CT and RPLND or RPLND alone) and the long-term recovery of normal SF. Age at orchiectomy was included in the analyses as segregated into age quartiles (first quartile: ages ≤34 years; second quartile: ages 35–42 years; third quartile: ages 43–50 years; fourth quartile: ages ≥51 years). The CCI was included in the model as a continuous variable.
Statistical tests were performed using SPSS version 19 (IBM Corp., Armonk, NY, USA). All tests were two-sided, with a significance level set at 0.05.
RESULTS
Sexual functioning outcomes were assessed in 143 TCSs at an 86 months mean (71 months median) FU (range 3–348 months).
Table 1 lists the characteristics and descriptive statistics of the entire cohort of patients. Table 2 reports patients’ psychometric characteristics and descriptive statistics at long-term FU. In total, 35 (24.5%) individuals suffered from ED (Table 2). Rates of arbitrarily defined normal IIEF-SD, IIEF-OF, IIEF-IS, and IIEF-OS were 59.4%, 65.7%, 62.2%, and 64.3%, respectively.
Table 1.
Patients characteristics and descriptive statistics (n=143)
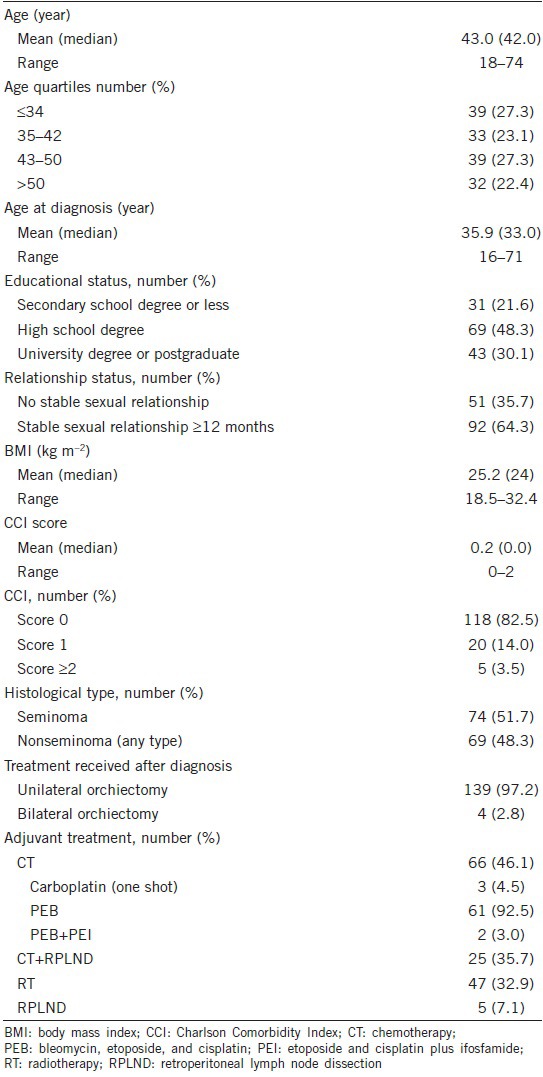
Table 2.
Patients psychometric characteristics at long-term follow-up
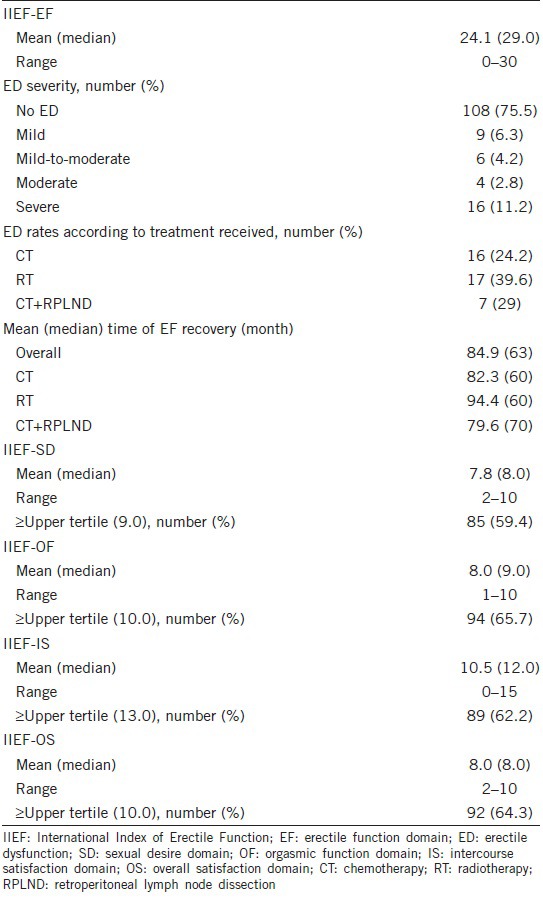
Table 3 details cox regression models predicting normal SF at long-term FU. According to the univariable analysis, normal long-term IIEF-EF was inversely associated with CCI, BMI, and adjuvant RT (all P ≤ 0.04). Conversely, age at the orchiectomy, adjuvant CT, and RPLND did not achieve the univariable predictor status. Figure 1 depicts log-rank (Mantel-Cox) long-term recovery of normal IIEF-EF scores according to adjuvant RT, adjuvant CT, and CT coupled with RPLND or RPLND alone, respectively.
Table 3.
Cox regression models predicting normal sexual function recovery at long-term follow-up (HR; P value; CI 95%)
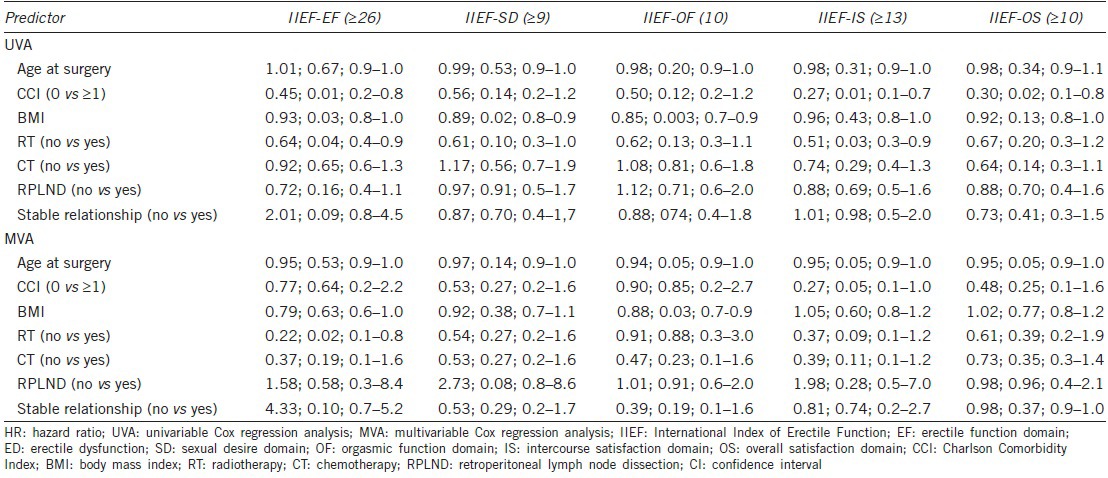
Figure 1.
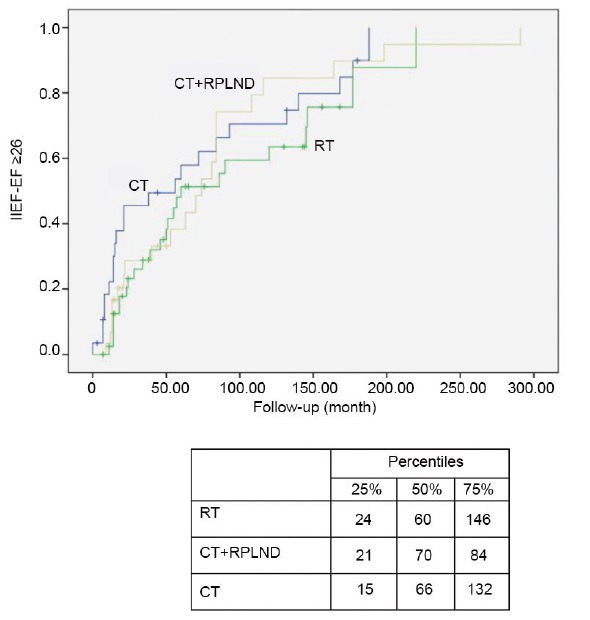
Log Rank (Mantel-Cox) normal IIEF-EF recovery (i.e., IIEF-EF ≥ 26) according to adjuvant treatment modalities. IIEF: International Index of Erectile Function; EF: erectile function domain.
At univariable analysis, BMI was negatively associated with both normal IIEF-SD and IIEF-OF domains (all P ≤ 0.02), whereas no further associations were found with the other variables. Likewise, a significant inverse association was observed between IIEF-IS domain and CCI and adjuvant RT (all P ≤ 0.03), and between IIEF-OS domain and CCI (P = 0.02). No further univariable associations were found.
The multivariable analyses showed that adjuvant RT was found to be independently associated with worse long-term IIEF-EF (P = 0.01) (Table 3). Conversely, none of the other predictors were significantly associated with recovery of normal IIEF-EF. Similarly, none of the analyzed variables emerged as independent predictors for the other IIEF domains, except for BMI, which was significantly correlated with the recovery of a long-term normal value of IIEF-OF (Table 3).
DISCUSSION
A number of studies have examined rates of SF impairment of TCSs who had received adjuvant treatments, with no unambiguous evidence.4,5,6,16,17,18,19 We retrospectively tested potential predictors of impairment of recovery of long-term normal SF values in a cohort of Caucasian-European TCSs, who had received either adjuvant RT, or CT, or CT followed by RPLND, or RPLND alone. Our exploratory analyses demonstrated that at a median FU of 71 months almost one out of four TCSs suffered from ED, with more than 10% of individuals with scores suggestive for severe ED. In this context, adjuvant RT emerged as the only independent predictor of nonrecovery of normal EF. Conversely, and in contrast with previous observations,10 neither adjuvant CT nor CT followed by RPLND or RPLND alone had a significant impact on the recovery of normal EF. Moreover, none of the adjuvant therapies after orchiectomy were found to be independently associated with impaired recovery of normal sexuality as a whole, considering the IIEF-SD, -OF, -IS, and -OS domains.
One strength is that the current study represents a single institute survey with a cohort of homogeneous, same-race patients for whom a long-term retrospective psychometric assessment was performed using a validated tool and a consistent method. Moreover, a point of originality was the fact that we did not assess the prevalence of sexual disorders, but the rate of failure to return to normality, which should be considered the true goal when taking into account relatively young individuals in terms of sexual health. In this sense, about 75% of TCSs in our cohort had normal EF when assessed at long-term FU. Likewise - and though the evaluation was done using an arbitrary cut-off for the various IIEF domains - normal SD was recovered by almost 60% of individuals, while 66% and about 62% men showed recovery of an adequate OF and a normal IS, respectively. Finally, just over 64% of TCSs reported to be adequately satisfied with the overall SF at the same long-term FU assessment.
This is most likely the evaluation of sexual health with the longest FU ever published in TCSs. In this context, longitudinally investigating SF in a cohort of TCSs throughout the first year after orchiectomy, Tuinman et al.20 observed significant time effects on EF, OF, IS, and OS, with a nonsignificant trend in overall improvement. Interestingly, TCSs experienced changes in all aspects of SF, except in the desire, which maintained an adequate level over time.20
It has been correctly observed that after TC patients may note a significant change in their bodies, which may subsequently lead to poorer self-esteem, sexual dysfunction, and psychological distress.4,5,12,13,17,19,21,22,23,24,25,26 Since TC per se is a disease that may directly affect individual sense of masculinity, it is easy to understand how in TCSs even the least invasive procedure – namely, surveillance, may lead to some biologically-linked sequelae, such as ejaculatory dysfunction and ED.8,17,18,19 Conversely, issues like low sexual interest are certainly mostly deemed as psychologically-based in TCSs.19,22,24 Overall, however, knowledge regarding SF outcome in TCSs is uncertain and not unique. Using data from a case-control study among US military servicemen, Kim et al.19 provided evidence that TCSs are more likely to have impaired SF than demographically matched healthy controls, with poorer quality erection and ejaculation. Conversely, there were no significant differences in sex drive and overall SF between cases and controls.19 Eberhard et al.6 showed that 3–5 years after cancer treatment TCSs were likely to report both manifest low SD (4%) and ED (20%) significantly more often than age-matched controls. Worryingly, low SD and ED co-occurred in 25% of the patients.6 Furthermore, in a long-term FU study of TCSs in Denmark, Rossen et al.5 reported a prevalence of perceived ejaculatory dysfunction of 7% and an ED prevalence of 18%, which was higher than in the general population. The authors concluded that although the explanation of ED was unknown, it could certainly be rooted in the psychological changes subsequent to the diagnosis and treatment of TC. It was also observed that roughly 17% of TCSs reported a negative change in body image after TC diagnosis and its treatment and that this finding was significantly associated with several parameters of sexual dysfunction, precisely including ED.5 Interestingly enough, type of treatment was not associated with SF (therefore including EF), except for ejaculatory disorders which more frequently resulted after RPLND.
Therefore, a first key point is the fact that the literature seems to underline that the psychological factor - thus including the feeling of loss of masculinity, together with anxiety and depression resulting from cancer diagnosis and therapy - may play a fundamental role in the determination of possible sexual sequelae in TCSs. Dahl et al.27 exploring SF in a large unselected sample of Norwegian TCSs by comparing the results with population data, observed that overall sexual complaints were independently associated with increasing age, lack of partner, and a higher anxiety score. Conversely, ejaculatory problems only showed a trend for CT and neurotoxic side effects. Similarly, Tuinman et al.20 confirmed that single TCSs complained of more sexual problems than patients in a stable relationship.
However, a responsible role of adjuvant treatment in promoting the onset, and the possible persistence of SF sequelae was noted on many occasions. Kim et al.19 for instance, showed that CT combined with surgery was more likely to result in impairment of libido and ejaculation, whereas a combination of RT and surgery was more frequently associated with ED. Similar findings were also reported in a meta-analysis by Jonker-Pool et al.17 ejaculatory dysfunction was most frequently reported after RPLND, while ED was mainly related to irradiation. These latter observations seem to confirm our findings. Conversely, Incrocci et al.22 reported that in patients who received infradiaphragmatic irradiation for stage I–II testicular seminoma, erectile difficulties and satisfaction with sexual life did not significantly differ from age-matched healthy controls; still, roughly 20% of TCSs in that cohort reported less interest and pleasure in sex, and less sexual activity as a whole, at a mean of 51 months after treatment. Moreover, up to 17% TCSs kept complaining of erectile difficulties at a considerable distance after RT, occurrences which the authors correlated with patients’ age. It is undoubtedly true that as many as 32% of patients who received RT reported that cancer treatment had negatively influenced their sexual life.22 Our impression seems to confirm previous observations of Tinkler et al.28 showing significantly reduced libido, lower intensity of orgasm, and difficulties in maintaining erection in seminoma patients treated with RT, as compared with a control group. As expected, age emerged as a factor of significant importance to SF, since it worsened the side effects of treatment.
Our study is not devoid of limitations. First, since only 32% of the patients we approached completed the survey – which was mostly a consequence of either a large number of incomplete psychometric information or of refusal to participate in the survey – a significant sampling bias may exist, therefore the results presented may not represent the long-term SF of patients overall. Second, the lack of data regarding the premorbid level of sexual dysfunction make it difficult to precisely detail the level of sexual dysfunction which could be associated with the TC diagnosis and treatments. Indeed, it has been observed that retrospective studies may report more sexual dysfunction as compared to prospective studies.17 Third, the analysis lacks a group of men who had been only subjected to postorchiectomy surveillance, whereas the simple diagnosis of TC per se was demonstrated to have a negative impact on SF.17,18,19 Fourth, we lacked valid tools dedicated to the assessment of psychological distress26,29 and self-body image.4,5,11,12 Finally, the retrospective nature of this psychometric survey did not allow for the collection of data on circulating testosterone levels in TCSs.18,30
Our data suggest that roughly one out of four TCSs suffered from ED at long-term FU; of these, more than 10% had scores suggestive of severe ED. Adjuvant RT was found to be independently associated with long-term nonrecovery of normal erectile functioning, after adjusting for potential confounding variables. Conversely, neither adjuvant CT nor CT followed by RPLND or RPLND alone demonstrated a significant impact on the recovery of normal erections. Moreover, none of the adjuvant therapies after orchiectomy were found to be independently associated with an impaired recovery of a normal sexuality as a whole, considering SD, OF, IS domains, as well as the sexual satisfaction. Further prospective studies are certainly needed to more comprehensively understand the complex interaction between biological and psychological facets of long-term SF in TCSs.
AUTHOR CONTRIBUTIONS
PC collected the data and drafted the manuscript; LB, MF, and GLC collected and manage the data; EV, UC, and AB analyzed and interpreted the data and performed the statistical analysis; RD and FM were responsible for the critical revision of the manuscript for important intellectual contents; AS was responsible for the study concept and design and drafted the manuscript.
COMPETING INTEREST
All authors declare no competing financial interests.
ACKNOWLEDGMENTS
The authors thank Ms. Chiara Molteni for reviewing the language in this manuscript.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62:283–98. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 2.Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–56. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 3.Fosså SD, Cvancarova M, Chen L, Allan AL, Oldenburg J, et al. Adverse prognostic factors for testicular cancer-specific survival: a population-based study of 27,948 patients. J Clin Oncol. 2011;29:963–70. doi: 10.1200/JCO.2010.32.3204. [DOI] [PubMed] [Google Scholar]
- 4.Abouassaly R, Fossa SD, Giwercman A, Kollmannsberger C, Motzer RJ, et al. Sequelae of treatment in long-term survivors of testis cancer. Eur Urol. 2011;60:516–26. doi: 10.1016/j.eururo.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Rossen P, Pedersen AF, Zachariae R, von der Maase H. Sexuality and body image in long-term survivors of testicular cancer. Eur J Cancer. 2012;48:571–8. doi: 10.1016/j.ejca.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Eberhard J, Ståhl O, Cohn-Cedermark G, Cavallin-Ståhl E, Giwercman Y, et al. Sexual function in men treated for testicular cancer. J Sex Med. 2009;6:1979–89. doi: 10.1111/j.1743-6109.2009.01298.x. [DOI] [PubMed] [Google Scholar]
- 7.Bumbasirevic U, Bojanic N, Pekmezovic T, Janjic A, Janicic A, et al. Health-related quality of life, depression, and sexual function in testicular cancer survivors in a developing country: a Serbian experience. Support Care Cancer. 2013;21:757–63. doi: 10.1007/s00520-012-1577-6. [DOI] [PubMed] [Google Scholar]
- 8.Tal R, Stember DS, Logmanieh N, Narus J, Mulhall JP. Erectile dysfunction in men treated for testicular cancer. BJU Int. 2014;113:907–10. doi: 10.1111/bju.12331. [DOI] [PubMed] [Google Scholar]
- 9.Glendenning JL, Barbachano Y, Norman AR, Dearnaley DP, Horwich A, et al. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer. 2010;116:2322–31. doi: 10.1002/cncr.24981. [DOI] [PubMed] [Google Scholar]
- 10.Tasdemir C, Firdolas F, Harputluoglu H, Altintas R, Gunes A. Erectile dysfunction in testicular cancer patients treated with chemotherapy. Andrologia. 2012;44:226–9. doi: 10.1111/j.1439-0272.2011.01271.x. [DOI] [PubMed] [Google Scholar]
- 11.Nazareth I, Lewin J, King M. Sexual dysfunction after treatment for testicular cancer: a systematic review. J Psychosom Res. 2001;51:735–43. doi: 10.1016/s0022-3999(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 12.Fleer J, Sleijfer D, Hoekstra H, Tuinman M, Klip E, et al. Objective and subjective predictors of cancer-related stress symptoms in testicular cancer survivors. Patient Educ Couns. 2006;64:142–50. doi: 10.1016/j.pec.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Pühse G, Wachsmuth JU, Kemper S, Husstedt IW, Evers S, et al. Chronic pain has a negative impact on sexuality in testis cancer survivors. J Androl. 2012;33:886–93. doi: 10.2164/jandrol.110.012500. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54:346–51. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 16.Arai Y, Kawakita M, Okada Y, Yoshida O. Sexuality and fertility in long-term survivors of testicular cancer. J Clin Oncol. 1997;15:1444–8. doi: 10.1200/JCO.1997.15.4.1444. [DOI] [PubMed] [Google Scholar]
- 17.Jonker-Pool G, Van de Wiel HB, Hoekstra HJ, Sleijfer DT, Van Driel MF, et al. Sexual functioning after treatment for testicular cancer - review and meta-analysis of 36 empirical studies between 1975-2000. Arch Sex Behav. 2001;30:55–74. doi: 10.1023/a:1026468707362. [DOI] [PubMed] [Google Scholar]
- 18.Pühse G, Secker A, Kemper S, Hertle L, Kliesch S. Testosterone deficiency in testicular germ-cell cancer patients is not influenced by oncological treatment. Int J Androl. 2011;34:e351–7. doi: 10.1111/j.1365-2605.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim C, McGlynn KA, McCorkle R, Li Y, Erickson RL, et al. Sexual functioning among testicular cancer survivors: a case-control study in the U.S. J Psychosom Res. 2012;73:68–73. doi: 10.1016/j.jpsychores.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuinman MA, Hoekstra HJ, Vidrine DJ, Gritz ER, Sleijfer DT, et al. Sexual function, depressive symptoms and marital status in nonseminoma testicular cancer patients: a longitudinal study. Psychooncology. 2010;19:238–47. doi: 10.1002/pon.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Incrocci L, Bosch JL, Slob AK. Testicular prostheses: body image and sexual functioning. BJU Int. 1999;84:1043–5. doi: 10.1046/j.1464-410x.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 22.Incrocci L, Hop WC, Wijnmaalen A, Slob AK. Treatment outcome, body image, and sexual functioning after orchiectomy and radiotherapy for Stage I-II testicular seminoma. Int J Radiat Oncol Biol Phys. 2002;53:1165–73. doi: 10.1016/s0360-3016(02)02849-3. [DOI] [PubMed] [Google Scholar]
- 23.Heidenreich A, Hofmann R. Quality-of-life issues in the treatment of testicular cancer. World J Urol. 1999;17:230–8. doi: 10.1007/s003450050138. [DOI] [PubMed] [Google Scholar]
- 24.Joly F, Héron JF, Kalusinski L, Bottet P, Brune D, et al. Quality of life in long-term survivors of testicular cancer: a population-based case-control study. J Clin Oncol. 2002;20:73–80. doi: 10.1200/JCO.2002.20.1.73. [DOI] [PubMed] [Google Scholar]
- 25.Fegg MJ, Gerl A, Vollmer TC, Gruber U, Jost C, et al. Subjective quality of life and sexual functioning after germ-cell tumour therapy. Br J Cancer. 2003;89:2202–6. doi: 10.1038/sj.bjc.6601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siafaka V, Hyphantis TN, Alamanos I, Fountzilas G, Skarlos D, et al. Personality factors associated with psychological distress in testicular cancer survivors. J Pers Assess. 2008;90:348–55. doi: 10.1080/00223890802107958. [DOI] [PubMed] [Google Scholar]
- 27.Dahl AA, Bremnes R, Dahl O, Klepp O, Wist E, et al. Is the sexual function compromised in long-term testicular cancer survivors? Eur Urol. 2007;52:1438–47. doi: 10.1016/j.eururo.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 28.Tinkler SD, Howard GC, Kerr GR. Sexual morbidity following radiotherapy for germ cell tumours of the testis. Radiother Oncol. 1992;25:207–12. doi: 10.1016/0167-8140(92)90270-5. [DOI] [PubMed] [Google Scholar]
- 29.Skaali T, Fosså SD, Andersson S, Langberg CW, Lehne G, et al. Is psychological distress in men recently diagnosed with testicular cancer associated with their neuropsychological test performance? Psychooncology. 2011;20:369–77. doi: 10.1002/pon.1737. [DOI] [PubMed] [Google Scholar]
- 30.Lackner JE, Märk I, Schatzl G, Marberger M, Kratzik C. Hypogonadism and androgen deficiency symptoms in testicular cancer survivors. Urology. 2007;69:754–8. doi: 10.1016/j.urology.2007.01.002. [DOI] [PubMed] [Google Scholar]


