Abstract
Aims. To examine the expression patterns of hydrogen sulphide- (H2S-) producing enzymes in ischaemic heart tissue and plasma levels of H2S after 2 weeks of NaHS treatment after myocardial infarction (MI) and to clarify the role of endogenous H2S in the MI process. Results. After MI surgery, 2 weeks of treatment with the H2S donor NaHS alleviated ischaemic injury. Meanwhile, in ischemia myocardium, three H2S-producing enzymes, cystathionine γ-lyase (CSE), cystathionine-β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST) significantly increased. Plasma H2S levels were also elevated. In vitro, NaHS treatment protected cardiomyocytes from hypoxic injury and raised CBS levels in a concentration-dependent manner. Different from in vivo results, however, CSE or 3-MST expression did not change. NaHS treatment increased the activity of CSE/CBS but not of 3-MST. When CSE was either knocked down (in vitro) or knocked out (in vivo), H2S levels significantly decreased, which subsequently exacerbated the ischaemic injury. Meanwhile, the expressions of CBS and 3-MST increased due to compensation. Conclusions. Exogenous H2S treatment changed the expressions of three H2S-producing enzymes and H2S levels after MI, suggesting a new and indirect regulatory mechanism for H2S production and its contribution to cardiac protection. Endogenous H2S plays an important role in protecting ischaemic tissue after MI.
1. Introduction
Hydrogen sulphide (H2S) has been recognized as the third gasotransmitter, following nitric oxide and carbon monoxide. It is synthesized endogenously by the catalysis of two pyridoxal-5-phosphate-dependent enzymes, namely, cystathionine-β-synthase (CBS) and cystathionine γ-lyase (CSE), both utilizing l-cysteine as substrate [1, 2]. CBS is mainly localized in the nervous system, whereas CSE has a crucial role in maintaining cardiovascular homeostasis [3, 4]. The third H2S-producing enzyme, 3-mercaptopyruvate sulfurtransferase (3-MST), has recently been discovered; this enzyme generates H2S in both the nervous and cardiovascular systems [5]. Accumulating evidence indicates that H2S is vital in regulating cardiovascular functions [6]. H2S can relax smooth muscle cells by activating ATP-dependent potassium (K+) channels (KATP), leading to a subsequent decrease in blood pressure [7]. It also has a substantial role in regulating cellular metabolism and alleviating heart fibrosis and inflammation [8]. H2S can reduce infarct size and preserve left ventricular function after MI or myocardial ischaemia/reperfusion in vivo [9–11]. It also regulates the outward K+ current (Ito) channels, extends action potential duration in epicardial myocytes, and shows efficacious protection against fatal arrhythmias in a rat model of myocardial infarction (MI) [12].
Qipshidze et al. reported that H2S promotes angiogenesis and significantly limits the extent of MI injury. They also observed a decrease in CSE expression after MI. NaHS treatment for 4 weeks upregulates CSE but not CBS expression [13]. Zhu et al. also reported the reduction of CSE mRNA in rats 48 h after MI [9]. However, less is known about the expression patterns of the three H2S-producing enzymes between 48 h and 4 weeks after MI, during which the fate of ischaemic cardiomyocytes is determined by many endogenous and exogenous signals. In this study, we tested the expressions of three H2S-producing enzymes in ischaemic heart tissues 2 weeks after MI and measured plasma H2S levels. We also clarified the role of endogenous H2S after MI.
2. Materials and Methods
2.1. The Mouse MI Model and NaHS Administration In Vivo
Adult male C57BL/6 mice (8 weeks) were purchased from the Institute of Laboratory Animal Science of the Chinese Academy of Medical Sciences (Shanghai, China). Experimental protocols were approved by the ethics committee for experimental research, Shanghai Medical College, Fudan University.
Mice were anaesthetized and ventilated with a rodent ventilator (Kent Scientific Corporation, USA). A parasternal incision was made with surgical scissors by cutting the skin and intercostal muscles between the left third and fourth ribs; MI was induced by occluding the left anterior descending coronary artery (LAD) using a 7-0 silk suture beneath the LAD, 1 mm inferior to the left auricle. The sham group experienced the same procedure without coronary artery occlusion.
After MI surgery, different concentrations of NaHS solution (0, 25, 50, and 100 μmol/kg/day) were injected intraperitoneally for 2 weeks (first administration was shortly after MI on day 1). Normal saline was used as control. The sham group received same NaHS or normal saline treatment as MI groups. On the 15th day (no NaHS treatment on that day), mice were sent to get echocardiographic assessment. After that mice were anesthetized and killed for plasma to measure H2S levels and for protein from ischemic area used for western blot to test the changes of three enzymes.
For acute infarct size determination, NaHS solution (0, 25, 50, and 100 μmol/kg) was administered 15 min before MI surgery. The infarct size is determined on day 2.
2.2. Echocardiographic Assessment
Echocardiography was performed as previously described [14]. Briefly, the mice were mildly anaesthetized with isoflurane (1%), and two-dimensional guided M-mode tracings were recorded using a Vevo 770 high-resolution system (Visualsonics, Toronto, Canada). Left ventricular internal dimension systole (LVIDs), left ventricular internal dimension diastole (LVIDd), left ventricular ejection fraction (LVEF), and left ventricular fractional shortening (LVFS) were measured to evaluate left ventricular function. All parameters were the average values from three cardiac cycles.
2.3. Determination of Infarct Size
Evans' Blue-2,3,5-triphenyltetrazolium chloride (TTC) double staining was introduced to measure the infarct size and the areas at risk as previously described [10]. Mice were injected with NaHS solution (0, 25, 50, and 100 μmol/kg) for 15 min before MI surgery. Twenty-four hours later, these mice were anaesthetized and their chests were opened again to expose the hearts. Evans' Blue (1.0%, Sigma-Aldrich, St. Louis, MO) solution was injected intravenously into the femoral vein to distinguish ischaemic from nonischaemic areas. The hearts were immediately excised and sliced into 1-2 mm thick slices parallel to the atrioventricular groove and immersed in 1% TTC (Sigma-Aldrich, St. Louis, MO) at 37°C for 15 min. The size of the area at risk (AAR) and the infarct area (INF) were quantified using ImageJ software by an observer blinded to the study.
2.4. Western Blot
Proteins were extracted from mouse left ventricles 2 weeks after MI or from cardiomyocytes pretreated with NaHS or vehicle 30 min before the 6 h exposure to hypoxic or normoxic conditions. Protein concentrations were determined by the BCA method (Shen Neng Bo Cai Corporation, Shanghai, China). Protein samples (50 μg) were subjected to 10% SDS-PAGE, transferred to polyvinylidene fluoride membranes (Millipore-Upstate, Billerica, MA, USA), and blocked with 5% nonfat milk. The membranes were then probed with primary antibodies against CSE, CBS (Abcam, Cambridge, UK), or 3-MST (Santa Cruz Biotechnology, CA). GAPDH was used as the internal control (Cell Signaling Technology). These experiments were performed at 4°C overnight. After washing with TBST 3X, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific-Pierce, Waltham, MA, USA) was then added and detected on radiographic films. Gray values of bands were quantified with the SmartViewer software.
2.5. Measurement of H2S Levels and the Activity of H2S-Producing Enzymes
Whole blood sample was collected, anticoagulated, and centrifuged at 3000 rpm for 15 min to obtain the plasma. The medium from cardiomyocyte cultures was collected. H2S levels in plasma or culture medium were measured as previously described [15]. H2S levels were calculated using a standard curve generated from a sodium sulphide solution (0–100 μM). The activities of CSE/CBS and 3-MST were measured according to the methods described by Tao et al. [16]. Briefly, cardiomyocytes were incubated with the substrate, l-cysteine, in the presence of pyridoxal-5′-phosphate for CSE/CBS or α-ketoglutarate for 3-MST. H2S levels were measured, and the amount of H2S produced by 1 μg protein/min was calculated, representing the activity of H2S-producing enzymes. However, because the detection of CSE and CBS activity shared the same reaction system, their activities could not be separated using this method.
2.6. Cell Culture and Treatment
Primary cultures of neonatal cardiomyocytes were established according to previously described methods [10]. The experimental protocol was approved by the ethics committee for experimental research, Shanghai Medical College, Fudan University. In brief, the hearts dissected from 1-day-old Sprague-Dawley rats were washed in cold phosphate-buffered saline (PBS) containing 130 mM NaCl, 3 mM KCl, 1 mM NaH2PO4, 4 mM glucose, and 20 mM HEPES (pH adjusted to 7.4 with NaOH) for three times to remove blood. The hearts were then minced in a drop of 0.125% trypsin (Sigma-Aldrich, St. Louis, MO, USA) to as small a size as possible and then subjected to a series of incubations at 37°C in a normal saline-buffered trypsin solution. After centrifugation, cell pellets were resuspended in Dulbecco's modified Eagle medium/F-12 (Gibco, Grand Island, NY) containing 10% heat-inactivated fatal bovine serum (Gibco, Grand Island, NY), 100 U/mL penicillin, and 100 μg/mL streptomycin. To increase the purity of cardiomyocytes, dissociated cells were plated in 100 mm culture dishes and kept in a 5% CO2 incubator at 37°C for 1 h. Nonmyocytes readily attached to the bottom of the dish, whereas most cardiomyocytes remained suspended in the medium. The resulting suspensions of cardiomyocytes were diluted to a density of 1 × 106 cells/mL and plated in 60 mm dishes or 6-well plates for protein extraction or 96-well plates for the Cell Counting Kit 8 (CCK8) and lactic dehydrogenase (LDH) tests and then cultured in a humidified 5% CO2 incubator at 37°C. 5-Bromo-2′-deoxyuridine (100 μM) was added during the first 48 h to prevent proliferation of residual nonmyocytes.
To establish the hypoxia model, cardiomyocytes were cultured in DMEM/F-12 without serum and exposed to hypoxic conditions (95% N2 + 5% CO2) for 6 h. For the NaHS treatment groups, 25, 50, and 100 μM of NaHS solution were added 30 min before hypoxia.
CSE small interfering RNAs (CSE siRNA) were purchased from Ambion (Austin, TX, USA). Cardiomyocytes were transfected with 25 nM CSE siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's guidelines.
2.7. CCK8 and LDH Activity
Cardiomyocytes were seeded on 96-well plate and treated with vehicle or NaHS for 30 min, followed by 6 h exposure to hypoxic or normoxic conditions. Culture medium was collected and LDH activity was tested according to manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Cardiomyocytes were used for CCK8 activity measurement according to manufacturer's instructions (Dojindo Molecular Technologies, Japan).
2.8. CSE Knock-Out Mice Identification
PCR-genotyping of CSE KO mice was identified according to the methods before [17]. Briefly, genomic DNA was extracted from heart tissues and subjected to PCR with paired primers: CSE knock-out mice (CSE KO) forward, 5′-CCTGGATATAAGCGCCAAAG-3′, and reverse, 5′-AGGAACCAGGGCGTATCTCT-3′, wild type (WT) forward, 5′-CCTGGATATAAGCGCCAAAG-3′, and reverse, 5′-CGAGAATTCCATTGCTCAGG. The amplified DNAs were then identified by DNA electrophoresis, from the representative photo of which we could separate CSE KO mice (single band, 309 bp) from WT mice (single band, 167 bp).
2.9. Statistical Analysis
Data are expressed as means ± SEM. Statistical analysis was performed using an SPSS software package, version 17.0 (SPSS, Inc., Chicago, IL, USA). The statistical comparisons among groups were performed by one-way ANOVA. Paired data were evaluated by two-tailed Student's t-test. P < 0.05 was considered statistically significant.
3. Results
3.1. The H2S Donor (NaHS) Protected Ischaemic Myocardium from Myocardial Infarction Injury
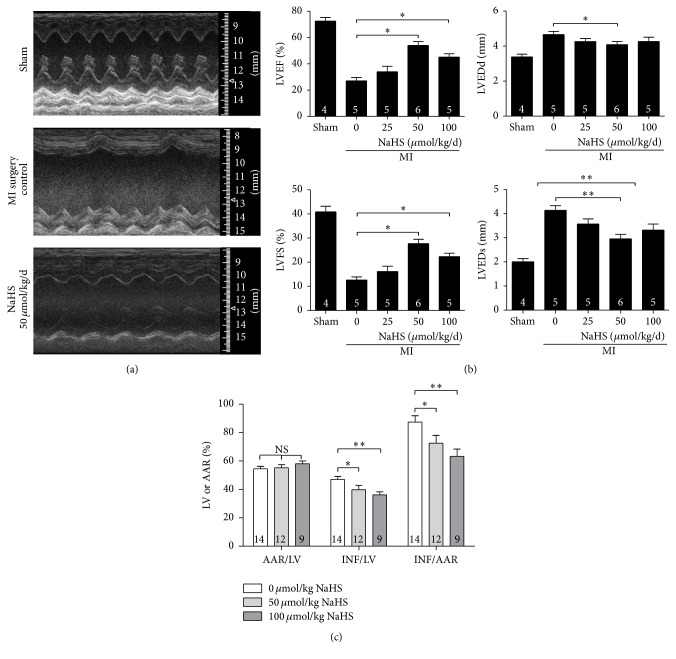
We used echocardiography to evaluate systolic and diastolic function. Echocardiography revealed that 2 weeks of NaHS treatment at 50 and 100 μmol/kg/day significantly increased cardiac function compared with cardiac function after treatment with normal saline. LVEF and LVFS increased, whereas LVIDs and LVIDd decreased (Figures 1(a) and 1(b)). Further, the infarct size was measured by Evans' Blue-TTC staining. NaHS pretreatment at 50 and 100 μmol/kg for 15 min significantly decreased the INF compared with the normal saline group (Figure 1(c)).
Figure 1.
The H2S donor NaHS protects ischaemic myocardium from myocardial infarction injury. (a) Representative echocardiogram from the sham, MI surgery control, and NaHS 50 μmol/kg/day treatment groups; (b) Echocardiographic parameter analysis. LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVIDs, left ventricular internal dimension systole; LVIDd, left ventricular internal dimension diastole. (c) Statistics of Evans' Blue-TTC staining to show the percentage of area at risk or infarct area. LV, left ventricle; AAR, area at risk; and INF, infarct area. Numbers inside bars denote the number of animals per group. Values are mean ± SE. ∗ P < 0.05 and ∗∗ P < 0.01.
The above results showed that NaHS treatment after MI surgery could efficiently protect the ischaemic heart compared with the untreated group.
3.2. H2S Levels and Expressions of H2S-Producing Enzymes Increased with NaHS Treatments
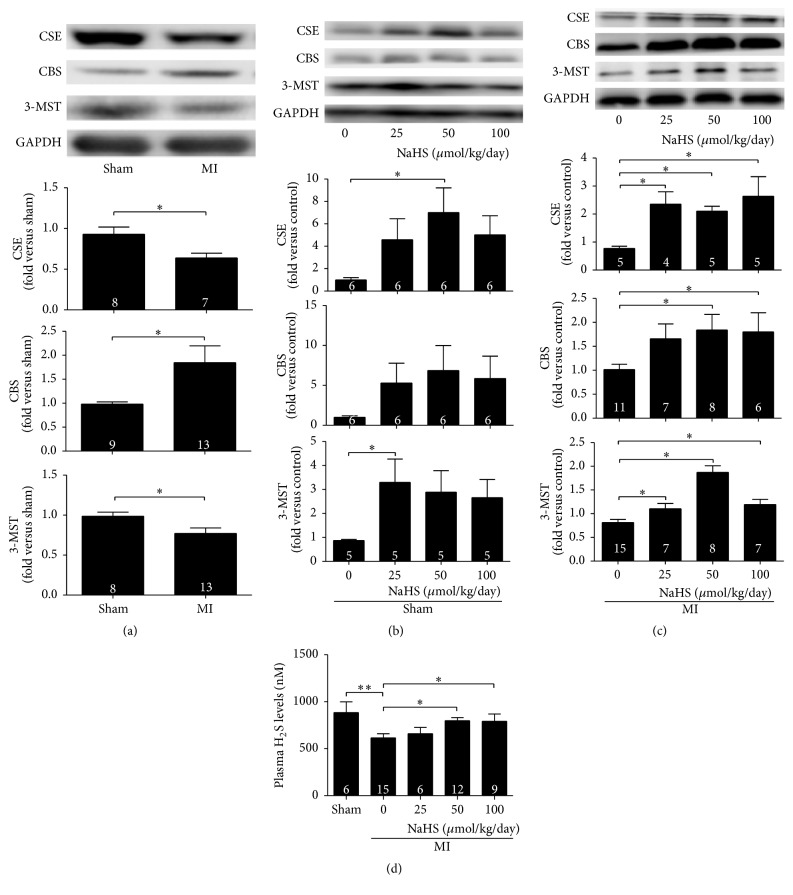
Ischaemic heart tissues from MI mice showed decreased CSE and 3-MST levels after 2 weeks; in contrast, CBS expression significantly increased. Two weeks of NaHS treatments in the range of 25–100 μmol/kg/d significantly increased the protein expression of all three enzymes in ischemia myocardium (Figures 2(a) and 2(c)). In noninfarcted control hearts, NaHS treatment for 2 weeks increased the expression of CSE (50 μmol/kg/day) and 3-MST (25 μmol/kg/day), while NaHS (25–100 μmol/kg/day) did not change expression of CBS (Figure 2(b)). Plasma H2S levels decreased after MI surgery, whereas 2 weeks of NaHS treatments alleviated the reduction. NaHS treatment (50 and 100 μmol/kg/day) significantly raised the plasma H2S levels after MI (Figure 2(d)).
Figure 2.
Exogenous H2S increased plasma H2S levels and the three H2S-producing enzymes expression in ischaemic myocardium. (a) The expressions of CSE and 3-MST significantly decreased, whereas CBS expression increased 2 weeks after MI surgery. (b) In noninfarcted myocardium, NaHS treatment for 2 weeks increased the expression of CSE (50 μmol/kg/day) and 3-MST (25 μmol/kg/day), while NaHS (25–100 μmol/kg/day) did not change expression of CBS; (c) H2S donor NaHS (25–100 μmol/kg/day) increased the expression of the three enzymes in ischaemic myocardium 2 weeks after MI. (d) Changes in plasma H2S levels in sham, MI surgery control, and NaHS treatment groups. Plasma H2S levels decreased 2 weeks after MI, whereas 50 and 100 μmol/kg/day NaHS treatment ameliorated this trend. Numbers inside bars denote the number of animals per group. Values are mean ± SE. ∗ P < 0.05.
3.3. The H2S Donor NaHS Protected Cardiomyocytes from Hypoxia-Induced Injury In Vitro
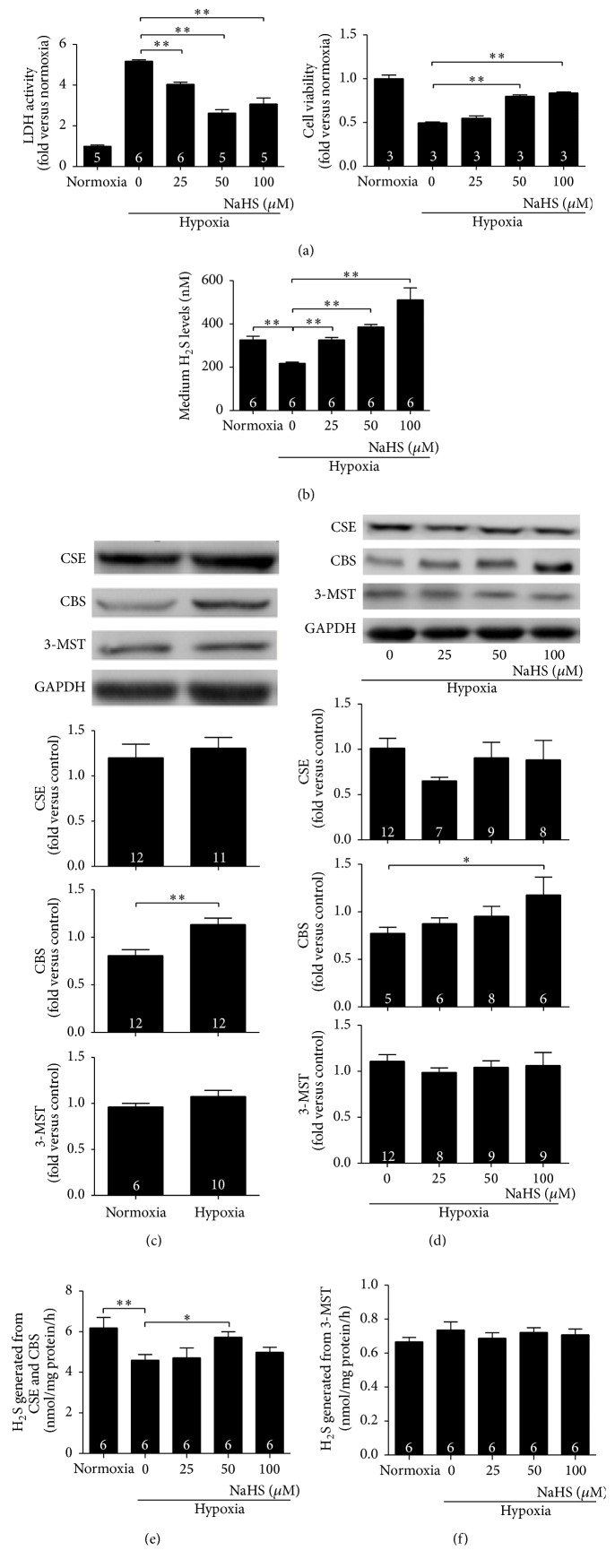
Cardiomyocytes are the major heart cells, and more importantly, they are highly terminated and cannot proliferate after birth. Protecting cardiomyocytes from ischaemic injury is essential. LDH and CCK8 tests are classic methods to detect cell viability and toxicity. Compared with the normoxic group, LDH activity in the culture medium of cardiomyocytes exposed to hypoxic conditions was much higher, whereas NaHS treatment in the range of 25–100 μM significantly decreased LDH activity. NaHS treatment at 50 and 100 μM also increased CCK8 values, indicating improved cardiomyocyte activity (Figure 3(a)). Cells produced less H2S under hypoxic conditions, whereas NaHS treatment significantly increased H2S production in a concentration-dependent manner (Figure 3(b)). Different from the enzyme expression patterns in ischaemic heart tissues, hypoxia did not change CSE or 3-MST expressions, whereas an increase in CBS expression still occurred in vitro. NaHS treatments did not change CSE or 3-MST expressions under hypoxic conditions but caused an increase in CBS levels in a concentration-dependent manner (Figures 3(c) and 3(d)). Hypoxia decreased the activity of CSE/CBS, and 50 μM NaHS treatment significantly diminished this decrease (Figure 3(e)). 3-MST activity was not influenced (Figure 3(f)).
Figure 3.
H2S donor NaHS protects cardiomyocytes from hypoxia-induced injury. (a) Cell viability was detected with LDH and CCK8 kits. Hypoxia exposure decreased cell viability, as evidenced by high LDH activity and low CCK8 values; 30 min of NaHS pretreatment alleviated the above injury, as demonstrated by the significant drop in LDH activity and increase in CCK8 values; (b) H2S level in the culture medium decreased after exposure to hypoxia, and 25–100 μM NaHS pretreatment in a concentration-dependent manner caused elevation of H2S levels; (c) 6 h hypoxia exposure did not change CSE or 3-MST expressions but significantly increased CBS expression; (d) NaHS pretreatment did not change the expression of either CSE or 3-MST but increased CBS expression at 100 μM. (e) The activity of CSE/CBS declined after exposure to hypoxia, whereas 50 μM NaHS treatment caused a significant increase in their activity; (f) Hypoxia with or without NaHS treatment did not change the activity of 3-MST. Numbers inside bars denote the number of animals per group. Values are mean ± SE. ∗ P < 0.05 and ∗∗ P < 0.01.
3.4. Endogenous H2S Protected Ischaemic Myocardium from Myocardial Infarction Injury
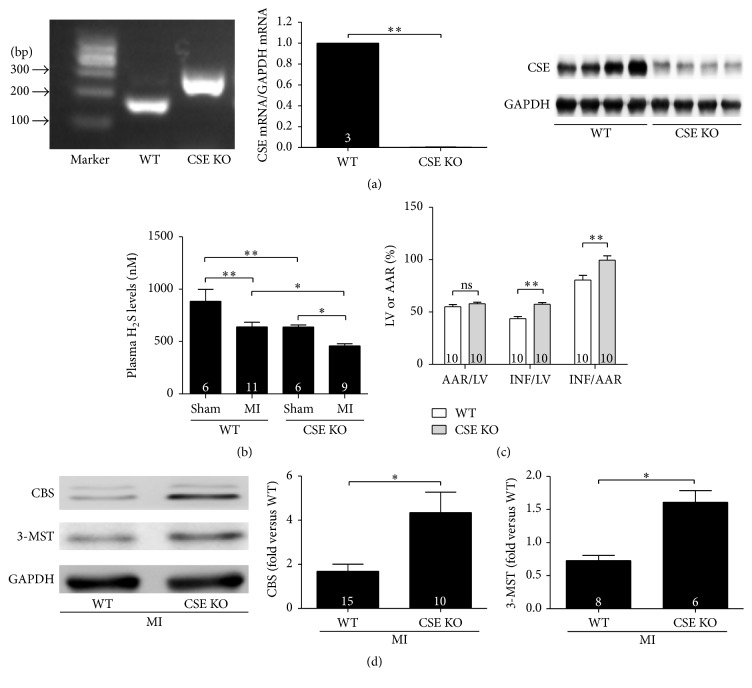
CSE is the main H2S-producing enzyme in the cardiovascular system, catalyzing the synthesis of endogenous H2S from l-cysteine; we used CSE KO mice to study whether endogenous H2S plays a role in cardiac protection after MI. CSE KO mice were characterized by analysing genomic DNA, CSE mRNA, and protein levels, which were compared with WT mice (Figure 4(a)). Plasma H2S levels in four groups were then tested: (1) CSE KO without MI, (2) CSE KO with MI, (3) WT without MI, and (4) WT with MI. As expected, plasma H2S levels were significantly lower in mice from the CSE KO without MI group than from the WT without MI group. Plasma H2S levels decreased significantly after MI both in WT and in CSE KO groups (Figure 4(b)). Furthermore, as shown in Figure 4(c), INFs in CSE KO mice were more extensive than those in WT mice. Protein expression of the other two enzymes, CBS and 3-MST, increased in CSE KO mice, most likely due to compensation (Figure 4(d)).
Figure 4.
Endogenous H2S protected ischaemic myocardium from myocardial infarction injury. (a) Characterization of CSE knock-out mice (CSE KO). Left panel: PCR analysis of genomic DNA from CSE KO mice and wide type mice (WT). Middle panel: real-time PCR analysis of CSE mRNA expression in the hearts from WT and CSE KO mice; right panel: representative western blot of CSE protein expression. (b) Plasma H2S levels in WT and CSE KO mice with (MI) or without (sham) surgery. Plasma H2S levels from the highest to lowest were as follows: WT without surgery, CSE KO without surgery, WT with MI surgery, and CSE KO with MI surgery; (c) infarct area in CSE KO group was larger than that in WT group detected with Evans' Blue-TTC staining; (d) expression of CBS and 3-MST increased in CSE KO mice. Numbers inside bars denote the number of animals per group. Values are mean ± SE. ∗ P < 0.05 and ∗∗ P < 0.01.
3.5. Endogenous H2S Protected Cardiomyocytes from Hypoxia-Induced Injury
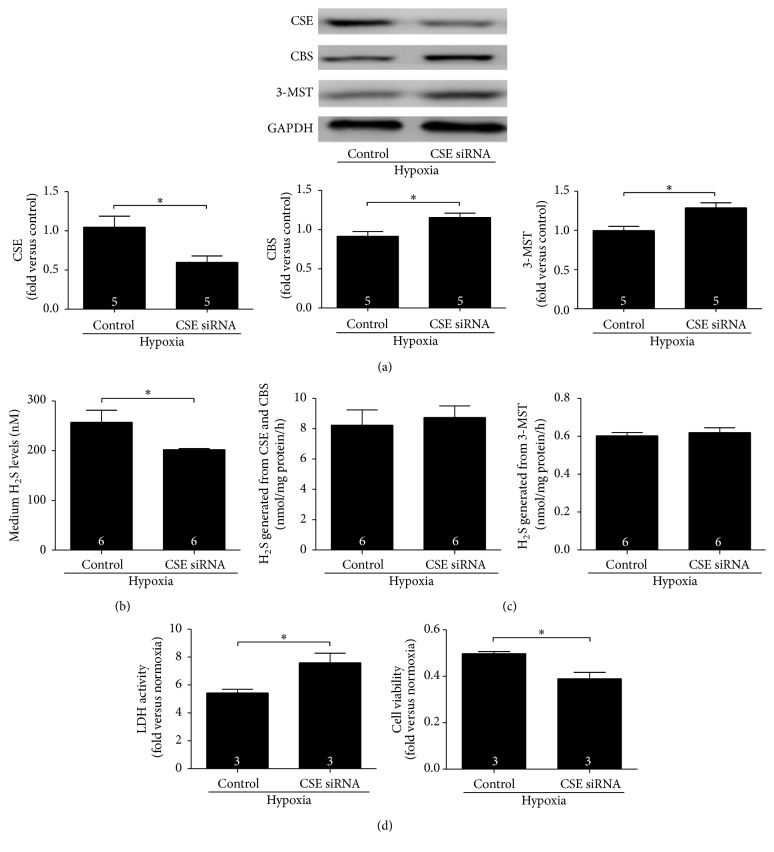
We used CSE siRNA to knockdown CSE expression and then monitored H2S levels in the culture medium. Compared with the hypoxia control, addition of CSE siRNA caused a significant decrease in H2S levels in culture medium and CSE expression in cardiomyocytes. The expressions of CBS and 3-MST in cardiomyocytes significantly increased after adding CSE siRNA (Figures 5(a) and 5(b)). The activity of the three H2S-producing enzymes did not change after the addition of CSE siRNA (Figure 5(c)). Cardiomyocyte injury was also evaluated after the addition of CSE siRNA. LDH activity was increased and CCK8 values were decreased, suggesting that knockdown of CSE expression exacerbated hypoxic injury (Figure 5(d)).
Figure 5.
Endogenous H2S protected cardiomyocytes from hypoxia-induced injury. (a and b) Compared with the hypoxia control, CSE siRNA significantly decreased CSE expression and H2S levels in the culture medium, whereas CBS and 3-MST expressions increased; (c) the activity of the three H2S-producing enzymes did not change after CSE siRNA treatment. (d) CSE siRNA exacerbated hypoxia injury, demonstrated by an increase in LDH activity and a decrease in CCK8 values. Numbers inside bars denote the number of animals per group. Values are mean ± SE. ∗ P < 0.05 and ∗∗ P < 0.01.
4. Discussion
In this study, we used an in vivo MI model and an in vitro hypoxic cardiomyocyte model to systemically investigate the regulation of exogenous H2S on the expression of the three H2S-producing enzymes. CSE KO mice were also used to study the effect of endogenous H2S on the ischaemic hearts. Cardiac expressions of the three enzymes were quantified 2 weeks after MI surgery. Our experiments indicated four important findings: (1) exogenous H2S significantly increased the expression of the three enzymes in vivo, (2) 25–100 μM NaHS pretreatment increased CBS levels in a concentration-dependent manner, and 50 μM NaHS treatment increased CSE/CBS activity in vitro, (3) endogenous H2S played an important role in protecting the ischaemic heart after MI, and (4) when CSE was either knocked down (in vitro) or knocked out (in vivo), the expressions of CBS and 3-MST increased due to compensation.
Accumulating evidence has confirmed that H2S is vital in regulating cardiovascular functions. H2S can relax smooth muscle cells by activating ATP-dependent K+ channels (KATP), leading to a subsequent decrease in blood pressure [7]. H2S reduces infarct size and preserves left ventricular function after MI or myocardial ischemia/reperfusion [9–11]. In this study, we used echocardiography and Evans' Blue-TTC double staining to detect the protective role of 2-week H2S treatment to mice suffering from MI injury. NaHS treatment at 50 and 100 μmol/kg after MI surgery effectively protected the heart from ischaemic damage. This protection was accompanied by greater CSE and 3-MST expression. From our results in Figure 3(c) those NaHS treatments did not change CSE or 3-MST expression under hypoxic condition in vitro; the elevated CSE and 3-MST expression after myocardial infarction by NaHS treatment in vivo is likely resulting from the improvement of cardiac function (e.g., less overload of noninfarcted area in NaHS-treated animals), but not from direct effect of H2S on these enzymes.
The mechanisms in which exogenous H2S participates in cardiac protection have been studied for years but still are not completely clear. H2S promotes the secretion of proangiogenic factors, such as VEGF, which appears to enhance angiogenesis in the ischaemic heart and improve blood supply [13, 18]. Inhibition of cardiomyocyte apoptosis also appears to play an important role in H2S-mediated cardiac protection [10, 11]. Recently, King reported eNOS-dependent cytoprotection by H2S in the setting of I/R injury [19]. The manner in which exogenous H2S affects another gaseous signalling molecule suggests that we should examine whether exogenous H2S influences endogenous H2S production to protect the ischaemic heart. In this study, we detected H2S levels in plasma and H2S-producing enzyme expression in the hearts.
After 2 weeks of intraperitoneal injection of NaHS (25–100 μmol/kg/day), the expressions of the three enzymes in ischemia myocardium increased but to different levels. In noninfarcted myocardium, NaHS treatment for 2 weeks increased the expression of CSE (50 μmol/kg/day) and 3-MST (25 μmol/kg/day), while NaHS (25–100 μmol/kg/day) did not change expression of CBS. Our results suggested that both myocardial infarction surgery (Figure 2(a)) and exogenous NaHS administration (Figures 2(b) and 2(c)) influenced the expression of H2S-synthesizing enzymes in infarcted hearts. ① Myocardial infarction decreased the CSE expression, while exogenous NaHS administration increased its expression. The overall effect of exogenous NaHS administration on CSE in infarcted heart was elevation. ② Myocardial infarction increased the CBS expression, while exogenous NaHS administration did not change its expression. The overall effect of exogenous NaHS administration on CBS in infarcted heart was elevation. ③ Myocardial infarction decreased the 3-MST expression, while exogenous NaHS administration increased its expression. The overall effect of exogenous NaHS administration on 3-MST in infarcted heart was elevation. The complicated in vivo situations that caused the effect of NaHS on CBS and CSE was not ideally dose-dependent and effect on 3-MST was maximal at 50 μmol/kg. NaHS treatment at 50 and 100 μmol/kg/day significantly increased plasma H2S levels. Qipshidze et al. previously reported that the expression of CSE increased and CBS decreased after drinking an H2S-releasing aqueous solution for 4 weeks [13]. The possible explanation for the different results between Qipshidze et al.'s study and ours could be the different duration of NaHS treatment; 2 weeks was used in the present study, whereas Qipshidze et al.'s research extended to 4 weeks. Changes in plasma H2S levels might also affect the expressions of the three enzymes.
Cardiomyocytes are the major cell type in the heart. We next investigated whether H2S could protect cardiomyocytes from hypoxia-induced injury in vitro. Similar to the in vivo results, H2S protected cardiomyocytes from hypoxic injury, as was evidenced by lower LDH activity in the culture medium and higher cellular CCK8 values. Simultaneously, H2S levels in NaHS-treated groups significantly increased. Because the expression and activity of both enzymes contribute to H2S production, we next tested the expression as well as the activity of the three enzymes. While the expressions of CSE or 3-MST did not change, CBS expression increased in a concentration-dependent manner. Hypoxia decreased the activity of CSE/CBS without affecting 3-MST, which may have contributed to the decrease in H2S levels under hypoxic conditions. Similarly, NaHS treatment may increase the activity of CSE/CBS to produce more H2S.
The difference between in vivo (H2S enhanced the expression of all three enzymes) and in vitro (H2S only enhanced the expression of CBS) studies may be due to the fact that the heart contains several kinds of cells and that H2S can be synthesized in cells other than cardiomyocytes. CSE, the major H2S-producing enzyme in cardiovascular systems, is also expressed in smooth muscle cells [7, 19], and 3-MST also exists in endothelial cells [5]. Cells surrounding cardiomyocytes can release H2S to maintain local H2S concentrations. Exogenous H2S can change the expressions of H2S-producing enzymes in other cell types. For example, Na2S (another H2S donor) also enhances CSE expression in ischemia/reperfusion-stimulated brain endothelial cells [20]. Results from tissue samples revealed overall H2S production, not just that from cardiomyocytes.
The mechanisms of upregulation of CBS by exogenous H2S need to be studied in the future. Exogenous H2S could specifically increase CBS expression with no effect on CSE or 3-MST, which might elevate local H2S concentrations and thus result in cardiac protection for different reasons. Wang et al. reported that exogenous H2S (10–80 μM) downregulates CSE expression, whereas hypoxia upregulates CSE expression in mammalian cell lines [21, 22]; these results differed from those obtained in the study. The probable explanation is that they used cell lines and NaHS treatment or hypoxia was administered separately. In our experimental conditions, we used primary cardiomyocytes and studied H2S effects under hypoxic conditions (a combination of hypoxia and NaHS treatment), which better mimicked post-MI conditions; this may reveal the mechanism of cardiac protection of H2S.
Finally, we used CSE KO mice or CSE siRNA to study the protective role of endogenous H2S. When CSE was either knocked out in vivo or knocked down in vitro, the ischaemic heart or cardiomyocytes displayed more damage; at the same time, although CBS and 3-MST expression increased due to compensation, H2S concentration still significantly decreased, confirming that CSE is the main H2S-producing enzyme in the heart. In cardiomyocytes, CSE siRNA significantly decreased CSE protein levels, but the activity of the three enzymes did not change. Low H2S levels in the medium may be the result of decreased CSE protein expression. In another study, King et al. also showed that CSE KO mice had large INFs, and the infarct size became less extensive when exogenous H2S was added [19]. King's experiments also confirmed that sufficient local H2S production around ischaemic tissues appears to be important for cardioprotection. When the major H2S-producing enzyme did not function adequately, H2S levels dropped and the protective effects of H2S were impaired.
5. Conclusion
In summary, we demonstrated for the first time that 2 weeks of exogenous H2S treatment can increase the expression of the three H2S-producing enzymes in ischaemic heart tissue and alleviate ischaemic damage. Endogenous H2S also appears to have a major role in protecting the ischaemic heart. This study suggested a new and indirect regulatory pathway for cardiac protection; however, the underlying mechanism needs further investigation.
Acknowledgments
The authors thank Bo Tan for his excellent technical assistance in H2S measurements and Rui Wang for his help during revision. This present study was supported by the National Natural Science Foundation of China (NSFC) (no. 81230003 to Yi-Chun Zhu and no. 81000045 to Ming-Jie Wang), the Ministry of Education (20110071130009 to Yi-Chun Zhu), Shanghai Pujiang Program (15PJ1400700 to Ming-Jie Wang), Research Center on Aging and Medicine Fudan University (13dz2260700 to Ming-Jie Wang), and a Key Laboratory Program of the Education Commission of Shanghai Municipality (ZDSYS14005 to Yi-Chun Zhu).
Conflict of Interests
The authors declare no conflict of interests on the publication of this paper.
Authors' Contribution
Na Li and Ming-Jie Wang contributed equally to this work.
References
- 1.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? The FASEB Journal. 2002;16(13):1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 2.Kimura H. Hydrogen sulfide: its production and functions. Experimental Physiology. 2011;96(9):833–835. doi: 10.1113/expphysiol.2011.057455. [DOI] [PubMed] [Google Scholar]
- 3.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. Journal of Neuroscience. 1996;16(3):1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosoki R., Matsuki N., Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and Biophysical Research Communications. 1997;237(3):527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 5.Shibuya N., Mikami Y., Kimura Y., Nagahara N., Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. Journal of Biochemistry. 2009;146(5):623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 6.Pan L. L., Liu X. H., Gong Q. H., Yang H. B., Zhu Y. Z. Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxidants and Redox Signaling. 2012;17(1):106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO Journal. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song K., Li Q., Yin X.-Y., Lu Y., Liu C.-F., Hu L.-F. Hydrogen sulfide: a therapeutic candidate for fibrotic disease? Oxidative Medicine and Cellular Longevity. 2015;2015:10. doi: 10.1155/2015/458720.458720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y. Z., Zhong J. W., Ho P., et al. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. Journal of Applied Physiology. 2007;102(1):261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 10.Yao L.-L., Huang X.-W., Wang Y.-G., Cao Y.-X., Zhang C.-C., Zhu Y.-C. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3β-dependent opening of mPTP. American Journal of Physiology—Heart and Circulatory Physiology. 2010;298(5):H1310–H1319. doi: 10.1152/ajpheart.00339.2009. [DOI] [PubMed] [Google Scholar]
- 11.Elrod J. W., Calvert J. W., Morrison J., et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(39):15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma S.-F., Luo Y., Ding Y.-J., et al. Hydrogen sulfide targets the Cys320/Cys529 motif in Kv4.2 to inhibit the I to potassium channels in cardiomyocytes and regularizes fatal arrhythmia in myocardial infarction. Antioxidants & Redox Signaling. 2015;23(2):129–147. doi: 10.1089/ars.2014.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qipshidze N., Metreveli N., Mishra P. K., Lominadze D., Tyagi S. C. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. International Journal of Biological Sciences. 2012;8(4):430–441. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z., Murtaza I., Wang K., Jiao J., Gao J., Lia P.-F. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(29):12103–12108. doi: 10.1073/pnas.0811371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen X., Pattillo C. B., Pardue S., Bir S. C., Wang R., Kevil C. G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radical Biology and Medicine. 2011;50(9):1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao B.-B., Liu S.-Y., Zhang C.-C., et al. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxidants & Redox Signaling. 2013;19(5):448–464. doi: 10.1089/ars.2012.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G., Wu L., Jiang B., et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kan J., Guo W., Huang C., Bao G., Zhu Y., Zhu Y. Z. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxidants and Redox Signaling. 2014;20(15):2303–2316. doi: 10.1089/ars.2013.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King A. L., Polhemus D. J., Bhushan S., et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(8):3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y., Li R., Yang H., Luo H., Chen Z. Sirtuin 6 is essential for sodium sulfide-mediated cytoprotective effect in ischemia/reperfusion-stimulated brain endothelial cells. Journal of Stroke and Cerebrovascular Diseases. 2015;24(3):601–609. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang M., Guo Z., Wang S. The effect of certain conditions in the regulation of cystathionine γ-lyase by exogenous hydrogen sulfide in mammalian cells. Biochemical Genetics. 2013;51(7-8):503–513. doi: 10.1007/s10528-013-9581-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang M., Guo Z., Wang S. Regulation of cystathionine γ-lyase in mammalian cells by hypoxia. Biochemical Genetics. 2014;52(1-2):29–37. doi: 10.1007/s10528-013-9624-7. [DOI] [PubMed] [Google Scholar]