Abstract
The atherosclerotic mouse aorta consists of a heterogeneous population of cells, including macrophages, endothelial cells (EC) and smooth muscle cells (SMC), that play critical roles in cardiovascular disease. Identification of these vascular cells in the vessel wall is important to understanding their function in pathological conditions. Immunohistochemistry is an invaluable technique used to detect the presence of cells in different tissues. Here, we describe immunohistochemical techniques commonly used for the detection of the vascular cells in the atherosclerotic mouse aorta using cell specific markers.
Keywords: atherosclerosis, mouse aorta, immunohistochemistry, macrophages, endothelial cells, smooth muscle cells
1. INTRODUCTION
Atherosclerotic lesions in hypercholesterolemic mice develop diffusely in the arteries. Different sites of the aorta, namely the aortic root, ascending aorta, the brachiocephalic artery (BCA), the aortic arch and the abdominal aorta are preferentially susceptible to lesion formation depending on the experimental conditions and the mouse model employed. The undiseased aorta is composed mainly of endothelial cells (EC) and a medial layer of smooth muscle cells (SMC). As lesions advance, they contain a heterogenous population of cells, including macrophages, EC, SMC, and T- and B-lymphocytes (1, 2). Immunohistochemistry (IHC) is an invaluable technique used for the detection and localization of these different cell types using labeled antibodies specifically directed against proteins associated with particular cell types. This technique uses the principle of visualization of antigen-antibody interactions either by fluorescent dyes or enzyme conversion of substrates to colored compounds (3). This chapter focuses on the methods used to detect macrophages, EC and SMC in atherosclerotic plaques using cell specific markers in mouse aortic sections. In addition, troubleshooting strategies and optimization notes are provided.
2. MATERIALS
2.1 Animals, instruments, and equipment
-Control and experimentally treated mouse models of atherosclerosis, typically apolipoprotein E or LDL receptor-deficient mice (or their variants)
- Forceps
- 70% ethanol
- micro scissors
- 25-gauge butterfly needle
- dissecting light microscope
- cryosection plastic molds
- cryostat microtome
- poly-L –Lysine coated slides (Fisher scientific, Cat # NC9895478)
Additional reagents and equipment for euthanizing the mouse
2.2 Reagents and solutions
- acetone
- phosphate buffered saline (PBS)
NOTE: PBS used should not contain calcium or magnesium since the ions may interfere with the detection process.
- normal goat or rabbit serum for blocking or DAKO serum free protein block.
- primary and secondary antibodies (see protocol)
- vectastain ABC alkaline phosphatase kit (Vector laboratories, Cat# AK 5000) or vectastain ABC peroxidase kit (Vector laboratories, Cat # PK 4000)
- DAB (3,3’ Diaminobenzidine) peroxidase substrate kit (Vector laboratories, Cat #SK 4100) or vector red alkaline phosphatase substrate kit (Vector laboratories, Cat# SK5100)
- Meyer's hematoxylin (Sigma Aldrich #MHS1-100ml)
- xylene
- xylene-based mounting media
- coverslips
3. METHODS
Typically a mouse based on either deficiency of apolipoprotein E (apoE−/−) or the LDL receptor (LDLr −/−) is fed a fat and cholesterol-enriched diet for a period long enough to develop the stage of atherosclerosis desired to be studied. In our lab, we use a 16-week diet period so that the lesions that develop are complex and similar to those in people with coronary artery disease, with multiple cell types present and with a necrotic core beginning to form (4, 5). At the time of euthanasia it is important to perfuse (for frozen sections) or perfuse and fix (for paraffin sections) at a physiological pressure, so as to keep the arteries patent, but not over-distended. This is done by suspending the perfusate at a height of 60 cm above the mouse, thus maintaining a perfusion pressure of 60-100 mm Hg and flow rate of 1-3 ml/min (6).
The choice between frozen sections vs. fixed and paraffin-embedded ones depends on the final use of the samples. If the sections are to be used for both immunohistochemistry (IHC) and to isolate RNA from cells in the sections by laser capture microdissection (LCM), then frozen sectioning needs to be done (7). On the other hand, if RNA isolation is not a consideration and maximum retention of tissue architecture is desired, fixation and embedding in paraffin blocks is the better choice. It should be noted that although the final immunostaining protocols are essentially the same for both types of tissue preparation (frozen & paraffin), in practice, a particular antibody might work better with one type of preparation than the other. Sometimes this is noted by the supplier of the antibodies or by a technical specialist at the supplier.
3.1 Protocol for harvesting mouse aorta
Euthanize the mouse according to your institutional animal care and use committee approved protocol. Briefly, we anesthetize the mice using a cocktail of ketamine (75 mg/ml) and xylazine (5 mg/ml). The injectable dose recommended is 1ml/g of the body weight of the animal. After injection, allow 10-15 minutes (min) for the mice to be completely sedated. Check for loss of reflex response by toe pinching or tail pinching. This is necessary to judge the animal's level of responsiveness to painful stimuli under anesthesia.
Prior to perfusion, flush the tubes of the suspended container with the perfusant (saline) to remove air bubbles (as noted above, the perfusate is suspended at a height of 60 cm above the mouse to achieve a physiologic blood pressure no as not to stretch or collapse the arteries by using too high or low a perfusion pressure, respectively).
Once the animal is sedated, secure the four paws to the surface spreading them as far as possible with the help of an adhesive tape. Wet the area where the incision is to be made with 70% ethanol.
With the help of forceps and scissors, make an incision to open the thoracic cavity by cutting the ribs lateral to the sternum thus exposing the heart.
Insert the 25 gauge needle attached to the gravity flow apparatus into the left ventricle of the mouse to start perfusion. Make an incision at the right atrium or the hepatic vein. Maintain the flow rate of the perfusant (saline) at 1-3 ml/min so that the pressure is maintained. Perfuse till the liver turns pale in color, which indicates circulating red bllod cells have been removed systemically.
Remove the lungs, kidneys, gastrointestinal and reproductive organs.
Using a dissection microscope, isolate the aortic arch by carefully dissecting the ascending aortic, aortic arch along with BCA, left common carotid artery and left subclavian artery along with 1 mm of descending aorta attached to the aorta. Remove excess fat with the help of a cotton swab.
If the aortic root is required, make an incision at the level of the upper level of atria, and cut the heart/aortic root tissue away from the aorta.
Proceed to embedding as outlined below.
3.2 Fixing, embedding and sectioning of aortic tissues
Frozen sections
Frozen tissues can be prepared by embedding them in Optimal Cutting Temperature (OCT) compound and quickly freezing in the presence of isopentane on dry ice. Tissues can be snap frozen and this method is beneficial especially when tissues are used for detecting post-translational modifications of proteins, such as phosphorylation. Frozen tissue sections fixed in acetone are also used when direct or indirect immunofluorescence is the detection method, in cases where formalin fixation can sometimes produce weaker results depending on the antibody.
Frozen tissue molds can be stored at −80 °C for up to 1 year. They can then be trimmed and cut into cryosections with the help of microtome or cryostat. They can be re-processed in acetone and re-hydrated in buffer before immunohistochemistry. One of the primary reasons for using frozen tissue or sections is a faster examination that eliminates the time required for fixation, processing and de-waxing compared to paraffin sections. Frozen tissue sections are mostly fixed using acetone or alcohol that avoids the requirement to retrieve epitopes masked by formaldehyde cross-linking in paraffin sections. More importantly, RNA isolation can also be performed by laser capture microdissection in frozen sections if gene expression profiling is necessary. In paraffin sections, antigen-retrieving methods can destroy the epitopes sometimes making the antibodies unusable.
Protocol
Fill the separate cryoplastic plastic molds with OCT compound for embedding the heart/aortic tissue and the arch.
With the help of forceps, take the heart/aortic root tissue and embed it in the cryomold containing OCT at the axis of the aorta perpendicular to the base of the mold.
For the aortic arch, make an incision between the left common artery and left subclavian artery. Embed the two sections such that the aortic arches are perpendicular to the mold; ie, they are facing the user.
Freeze the tissues rapidly by placing the molds in dry ice to which isopentane is added.
Cover with aluminum foil and store the tissue molds in −80 °C freezer until cryosections are required.
To section frozen tissue, cut the tissue at 5-6 μm in thickness with a cryostat microtome. Using a thin brush, gently move the curled section of the section over the cryostat stage. Pick the sections with the help of brush and place on poly-L–lysine coated slides. Lysine coated slides are used for better tissue adhesion especially since the aortic sections can easily dislodge from the slides during the staining process. Place 4-5 sections per slide for IHC.
Store the cut cryosections in −80 °C freezer immediately. Do not fix the slides before freezing. Fix the slides only if proceeding immediately to immunostaining.
Perform IHC to detect different vascular cells (see below). Although we have used uncut frozen tissues for up to one year, once they are sectioned, they should be further processed with a week if RNA is to be isolated from some of the sections.
Paraffin sections
Paraffin embedding is the best method to preserve tissue morphology. However, before embedding, tissues have to be treated in fixatives like neutral buffered formalin (NBF) or 4% paraformaldehyde (PFA) in PBS overnight at 4 °C. Inadequately fixed tissues can dehydrate during tissue processing, resulting in hard and brittle specimens. Once embedded in paraffin, tissue blocks can be stored in room temperature (RT) for a long time (i.e., years). Paraffin sections can then be obtained by cutting the tissue blocks with the help of a microtome. As noted above, two limitations of paraffin sections is that it is hard to isolate RNA (though special kits are now available) and antigen retrieval processes can sometimes destroy the epitope of interest.
Protocol
Fix the harvested tissues in 4% PFA prepared in PBS or 10% neutral buffered formalin overnight at 4° C. Since the aortic tissues are small, overnight tissue fixation is optimal. Usually the fixative volume should be 10X times the tissue volume.
Wash the tissues gently with PBS three times.
Store the tissues in 70% ethanol till they are ready to be embedded in paraffin blocks.
- Transfer tissues to embedding cassettes and dehydrate accordingly
- - 70% ethanol, 2 changes, 1 h each
- - 80% ethanol, 2 changes, 1 h each
- - 95% ethanol, 2 changes, 1 h each
- - 100% ethanol, 3 changes, 1 h each
- - xylene, 3 changes, 1 h each
- - paraffin wax (56-58 °C), 2 changes, 1.5 h each
Embed the tissues into paraffin blocks
To section paraffin blocks, trim and mount the blocks on to the microtome. Take care to trim the paraffin blocks such that there is an optimal cutting surface area that includes the sample with a small paraffin frame.
Using the microtome, cut 3-10 μm slices of paraffin sections. 5 - 6 μm is commonly used thickness for IHC of mouse aorta. Use a brush to gently hold the sections. If the microtome is adjusted properly, the paraffin sections should come out as a ribbon.
Place paraffin ribbons or a slice in 40-45 °C water bath with a second wet brush. As the sections expand on the waterbath, the wrinkles on the sections vanish.
Gently scoop out the floating paraffin sections using glass slides and position the section with the help of a brush.
Dry the section sections O/N at 37°C (lower baking temperatures are better for subsequent antibody detection)
Sections can be stored at RT for an extended period of time.
3.3 Immunostaining detection methods
The main aim of immunostaining is to detect the expression of the antigen of interest with the least background without false positive results. Immunostaining can be done by direct or indirect methods.
Direct method
The primary antibody has the fluorophore or enzyme directly attached. This enables faster detection in that it is a one step procedure. The drawback, however, is that each antibody used needs to be derivatized.
Indirect methods
The indirect method involves a typically two-step process, and sometimes a multi-step process.
-
(a)
In the two-step detection method, the primary antibody is recognized by a secondary antibody conjugated with a fluorescent group or an enzyme, such as alkaline phosphatase. The primary antibody, which has the specificity for the antigen of interest, is from one species (such as a mouse), and the secondary antibody, which has specificity for the source of the primary antibody, is from another species (such as a rabbit). Binding of the secondary to the primary antibody is detected by either the fluorescence resulting from being excited at the recommended wavelength or by adding a colorimetric substrate for the enzyme. In this way, any primary antibody can be used, and commercially supplied secondary antibodies with the detection system are purchased.
-
(b)
A common multistep detection method involves the avidin-biotin complex (ABC) method and relies on the strong affinity of avidin or streptavidin for the vitamin biotin. Streptavidin (from Streptomyces avidinii) and avidin (from chicken egg) both possess four binding sites for biotin, which can be easily conjugated to proteins. In the ABC method, secondary antibodies are conjugated to biotin and function as links between tissue-bound primary antibodies and an avidin-peroxidase or phosphatase complex.
In general, the sensitivity or signal amplification of the assay increases with its complexity; i.e., multi-step > two-step > single step. As noted earlier, the atherosclerotic mouse aorta is composed of a heterogenous population of cells. Indirect methods that involve multiple steps where the signal is significantly amplified are commonly used to detect the different cells in the lesions. Here we outline the immunostaining protocol for macrophages, EC and SMC using a multi-step immunostaining protocol.
3.4 General protocol for immunstaining
For paraffin aortic sections
Bake slides in 56 °C oven for 1 h to melt the wax.
- Deparaffinize the tissues as outlined below
- - xylene : 3 changes, 5 min each
- - 100% alcohol: 2 changes, 2 min each
- - 95% alcohol: 2 washes, 2 min each
- - 70% alcohol: 3 changes, 2 min each
- - dd.H20 : 1 change, 2 min
Antigen retrieval: Antigens can become masked during the preparation of the tissue. In some cases, “antigen retrieval” methods are used to achieve better access to the epitopes recognized by the primary antibody. The method of antigen retrieval usually depends on the target antigen and the type of antibody used. Antigen retrieval can be done by multiple methods
-
(a)
Protease-induced Epitope Retrieval: Proteases including proteinase K, trypsin and pepsin have been used to unmask the epitopes in paraffin sections. Incubation with these enzymes results in the cleavage of the peptides masking the epitope. The optimal time and duration should be pre-determined for each antibody.
-
(b)
Heat-induced epitope retrieval: Heating is performed using microwave ovens or pressure cookers. This protocol tends to involve 5 min periods of heat followed by replacement of the buffer. The heat-induced method is especially time, temperature-, buffer-, and pH-sensitive, and the best method must be determined empirically by the user.
For aortic tissues, heat induced epitope retrieval is usually performed in 10 mM citrate buffer (pH 6.0) or 1 mM EDTA (pH 8.0) buffer for a total of 15 min. Slides must be cooled for 30-40 min before proceeding to immunostaining.
-
4.
Wash sections in PBS, 3 changes, 5 min each.
-
5.
Block endogenous peroxidase activity with 3% hydrogen peroxide (H2O2) in methanol or 0.3% H2O2 in PBS for 10 min at RT. Prepare this solution just before use. Presence of endogenous peroxidase enzymes in the tissue can severely limit the interpretation of immunoperoxidase staining in frozen tissues. Hence it is necessary to quench the endogenous peroxidase activity in the aortic tissues.
-
6.
Wash slides in double distilled water (dd. H20) for 5 min once.
-
7.
Rinse in PBS for 5 min with two changes. Change PBS between washes and wipe tissue sections with Kimwipes.
-
8.
Outline sections with a hydrophobic marker.
-
9.
Block nonspecific binding with DAKO blocking buffer (DAKO, Catalog #X0909) or 5% normal goat or rabbit serum for 20 min at RT.
-
10.
Gently knock excess liquid off the slides and wipe around tissues.
-
11.
Incubate with specific primary and secondary antibodies and proceed for the detection of different vascular cells as outlined in the following sections.
For frozen aortic sections
Take frozen slides out of −80 °C and let them warm up to RT for 2 min.
Fix sections in ice-cold acetone for 10 min.
Air dry sections for 30 seconds min at RT. Do not let the sections dry out.
Wash slides in PBS three times for 5 min each in a Coplin jar.
Follow steps from 5-10 as outlined for paraffin sections.
Incubate with specific primary and secondary antibodies and proceed to the detection of different vascular cells as outlined in the following sections.
(1) CD68 immunostaning protocol for macrophages
CD68 is a macrophage marker frequently used in mouse atherosclerosis studies. Other markers for macrophages include F4/80, CD64 (FCγR1), MerTK and CD115 (CSF1R) (8). If laser capture microdissection (LCM) is to be performed on the samples, a rapid CD68 IHC is performed to identify the cells of interest (tissues are incubated for 1 min with a high titer of CD68 antibody). Here we outline the protocol for CD68 staining of frozen mouse aortic sections.
Follow steps from (1) to (6) under Section 3.4 for frozen sections
For routine staining, incubate frozen sections for 1 h with monoclonal rat anti-mouse CD68 (1: 250, AbSerotec, Cat# MCA1957) diluted in 4% rabbit serum. This step is critical since over-incubation would result in false positive results. For rapid CD68 stain for LCM purposes, incubate sections for 1 min at an antibody concentration of 1:50 at RT and proceed as follows for either application (routine or LCM).
While the tissues are incubated with primary antibody, prepare Vectastain ABC solution in PBS. The solution should be incubated for 30 min at RT.
ABC reagent: To 5 ml PBS, add 2 drops of reagent A, vortex followed by 2 drop of reagent B.
-
3.
Rinse sections in PBS, three times, 5 min each.
-
4.
Secondary Antibody: Incubate sections in biotinylated rabbit anti-rat antibody (1: 200) diluted in blocking solution for 30 min.
-
5.
Rinse in PBS, three times, 5 min each.
-
6.
Incubate sections for 20 min with Vectastain-ABC alkaline phosphatase solution. Prepare vector red solution in Tris-HCl just before use.
Vector Red Solution: To 5 ml Tris buffer, pH 8.5 + 2 drops of each reagent A and of reagent B. Vortex after addition of each reagent.
-
7.
Rinse in PBS, three times, 5 min each.
-
8.
Incubate sections in Vector red solution approximately for 8-10 min to get the desired stain intensity.
-
9.
Place slides in dd.H20 for 5 min to stop the reaction.
-
10.Counter stain with hematoxylin and dehydrate sections as follows:
- - hematoxylin: 2 min
- - PBS: 2 changes, 3 min
- - 70% Ethanol: 1 change, 2 min.
- - 95% Ethanol: 1 change, 2 min.
- - 100% Ethanol: 1 change, 2 min.
- - xylene, 3 changes, 2 min.
-
11.
Mount section with DAKO mounting media and coverslip.
-
12.
Seal around the edges of the section with nail polish and place slides horizontal to dry.
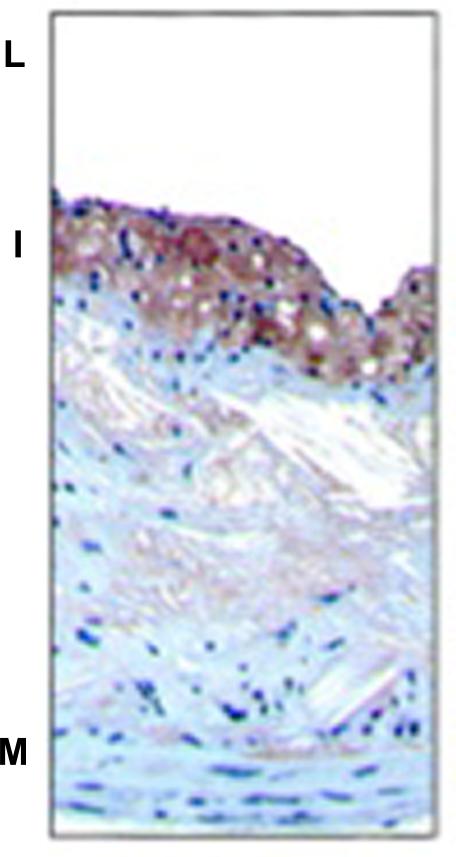
Staining pattern: The macrophages are stained red and are easy to identify in the light background. An example of the CD68 immunostaining is shown in Fig 1.
Figure 1.
Atherosclerotic lesions were immunostained for macrophage specific marker CD68 reddish-(brown stain) in ApoE−/− mice fed western diet for 16 weeks. L indicates lumen; I, intima; M, media. From Rong JX et al. Circulation.2001; 104:2447-2452.
(2) CD31/PECAM1 immunostaning protocol for EC
CD31 is a 130-kDa transmembrane glycoprotein also designated as PECAM-1 (platelet endothelial cell adhesion molecule 1). It is a constituent of the endothelial intercellular junction and plays a major role in the adhesion cascade between EC and the circulating monocytes, promoting their entry into atherosclerotic sites, where they become macrophages. CD31 is commonly used as a standard marker of EC. The other commonly used EC markers in the mouse aorta include VE-Cadherin, Von Willebrand factor (VWF), and VEGFR2 /Flk1/KDR [9, 10]. The protocol outlined below is for CD31 immunostaining using DAB peroxidase substrate, but a similar design can be used for any of these with the appropriate primary and secondary antibodies.
Protocol
Follow steps from (1) to (6) under Section 3.4 for frozen sections.
Incubate sections with monoclonal rat anti-CD31 antibody (BD Pharmingen, Cat # 550274) diluted at a concentration of 1:100 in blocking buffer for 1 h in a humidified chamber or at 4 °C overnight.
Rinse in PBS, three changes, 5 min each.
Add biotinylated anti-rat secondary antibody diluted to 1:200 in PBS and incubate for 30 min at RT in a humidified chamber.
Rinse in PBS, three changes, 5 min each.
ABC reagent must be prepared 30 min before use for complex formation.
Incubate sections in ABC for 30 min, at RT in humidified chamber.
Rinse in PBS, three changes, 3 min each. Change PBS between washes.
Stain slide with DAB peroxidase substrate (3-3’ diaminobenzidine) for 2-5 min till the desired intensity. Prepare the DAB solution just prior to use.
DAB solution
To 2.5 ml dd. H20, add 1 drop of buffer stock solution, 2 drops of DAB stock solution, 1 drop of hydrogen peroxide solution. Vortex and add to sections.
Note: DAB gives a brown reaction product. If a black reaction product is required, add 1 drop of nickel solution to the DAB solution. The solution should be prepared fresh.
-
9.
Counterstain with hematoxylin for 1-2 min. Wash slides in dd.H20. Staining time is important to reduce intensity of hematoxylin. If required, an extra wash may be performed to remove excess stain.
-
10.
Dehydrate slides and mount them with mounting media and sealing the edges as outlined in Section 3.4 under CD68 stain (Steps 9 -12).
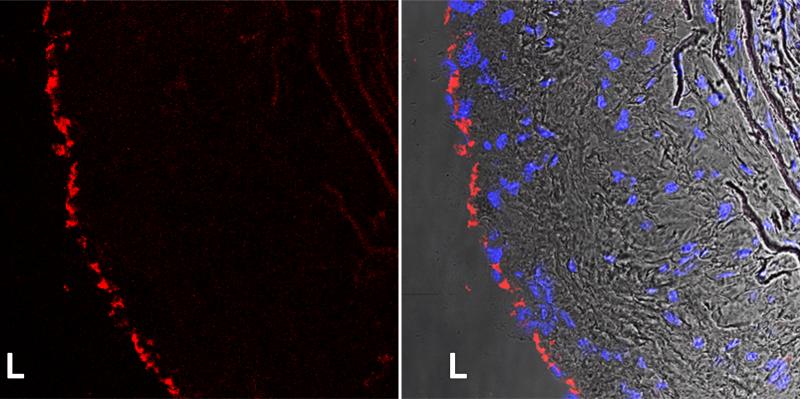
Staining pattern: EC appear as a thin layer of cells lining the luminal surface of the vessel wall. An example of CD31 staining in the mouse atherosclerotic lesion is shown in Fig 2.
Figure 2.
Confocal images of CD31 (red) immunofluorescent stain in the frozen atherosclerotic lesions of ApoE−/− mice fed western diet for 16 weeks (left panel). Nuclei are stained blue with DAPI and overlaid with CD31 in the bright field image (right panel). L. lumen. P. Menon and E. Fisher, unpublished data.
(3) α- smooth actin immunostaining protocol for SMC
α-smooth muscle (SMC) actin, an isoform typical of contractile SMC, is present in high amounts in vascular SMC (VSMC) and in pericytes (cells surrounding small vessels, such as capillaries). α-SMC actin is localized to microfilament bundles, consistent with its representing the contractile capacity of these cell types. It is one of the best markers of VSMC. Other markers for VSMC include myosin heavy chain, α-tropomyosin, and calponin (11, 12). The protocol outlined below will be for α-SMC actin, but a similar design can be used for any of these with the appropriate primary and secondary antibodies. Normally, VSMC stains localize to the media layer of an artery, but in atherosclerosis, staining can be found within complex plaques because of migration of VSMC from the media. Note though, that as the VSMC persist in the intimal space, their differentiation status can change and the expression of any one marker of the contractile state can decrease.
Follow steps from (1) to (6) outlined in Section 3.4 for frozen sections
Incubate sections with mouse monoclonal α-SMC actin antibody (Sigma, Clone1A4, Cat # A2547) diluted at a concentration of 1: 400 in blocking buffer for 1 h in a humidified chamber or overnight at 4°C.
Rinse in PBS, three changes, 5 min each.
Add biotinylated anti-mouse secondary antibody diluted to 1:200 in PBS. Incubate for 30 min at RT in a humidified chamber.
Rinse in PBS, three times, 5 min each.
Incubate sections in ABC for 30 min, at RT in humidified chamber.
Rinse in PBS, three times, 3 min each. Change PBS between washes.
Stain slide with DAB peroxidase substrate (3-3’ diaminobenzidine) for 2-5 min till the desired intensity. Prepare the DAB solution just prior to use as described above for CD31 stain.
Counterstain with 1X hematoxylin for 1-2 min. Wash slides in dd. H2O. Perform an extra wash to remove excess stain.
Dehydrate and mount with coverslip as described in Section 3.4.1, under CD68 stain (Steps 9 -12).
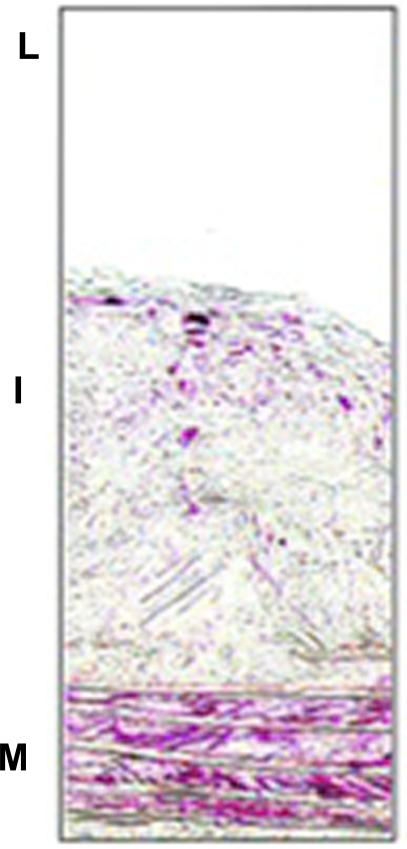
Staining pattern: Membrane/ cytoplasmic stain of α-smooth muscle actin is observed. An example of smooth muscle α-actin immunostaining is shown in Fig 3.
Figure 3.
Immunostaining of smooth muscle α-actin ( red stain) in atherosclerotic lesions of ApoE −/− mice fed western diet for 16 weeks. L indicates lumen; I, intima; M, media. Note the two positive areas: at the bottom of the section, where the bands of the internal elastic laminae are visible, are the VSMC in the medial layer (M) of the artery. In the intimal area (I) are VSMC that migrated from the media and continue to express smooth muscle α-actin. From Rong JX et al. Circulation.2001; 104:2447-2452.
4. TROUBLESHOOTING
Antibody standardization by IHC can be challenging especially in a mouse aorta where tissue surface area is small. The following troubleshooting protocol assumes that aortic tissues are frozen sections and fixed in acetone.
(a) Tissue detachment from slides
Use lysine coated adhesive slides for more adherence.
Inadequate fixing of sections: Increase fixing time in acetone. Rinse well with PBS to remove residual acetone. Inadequate or over fixing will impair the primary antibody binding to the epitope.
Drying out of tissues: Incubate sections in a humidified chamber through out the staining process to prevent tissues from drying.
Transfer slides gently between washes to avoid sections from coming off the slides.
(b) High Background
Endogenous enzyme activity not quenched: Block endogenous activity by increasing incubation time with, or the concentration of, the blocking reagent.
While using normal serum as blocking reagent, make sure that it is from the same species in which the secondary antibody is generated. For example, normal goat serum is used as a blocking reagent when the secondary antibody is generated in goat. Alternatively, if high background still persists, DAKO serum-free protein block can be used.
Primary antibody concentration is too high: Decrease the concentration and/or incubation time. Increase wash times.
Secondary antibody concentration is too high: Decrease the concentration and/or incubation time. Secondary antibodies can sometimes recognize endogenous tissue immunoglobulins. To rule out false positive results, run a trial experiment by incubating tissues with secondary antibody only to observe background stain.
Chromogen concentration is high (for enzyme-based detection systems): Reduce concentration and incubation time.
Buffer washes are not sufficient. Add an extra wash or extend wash time between washes.
Counterstain stain too strong. Decrease incubation time or increase wash time. Counterstains can sometime obscure the IHC reaction. Use a counterstain that does not interfere with the IHC staining.
(C) Weak staining in samples
Over or under-fixed tissue. Optimize fixing protocol by increasing/decreasing fixing times
Primary antibody concentration is too low: Increase concentration or incubation time or both.
Secondary antibody concentration is too low: Increase concentration or incubation time or both.
Chromogen incubation time is too short: Increase incubation till desired intensity is obtained.
Acknowledgements
This work is supported by NIH HL 084312 and HL098055 to E.A.F, and NIH T32 grant 5T32HL098129-05 to P.M.
Footnotes
- Mouse aorta embedding and processing is a critical step to get consistent results. All aortic tissues (control & experimental) should be processed exactly the same way since variations in this process can lead to inconsistencies.
- Control tissue should be from the same species as the experimental tissues.
- Controls using IgG or no primary antibodies should be performed for all IHC stains to rule out false positive results. To obtain best results, it is recommended that each user determine the optimum primary working dilution of bot the primary and secondary antibodies by titration assays.
- Positive and negative controls whenever possible should be added to the staining protocol.
- Incubation time for the primary antibody usually depends on the antigen of interest. The protocol outlined here is based on a default incubation time of 30-60 min. When increasing or decreasing the antibody concentration, incubation times may be needed to be adjusted accordingly.
- ABC complex is best when freshly prepared. Allow 30 min for complex formation before incubation with sections.
- Incubation time with DAB depends the concentration and incubation time of antibodies used and amount of the antigen of interest present in the tissue. Optimize time accordingly to prevent over staining and high background.
- DAB and nickel chloride are toxic and carcinogenic. Appropriate care such as gloves, eye protection and lab coats must be worn while performing the experiment. Dispose according to institutional regulations.
References
- 1.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14(1):141–7. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 2.Rong JX, Li J, Reis ED, et al. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104(20):2447–52. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-Vara JA. Principles and methods of immunohistochemistry. Methods Mol Biol. 2011;691:83–96. doi: 10.1007/978-1-60761-849-2_5. [DOI] [PubMed] [Google Scholar]
- 4.Feig JE, Rong JX, Shamir R, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108(17):7166–71. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslow JL. Mouse models of atherosclerosis. Science. 1996;272(5262):685–8. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 6.Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. J Vis Exp. 2012;(65) doi: 10.3791/3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trogan E, Choudhury RP, Dansky HM, et al. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2002;99(4):2234–9. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimbrone MA., Jr. Vascular endothelium: an integrator of pathophysiologic stimuli in atherosclerosis. Am J Cardiol. 1995;75(6):67B–70B. doi: 10.1016/0002-9149(95)80016-l. [DOI] [PubMed] [Google Scholar]
- 10.Muller AM, Hermanns MI, Skrzynski C, et al. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72(3):221–9. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- 11.Skalli O, Ropraz P, Trzeciak A, et al. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103(6 Pt 2):2787–96. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96(3):280–91. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]