Abstract
Background
Health insurance claims data may play an important role for healthcare systems and payers in monitoring the non-medical use of prescription opioids (NMPO) among patients. However, these systems require valid methods for identifying NMPO if they are to target individuals for intervention. Limited efforts have been made to define NMPO using administrative data available to health systems and payers. We conducted a systematic review of publications that defined and measured NMPO within health insurance claims databases in order to describe definitions of NMPO and identify areas for improvement.
Methods
We searched eight electronic databases for articles that included terms related to NMPO and health insurance claims. A total of 2,613 articles were identified in our search. Titles, abstracts, and article full texts were assessed according to predetermined inclusion/exclusion criteria. Following article selection, we extracted general information, conceptual and operational definitions of NMPO, methods used to validate operational definitions of NMPO, and rates of NMPO.
Results
A total of seven studies met all inclusion criteria. A range of conceptual NMPO definitions emerged, from concrete concepts of abuse to qualified definitions of probable misuse. Operational definitions also varied, ranging from variables that rely on diagnostic codes to those that rely on opioid dosage and/or filling patterns. Quantitative validation of NMPO definitions was reported in three studies (e.g., receiver operating curves or logistic regression), with each study indicating adequate validity. Three studies reported qualitative validation, using face and content validity. One study reported no validation efforts. Rates of NMPO among the studies’ populations ranged from 0.75–10.32%.
Conclusions
Disparate definitions of NMPO emerged from the literature, with little uniformity in conceptualization and operationalization. Validation approaches were also limited, and rates of NMPO varied across studies. Future research should prospectively test and validate a construct of NMPO to disseminate to payers and health officials.
INTRODUCTION
Misuse, abuse, and dependence on opioid analgesic medications (referred to herein as the non-medical use of prescription opioids [NMPO]) and related consequences has become a critical public health issue in the US.1,2 From 1997 to 2007, there was a 402% increase in the average per person milligrams of prescription opioids sold in the US.3 In 2012, the number of persons who reported NMPO was 4.9 million, second only to marijuana use at 18.9 million.4 Serious health ramifications have been associated with the NMPO epidemic.5–7 Those engaged in NMPO are likely to have mental and behavioral health comorbidities, such as post-traumatic stress, mood, anxiety, personality,8–12 and substance use disorders.9,10,13–16 Other common health problems include hepatitis10,13 and overall poorer health.9,17 The most severe health consequence resulting from the rapid escalation of NMPO has been the increase in opioid-related overdose deaths.18 From 1999–2008, overdose deaths increased fourfold,1 and in 2010, opioid overdoses claimed nearly 50 lives per day.18
Individuals engaged in NMPO are very likely to seek health care/services and subsequently represent a significant burden on the health care system. In the US, estimates of total societal costs of NMPO were $55.7 billion in 2007, with health care services (medical and prescription) accounting for 45% of those costs.19 To reduce the excess costs of NMPO and improve the health of populations served, health care systems and payers may benefit from using population-level administrative claims data to identify and intervene with those engaged in or at risk for NMPO. These systems require valid methods for identifying NMPO if they are to target patients or providers for intervention. However, definitions of NMPO vary across sources.20 Examples of benefits of a valid operational definition of NMPO could include payers having the capacity to identify patients at-risk or engaged in misuse then subsequently notifying providers. With such data, providers could provide prevention interventions (opioid contracts) or referrals and/or office-based treatment options (buprenorphine maintenance therapy).
The primary purpose of this article is to report findings from our systematic review of peer-reviewed research publications that have defined and measured NMPO within administrative claims databases in order to describe: the existing variation in definitions, efforts to validate the various definitions of NMPO previously implemented in health insurance claims data, and the prevalence of NMPO.
Defining the Problem
In spite of the far reaching impact of NMPO on individuals and the health care systems, current consensus does not exist for how to accurately identify NMPO within administrative claims data. Furthermore, NMPO has a variety of definitions within the literature, which leads to challenges for comparing prevalence rates and results across studies and reports. Table 1 contains examples of definitions or questions from leading national organizations in the US that are designed to describe or capture NMPO. The National Institute on Drug Abuse (NIDA) defines prescription medication abuse as: “…[T]he use of a medication without a prescription, in a way other than as prescribed, or for the experience or feelings elicited.”21 An additional commonly cited conceptualization of NMPO comes from the Substance Abuse and Mental Health Services Administration’s (SAMHSA) annual National Survey on Drug Use and Health (NSDUH). This survey asks participants regarding their: “…[U]se of any form of prescription pain relievers that were not prescribed for you or that you took only for the experience or feeling they caused.”22 The World Health Organization Composite International Diagnostic Interview23 asks patients if they “Have … ever used a pain killer nonmedically?” In addition to these conceptualizations of NMPO, one of the largest barriers to identifying patients with NMPO in administrative claims data is that while opioid use, abuse, and dependence are identifiable within the diagnostic codes of the International Classification of Diseases (ICD),24 NMPO is not a classified condition. Given the serious problems faced in the US regarding NMPO and the variation and limitations that exist regarding its definition, we initiated the current systematic review.
Table 1.
Examples of definitions of opioid medication misuse
| Organization | Definitions and questions |
|---|---|
| American College of Preventive Medicine38 | Non-medical use (prescription drug abuse, illicit use): Intentional or unintentional use of legitimately prescribed medication in an unprescribed manner for its psychic effect (either experimentation or recreationally), deciding to increase the dose of one’s own medication, unknowingly taking a larger dose than directed, engaging in a suicidal attempt or gesture, and inadvertent poisoning. The non-medical use of prescription medications implies that the person is using the drug for reasons other than those indicated in the prescribing literature or other off-label uses prescribed by a clinician. Nonmedical use includes procurement of drugs for abuse, bartering, suicide, homicide, or accidental ingestion. |
| MedlinePlus39 | If you take a medicine in a way that is different from what the doctor prescribed, it is called prescription drug abuse. It could be: 1) Taking a medicine that was prescribed for someone else; 2) Taking a larger dose than you are supposed to; 3) Taking the medicine in a different way than you are supposed to, this might be crushing tablets and then snorting or injecting them; 4) Using the medication for another purpose, such as getting high. |
| National Institute on Drug Abuse21 | Prescription drug abuse is the use of a medication without a prescription, in a way other than as prescribed, or for the experience or feelings elicited. |
| Substance Abuse and Mental Health Services Administration’s annual National Survey on Drug Use and Health22 | Use of any form of prescription pain relievers that were not prescribed for you or that you took only for the experience or feeling they caused. |
| World Health Organization: Composite International Diagnostic Interview23 | Have you ever used a pain killer nonmedically? |
METHODS
Search Strategy
We began by searching eight electronic databases (CINAHL, Health Source: Nursing/Academic Edition, Medline, PAIS International, PsychINFO, PsycArticles, PubMed, and Social Work Abstracts). These databases search a wealth of sources, including: peer-reviewed literature, book chapters, conference proceedings, web content, dissertation publications, professional materials, legal sources, monographs, governmental documents, and technical reports. Within these databases, we searched for three broad categories of terms related to opioids (e.g., painkiller, analgesic), health insurance claims (e.g., benefits, claims, insurance), and non-medical use (e.g., misuse; dependence; Table 2). Given the fact that a variety of terms have been used to describe NMPO within the literature, a range of search terms were employed attempting to capture a majority of synonyms and subgroups. For example, based on the study of Manshikanti et al.,3 a list of opioid medications for which consumption has greatly increased, such as fentanyl and hydromorphone, were included as key terms under the opioids. Furthermore, we searched the National Library of Medicine Medical Subject Headings (MeSH) for relevant synonyms to our search terms related to opioids and administrative data. In addition to our broad conceptualization of opioid-related terms, we likewise chose broad terms to depict misuse (e.g., abuse, dependence, misuse) given the varied terminology used within the field. Our choice to maintain broad search terms stemmed from our interest in gathering all definitions of NMPO in health claims analyses rather than attempting to only identify a single a priori conceptualization. Search strings were organized by combining terms with Boolean operators (i.e., AND and OR) in each of the eight databases. The three broad categories of terms were combined using the operator AND, while the subgroups of keywords within categories were listed using the operator OR (Table 2).
Table 2.
Search strings/commanda
| Opioid category | AB (Analges* OR Buprenorphine OR Fentanyl OR Hydromorphone OR Morphine OR Opi* OR Oxycodone OR Oxymorphone OR Oxycontin OR Painkiller OR Pain Management OR Pain Medication OR Suboxone OR Subtex) |
| AND | |
| Health insurance claims category | (Admin* OR Benefi* OR Claim* OR Diversion* OR Enrollee OR Insur* OR Medicaid OR Medicare OR Pay*) |
| AND | |
| Non-medically use category | (Abuse OR Chronic OR Dependence OR Long-term OR Misuse OR Overuse) |
Terms related to opioid medications were searched using the electronic database search engines within the title and abstract, and terms related to claims and misuse were searched using the electronic database search engines from any part of the article. This decision was based on very limited search results generated when all terms were only searched within titles and abstracts.
= Exploded mesh term encompassing all MeSH sub-headings.
We reviewed publications from 2000 through February 2014. The year 2000 was selected as the first year for our search given escalating trends around that timeframe in the US for both elevated levels of NMPO25 and overdose death.26 Lastly, because the study aimed to investigate NMPO among enrollees of health insurance in the US, we limited our electronic search to studies conducted in the US.
Selection of Studies
Our initial search yielded a total of 2,613 studies (Figure 1), which were imported into an electronic database (Endnote X7.1) for review. We selected studies from this initial search in two steps. For the first step, two independent reviewers selected articles in which the titles and abstracts discussed prescription opioids or a conceptualization of NMPO, used administrative claims data, studied human patients, and utilized quantitative methodologies. Reviewers eliminated studies that were non-US based, duplicates, and conceptual/qualitative in nature (e.g., literature reviews and editorials; Figure 1). Conceptual/qualitative articles were not included given our specific intent to identify studies that developed definitions of NMPO for use within health claims data and subsequently reported results of the empirical implementation of those definitions. For articles found to be unclear in terms of the inclusion/exclusion criteria, the methods sections of these articles were examined. This step also involved discussions between reviewers to resolve disagreement and to protect the review from individual reviewer selection bias. A total of 2,569 studies were eliminated in this step, which resulted in 44 remaining studies (Figure 1). The second step of the study selection process was similar to the first, with the exception that the 44 remaining articles from the initial step were examined by the reviewers by reading both the abstract and full text and specifically only including studies that contained a conceptual and an operational definition of NMPO. Our final step also included examination of the reference lists within our selected studies to identify additional possible publications for inclusion, which yielded no additional studies that met our inclusion criteria.
Figure 1.
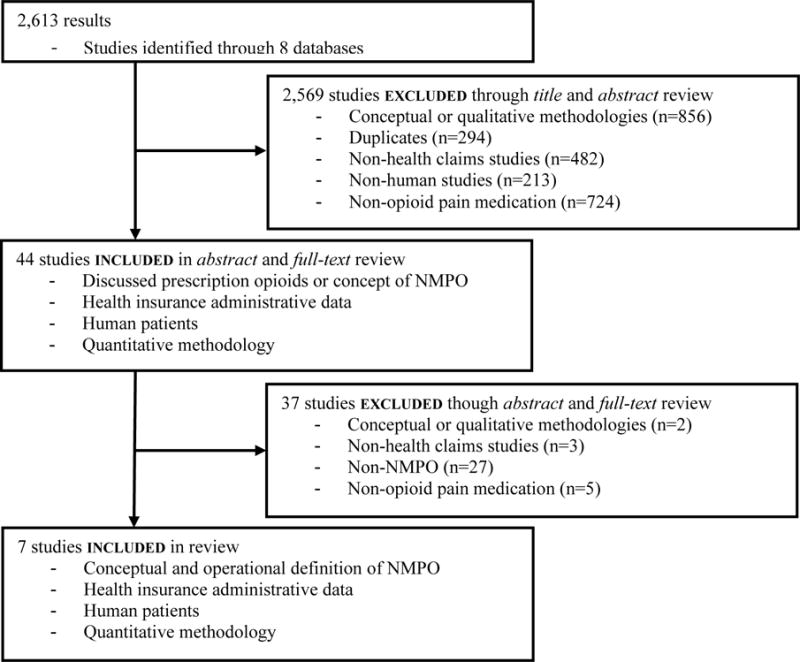
Consort flow diagram of selected studies
Data Extraction and Analysis
The data extraction template for the final publications identified in the review was developed by the authors and was used by two reviewers during the extraction process to record general information; including first author, year of publication, and data source. Furthermore, the NMPO conceptualization used within the article title or abstract and operational definition of NMPO were also extracted. By NMPO conceptualization, we mean the brief nominal label or term the authors of the articles utilized to summarily describe their NMPO concept in the titles or abstracts (e.g., “prescription opioid abuse”). By operational definition, we mean the specific observed indicators utilized by the authors to operationalize and construct the NMPO variables from within their data sources (e.g., ICD-9 codes, dosage of medication). In addition, validation methods of the operationalized definition of NMPO (if one was included in the article), the general purpose of the analysis plan related to the NMPO variable(s), a general description of the main outcomes, and the rates of NMPO reported were also added to the data extraction template.
RESULTS
We identified seven articles fitting our inclusion/exclusion criteria, including data from publicly funded insurance (n=2; Braker et al.,27 Leider et al.28), commercial health plans (n=3; Logan et al.,29 Rice et al.,13 White et al.10), and a mix of both publicly funded and commercial health plans (n=2; Roland et al.,30 Sullivan et al.31; Table 3).
Table 3.
NMPO across studies
| Operationalization of NMPO | |||||||
|---|---|---|---|---|---|---|---|
| First author/ year | Stated NMPOa concept | Diagnosis-based measure | Number of providers and pharmacies | Prescription-fill based measure | Urine toxicology | Exclusions | Data source(s) |
| Braker 200927 | Potential Rxb opioid misuse | – | Received ≥3 opioid Rxs from ≥2 providers; ≥6 opioid Rxs within 6-monthsc | Yes/no record of opioid Rx | – | Pts d receiving short terms pharmacotherapy and those with cancer diagnosis | Publicly funded insurance: Upper Peninsula Health Plan (Medicaid MCO e) patients from a single family medicine practice |
| Leider 201128 | Non-adherence among chronic opioid users | – | – | 120 days of a qualifying opioid within 6-months | Medication match and levels within expected ranges | Pts not matched to controls were excluded | Publicly funded insurance: Medicare advantage, and Medicaid plans; independent database of urine drug testing results |
| Logan 201329 | Potential opioid misuse/ inappropriate Rx practices | – | – | Opioid Rxs overlapping ≥1 week; overlapping opioid and benzodiazepine Rxs; long-acting/ extended- release opioids for acute pain f; or ≥100 morphine milligram equivalent/ day | – | Pts not continuously enrolled in their plans for 1 year and those receiving opioid Rx for cancer pain | Commercial insurance: MarketScan® Commercial Claims and Encounter Database prepared by Truven Health |
| Rice 201213 | Rx opioid abuse | 304.0X (opioid-type dependence), 304.7X (combinations of opioid-type dependence with any other drug dependence), 305.5X (nondependent opioid abuse), and 965.0 (poisoning by opiates/related narcotics) | – | Yes/no record of opioid Rx | – | Pts with heroin poisoning | Commercial insurance: Administrative claims data from a privately insured population (Ingenix Employer Solutoins, Eden Prarie, MN, USA) |
| Roland 201330 | Diagnosed Rx opioid abuse | 304.0X, 304.7X, 305.5X, 965.00, 965.02 (methadone poisoning), and 965.09 (opiates poisoning not elsewhere classified) | – | – | – | No exclusions | Publicly funded and commercial insurance: MarketScan Commercial Claims and Encounters and Medicare Supplemental databases (Thomson Reuters®) |
| Sullivan 2010 31 | Probable opioid misuse among chronic opioid users g | – | Number of prescribers (≤2, 3–4, ≥5); number of pharmacies (≤ 2, 3–4, ≥5) | Days of short acting opioids (91–185, 186–240, >240) and days of long acting opioids (91–185, 186–240, >240) within 6-months | – | Pts with cancer diagnosis (other than non-melanoma skin cancer), living in nursing homes, receiving hospice treatment, | Publicly funded and commercial insurance: HealthCore and Arkansas Medicaid |
| White 200910 | Rx opioid abuse | 304.0, 304.7, 305.5, or 965.0 | – | Yes/no record of opioid Rx | – | Pts with heroin poisoning | Commercial insurance: Maine Health Data Organization |
NMPO=Non-medical use of prescription opioids.
Rx=Prescription.
A misuse score was generated based on individual variables predicting ≥6 opioid Rx within a 6 month period among those who had received ≥3 Rxs from ≥2 providers.
Pt=Patient.
MCO=Managed Care Organization.
See Leider et al. Table 2 footnote for definition and list of ICD-9 codes for conditions included as acute pain.
Composite misuse scores were generated and categorized: no misuse (≤ 1), possible misuse (2–4), and probable misuse (≥5).
Conceptualization of NMPO
The conceptual definitions of NMPO varied widely (Table 3). White et al.10, Rice et al.13, and Roland et al.30 all conceptually present NMPO as identifying prescription opioid abusers. Braker et al.27, Logan et al.,29 and Sullivan et al.31 discuss their conceptualizations of NMPO in terms of potential or probable misuse of opioid medications. Leider et al.28 conceptualize their NMPO population as persons who chronically use opioids and are non-adherent to their prescribed regimen.
Operationalization of NMPO
The operational definition of NMPO also varied widely across studies (Table 3). The operational definition of NMPO is constituted by the specific observed indicators utilized by the authors to construct the NMPO variables from within their data. Four general types of indicators were used by the authors to operationalize NMPO within the claims data: (a) ICD-9 diagnosis codes, (b) opioid medication prescription records, (c) provider/pharmacist records, and (d) urine toxicology information.
Roland et al.,30 White et al.10 et al., and Rice et al.13 each utilize diagnoses of opioid use disorders in their operational definitions of prescription drug abuse. In addition, White et al.10 et al. and Rice et al.13 also include in their definitions that patients must also possess evidence of an opioid medication prescription. Sullivan et al.31 and Braker et al.27 employ numbers of providers and pharmacists as part of their definitions of potential or probable NMPO. For instance, Braker et al.27 indicate patients must have had opioid medication prescriptions from two or more providers. However, added to the numbers of providers and pharmacists, Sullivan et al.31 also include information on days of opioids supplied (e.g., 91–185, 186–240, >240 days), and Braker et al.27 include that patients must possess a record of having an opioid prescription. Similarly, Logan et al.29 utilize days of opioids supplied as well as doses of medications within their definition. Leider et al.28 likewise include days of opioids supplied combined with urine toxicology to verify non-adherence to the medication regimen (Table 3).
Validity
Two primary approaches, one quantitative and one qualitative, were employed in validating the operational definitions of NMPO in these studies (Table 4). Braker et al.27 employ a quantitative approach in which they performed a criterion validation using a receiver operating curve (ROC) analysis to identify how well their misuse score predicted whether or not a patient had filled more than six opioid prescriptions in a six-month period. These authors reported adequate validity (sensitivity=0.82, specificity=0.70). Similarly, White et al.10 tested three statistical models (2 models with prescription claims information only as independent variables and 1 with integrated prescription and medical claims information as independent variables) to predict their NMPO variable. The prescription claims models contained similar opioid medication information (e.g., dosages) but differed in that one contained prescriber information and the other did not contain prescriber information. The integrated model included opioid medication information (similar to the 2 prescription information models) as well as health and mental health diagnoses previously demonstrated to be related to NMPO (e.g., depression, hepatitis).10 Employing an ROC analysis to identify model fit, these authors reported their integrated analytical model possessed the highest degree of validity in predicting their NMPO variable (C=0.93, r2=0.37). Sullivan et al.31 also performed a quantitative criterion validity analysis, and relied on logistic regression models that associated comorbid opioid use disorder diagnoses with their NMPO outcome variable (which was defined by pharmacy/provider data and days of opioid supplied). Results showed adequate support based on significant associations of the opioid use disorder diagnoses with their NMPO variable (commercial claims data [Health core]: Odds Ratio [OR] = 3.53, 95% Confidence Interval [CI] = 2.4–5.2; public claims data [Medicaid]: OR = 2.66, (95% CI= 1.1–6.2).
Table 4.
Validity analyses and rates of misuse
| First author/ year | N | Validity | General analysis plan related to NMPO variable | Main outcomes | Rates of misuse | ||
|---|---|---|---|---|---|---|---|
| Type and method | Criterion | Outcome | |||||
| Braker 200927 | 61 | Criterion validity using ROC curve for model/score | ≥6 opioid Rxsa filled in 6-months | Sensitivity of 0.82 (95% CI=0.7–0.9) and specificity of 0.70 (95%CI=0.5–0.9) | Associations between the number of opioid Rxs in a 6-month period or ≥6 opioid Rxs filled in 6-months and factors possibly associated with misuse. | Number of opioid Rxs were most strongly associated with use of non-opioid pain medications, increasing opioid dosages, and multiple prescribers. | NAb |
| Leider 201128 | 49,425 | Face validity | Comparison of healthcare utilization/costs of adherent vs. non-adherent chronic opioid using patients. | Non-adherent opioid users who overuse opioids have significantly higher health care costs | 3.35% | ||
| Logan 201329 | 400,288 | Content validity c | Analysis of health services and opioid misuse/ inappropriate prescribing among males vs. female enrollees prescribed opioid analgesics in emergency departments. | Emergency department opioid prescribing practices were generally not problematic; problematic patterns were found only among a minority of patients | 10.32% | ||
| Rice 201213 | 821,916 | Content validity c | Estimates of associations between Rx opioid abuse and patient characteristics, Rx drug use, filling behaviors, comorbidities, medical resource use, and family member characteristics. | The strongest predictors of abuse were previous opioid Rxs, a previous opioid agonist Rx, non-opioid substance use, psychiatric conditions, hepatitis, or a family member with opioid abuse. | 0.78% | ||
| Roland 201330 | 61,592 | NA d | Comparisons of demographic and baseline characteristics between opioids abusers and matched non-abusers. | Rx opioid abuse increased from 2005–2010, and those who abuse have significantly higher health care utilization. | 0.20% | ||
| Sullivan 2010 31 | 31,844 | Criterion validity misuse indicator | Opioid abuse or dependence diagnosis | ORe = 3.53 (95%CI, 2.4–5.2) in Health Core and an OR = 2.66 (95%CI, 1.1–6.2) in AR Medicaid | Associations between categorical misuse and the independent variables hypothesized to be associated with opioid misuse, including: demographic variables, health and pain variables, mental health/ substance abuse diagnoses, and pharmacological variables. | Individuals who were younger, have pain conditions, have substance use disorders, receive >120mg opioids/day, or receive short acting schedule 2 opioid have increased risk for misuse. | Healthcore: 6%; AR Medicaid: 3% f |
| White 200910 | 134,542 | Criterion validity using ROC curves comparing model fit | Three misuse models | Integrated drug and health services claims to predict Rx opioid misuse was the best fitting model (C=0.93, r2=0.37) | Identified possible risk factors for Rx opioid abuse, including: demographic, health services, and mental health/ substance use disorders. | Individuals with early opioid Rxs refills, substance use disorders, mental health diagnoses are at highest risk for abuse. | 0.75% g |
Rx=Prescription.
Receiving ≥3 Rxs from ≥2 providers was also the selection criteria for the study sample, and the number of those with ≥6 opioid Rxs filled in 6-month within that sample was not reported.
We assessed this article as content validity given the authors of the article having cited previous research as the primary base of their definition.
NA=Not available; i.e., discussion of validity is not included in the article.
OR= odds ratio.
Rates of misuse reported herein are those associated with the “probable misuse” category—not the “possible misuse” category.
Rate of misuse is based on the drug claims model (n=116,382) due to the fact that the total number of abusers for the integrated model are not reported.
In contrast to these quantitative approaches, the second validation approach appears to be more conceptual (i.e., face and content validity; Table 4). These authors provided either rational justification of operational definitions and/or cited previous literature that showed support for the indicator they created. The only case wherein an operational definition with conceptual validation cited a previous study that had performed a quantitative validation was Rice et al.13 citing the previous work of White et al.10
Rates of NMPO
We also extracted rates of reported misuse from the articles by Logan et al.,29 Sullivan et al.,31 and Roland et al.30 The studies by Leider et al.,28 Rice et al.,13 and White et al.,10 did not specifically report rates of NMPO; however, the authors reported sufficient data to allow rates of NMPO to be calculated from their samples. The Braker et al.27 study neither reported rates of NMPO nor contained sufficient data to calculate rates of misuse (Table 4). Differences in rates range from the lowest at 0.75% (White et al.10 defining NMPO using a record of a opioid prescription combined with opioid use disorder diagnoses) to 10.32% (Logan et al.29 defining NMPO using prescription drug fill indicators). The calculation of the NMPO rate for Leider et al.28 was the total number of those likely non-adherent patients divided by total chronic users. The rate of NMPO for the Logan et al.29 study was the number of patients who had any one of four misuse/inappropriate prescribing factors divided by the total patients that received one or more prescription opioid medications from an emergency department. The rate of misuse from Sullivan et al.31 was calculated from the number of patients who were designated as having probable misuse divided by the total sample of chronic opioid users. For Rice et al. and Roland et al.,30 rates of misuse were based on the number of patients designated as abusers divided by the total patients within their samples. Rate of misuse for White et al.10 was also based on the number of patients designated as abusers (n=875) divided by the total patients within their sample (n=116,382). This rate of misuse is from the drug claims model and not the integrated model due to the fact that the total number of abusers for the integrated model is not clearly reported.
DISCUSSION
This systematic review identified seven peer-reviewed research articles from 2000 to 2014 that contain conceptual and operational definitions of NMPO that have been employed within public and private health insurance claims databases. Valid claims-based definitions of NMPO could help promote improved strategies for detecting and decreasing prescription opioid diversion and aid patients reduce NMPO and associated health problems. Our review found: (a) both the conceptual and operational definitions of NMPO are variable; (b) efforts have been made by authors of the studies to validate their definitions of NMPO, although these efforts are also variable, and (c) the variation among these studies likely has resulted in disparate rates of NMPO detected among the study samples drawn from the various claims databases.
Despite a focus in the reviewed articles on NMPO, the studies herein were unable to optimally validate NMPO using ‘gold standard’ measures. For example, while Sullivan et al.31 examined the association between their measure of NMPO and ICD-9 codes for opioid abuse and dependence, these codes include many instances of illicit opioid use (e.g. heroin) in addition to prescription abuse. Braker et al.27 examined the association of their NMPO variable with having six or more opioid prescriptions filled within six months, producing more of a validation of high use rather than misuse. Ideally, researchers could compare measures such as the Prescription Opioid Misuse Index32 or the Current Opioid Misuse Measure33 with the reviewed NMPO indicators to establish their validity. Although such a study is not easily accomplished, a prospective study that recruited patients within health plans for NMPO assessment would be feasible and could yield highly valuable data. Given the high level of heterogeneity of the definitions of NMPO within this review, the varying sources of data, the different analytical approaches used to quantify NMPO, and the lack of a gold-standard comparison, it is not possible to identify which particular definition NMPO should be recommended for general use.
The definitions of NMPO herein more likely approximate some degree of potential opioid medication misuse through patient behaviors and/or use disorder diagnoses. For instance Rice et al. and White et al.10 employed ICD diagnostic codes of opioid abuse, dependence, and overdose among individuals prescribed an opioid medication, which point to psychosocial factors likely at work within these patients that put them into a misuse category. However, a clear limitation is this definition does not account for those individuals with opioid use disorders who justifiably need and receive opioid pain management. Furthermore, the Logan et al.29 definition is likely indicative of problematic prescribing practices. However, given that the authors labeled their definition as including “misuse,” and the fact that they included behaviors such as overlapping opioid prescriptions; their conceptualization and operationalization of NMPO fell within our search parameters and aligned with similar behaviors noted within other studies included herein (e.g., Sullivan et al.31 included >186 days supply of opioid within 6 months).
Other operational definitions rely on behavioral or medication utilization patterns that could be indicative of misuse. Accessing multiple opioid medications from multiple prescribers and pharmacies are important components of designating misuse.34,35 Furthermore, quantities of pills and dosages are also apparent indicators of misuse.36 However, among the authors that employed these parameters, only Sullivan et al.31 combine each of the ideas of multiple medications, prescribers, and pharmacies along with quantity. The combination of this nexus of indicators seems likely to reduce the number of false positive cases of NMPO. However, despite the value for using composite indicators, none of the studies using only health insurance administrative data can uncover whether individuals took the opioids for the purpose of getting “high” or the feelings produced thereby, as is central to definitions of NMPO utilized by SAMHSA or NIDA (Table 1). Furthermore, the addition of electronic health record information to a composite definition of NMPO may enhance the robustness and accuracy of a measure for identifying NMPO among patients.
Given the variety of definitions of NMPO among the studies examined within this review and the varying degrees of validation, rates of NMPO also appear to differ widely between studies. Rates of NMPO also are likely influenced by the populations studied. Specifically, the studies reviewed in this project were from public, private, and combined public and private sources, each sample adding a level of complexity for comparing NMPO rates. One possible solution to the variation in definitions we have presented herein would be for payers, health care systems, and researchers to unify constructs of NMPO under the auspices of organizing frameworks; such as that presented by the Analgesic, Anesthetic, and Addiction Clinical Trials, Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership.37 Such an organizing framework could help delineate and systematize approaches to conceptualizing and operationalizing NMPO. However, the robustness of such approaches is somewhat restricted within the health claims environment based on limitations of data available for creating indicators— thus calling for possible changes in coding schemes and additional research to augment current practice.
Limitations
A primary limitation of this review is the high degree of variation within the individual studies identified, which has impacted our efforts to systematically categorize the definition of NMPO, validation approaches, and rates of NMPO. Indeed, we acknowledge our search terms were intentionally broad in order to capture all studies that examined a conceptualization of NMPO within claims data. As a result, we recognize we are not comparing studies with similar research aims, populations, and methods. However, given that the earliest studies for our current review were from 2009, this appears to indicate that the analysis of health insurance administrative data with the intention of identifying NMPO is new within the field, and therefore, the observed variation among definitions of the NMPO is expected. This limitation, nonetheless, underscores the fact that our review is timely in bringing together existing literature in the field.
A related limitation from our review is that a relatively small number of studies (N=7) were found for analysis. It may be the case that payers or investigators have examined this data, but have not published their findings, introducing publication bias in our review. However, given the extensive and broad areas our selected databases search, we feel that non-peer-reviewed materials or grey literature would likely have appeared in our final results had they met study inclusion criteria. Nevertheless, the number of studies we found presents challenges for categorizing clear patterns in the definitions of NMPO using claims data. However, as was noted, the relatively small number of studies likely is a product of the number of years that studies of NMPO in administrative claims data has been underway in the literature—calling for advancement in this field. We also recognize that identifying NMPO within health care claims data is inherently limited, not having the capacity to capture misuse patterns among individuals who obtain opioid medications without a prescription. Therefore, generalizability of findings from the studies reviewed herein and from our review is limited to populations within health care claims data.
Conclusion
The behaviors that constitute NMPO are unclear. In our review of studies, we found varying definitions of NMPO with limited consistency in conceptualization and operationalization. We found that validation of these definitions was also limited, and estimates of rates of opioid misuse were inconsistent. Future research should build on the current definitions of misuse presented herein and work to prospectively validate a construct of NMPO for dissemination to payers, clinicians, health care system administrators, and public health officials to accurately identify NMPO from claims data in order to better confront this national public health crisis.
Acknowledgments
FUNDING
Drs. Cochran, Donohue, Gordon, and Gellad are supported by CDC/NIDA U01CE002496-01. Dr. Gellad is also supported by a VA HSR&D Career Development Award (09-207). Funders were not involved in study design, the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the manuscript for publication.
Footnotes
AUTHOR CONTRIBUTIONS
Gerald Cochran conceptualized, collaborated in performing the systematic review and analysis, and wrote the largest portions of the article. Bongki Woo collaborated in performing the systematic review and analysis and wrote the methods section of the article. Wei-Hsuan Lo-Ciganic, Adam Gordon, Julie Donohue, and Walid Gellad helped conceptualize and provided critical feedback and revisions on the article.
The authors declare that they have no conflicts of interest.
References
- 1.Centers for Disease Control. Vital Signs: Overdoses of Prescription Opioid Pain Relievers — United States, 1999–2008. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Morbidity and Mortality Weekly Report. [PubMed] [Google Scholar]
- 2.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain physician. 2008;11(2 suppl):S63–88. [PubMed] [Google Scholar]
- 3.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: A ten-year perspective. Pain Physician. 2010;12(5):401–435. [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 5.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(5):747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 6.Hahn KL. Strategies to prevent opioid misuse, abuse, and diversion that may also reduce the associated costs. Am Health Drug Benefits. 2011;4(2):107–113. [PMC free article] [PubMed] [Google Scholar]
- 7.AAFP. FDA approves labeling for reformulated, abuse-deterrent version of oxycontin. Am Fam Physician. 2013;87(10):730. [Google Scholar]
- 8.Huang B, Dawson DA, Stinson FS, et al. Prevalence, correlates, and comorbidity of nonmedical prescription drug use and drug use disorders in the United States: Results of the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67(7):1062–1073. doi: 10.4088/jcp.v67n0708. [DOI] [PubMed] [Google Scholar]
- 9.Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: Psychiatric, medical and substance use correlates. Drug and Alcohol Dependence. 2008;94(1):38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 10.White AG, Birnbaum HG, Schiller M, Tang J, Katz NP. Analytic models to identify patients at risk for prescription opioid abuse. The American Journal Of Managed Care. 2009;15(12):897–906. [PubMed] [Google Scholar]
- 11.Martins SS, Keyes KM, Storr CL, Zhu H, Chilcoat HD. Pathways between nonmedical opioid use/dependence and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug And Alcohol Dependence. 2009;103(1–2):16–24. doi: 10.1016/j.drugalcdep.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novak SP, Herman-Stahl M, Flannery B, Zimmerman M. Physical pain, common psychiatric and substance use disorders, and the non-medical use of prescription analgesics in the United States. Drug And Alcohol Dependence. 2009;100(1–2):63–70. doi: 10.1016/j.drugalcdep.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice JB, White AG, Birnbaum HG, Schiller M, Brown DA, Roland CL. A Model to Identify Patients at Risk for Prescription Opioid Abuse, Dependence, and Misuse. Pain Medicine. 2012;13(9):1162–1173. doi: 10.1111/j.1526-4637.2012.01450.x. [DOI] [PubMed] [Google Scholar]
- 14.Unick GJ, Rosenblum D, Mars S, Ciccarone D. Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993–2009. Plos One. 2013;8(2):e54496–e54496. doi: 10.1371/journal.pone.0054496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan MD, Edlund MJ, Fan M-Y, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amari E, Rehm J, Goldner E, Fischer B. Nonmedical prescription opioid use and mental health and pain comorbidities: a narrative review. Can J Psychiatry. 2011;56(8):495. doi: 10.1177/070674371105600808. [DOI] [PubMed] [Google Scholar]
- 17.Hudson TJ, Edlund MJ, Steffick DE, Tripathi SP, Sullivan MD. Epidemiology of regular prescribed opioid use: results from a national, population-based survey. J Pain Symptom Manage. 2008;36(3):280–288. doi: 10.1016/j.jpainsymman.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, united states, 2010. JAMA. 2013;309(7):657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 19.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 20.Biondo G, Chilcoat HD. Discrepancies in prevalence estimates in two national surveys for nonmedical use of a specific opioid product versus any prescription pain reliever. Drug Alcohol Depend. 2014;134:396–400. doi: 10.1016/j.drugalcdep.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 21.National Institute on Drug Abuse. Prescription Drug Abuse? What is prescription drug abuse. 2011 http://www.drugabuse.gov/publications/research-reports/prescription-drugs/what-prescription-drug-abuse. Accessed April 25, 2014.
- 22.LeBaron P, Dean E. 2012 National Survey on Drug Use and Health: CAI Specifications for Programming. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. [Google Scholar]
- 23.Kessler RC, Abelson J, Demler O, et al. Clinical calibration of DSM-IV diagnoses in the World Mental Health version of the World Health Organization Composite International Diagnostic Interview. Int J Methods Psychiatr Res. 2004;13(2):122–139. doi: 10.1002/mpr.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Geneva: World Health Organization; 2009. [Google Scholar]
- 25.Substance Abuse and Mental Health Services Administration. Overview of Findings from the 2003 National Survey on Drug Use and Health. Rockville, MD: SAMHSA; 2004. (Office of Applied Studies, NSDUH Series H–24, DHHS Publication No. SMA 04-3963). [Google Scholar]
- 26.National Institute on Drug Abuse. Research Report Series, Prescription Drugs: Abuse and Addiction. Bethesda, MD: National Institute on Drug Abuse; 2011. [Google Scholar]
- 27.Braker LS, Reese AE, Card RO, Van Howe RS. Screening for potential prescription opioid misuse in a michigan medicaid population. Family Medicine. 2009;41(10):729. [PubMed] [Google Scholar]
- 28.Leider HL, Dhaliwal J, Davis EJ, Kulakodlu M, Buikema AR. Healthcare costs and nonadherence among chronic opioid users. Am J Manag Care. 2011;17(1):32–40. [PubMed] [Google Scholar]
- 29.Logan J, Liu Y, Paulozzi L, Zhang K, Jones C. Opioid prescribing in emergency departments: the prevalence of potentially inappropriate prescribing and misuse. Med Care. 2013;51(8):646–653. doi: 10.1097/MLR.0b013e318293c2c0. [DOI] [PubMed] [Google Scholar]
- 30.Roland CL, Joshi AV, Mardekian J, Walden SC, Harnett J. Prevalence and cost of diagnosed opioid abuse in a privately insured population in the United States. J Opioid Manag. 2013;9(3):161–175. doi: 10.5055/jom.2013.0158. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan MD, Edlund MJ, Fan M-Y, DeVries A, Braden JB, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and Medicaid insurance plans: The TROUP study. Pain. 2010;150(2):332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knisely JS, Wunsch MJ, Cropsey KL, Campbell ED. Prescription Opioid Misuse Index: A brief questionnaire to assess misuse. J Subst Abuse Treat. 2008;35(4):380–386. doi: 10.1016/j.jsat.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130(1–2):144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Assessing opioid shopping behaviour: a large cohort study from a medication dispensing database in the US. Drug safety: an international journal of medical toxicology and drug experience. 2012;35(4):325–334. doi: 10.2165/11596600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Hall AJ, Logan JE, Toblin RL, et al. PAtterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 36.Baumblatt J, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. HIgh-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Internal Medicine. 2014 doi: 10.1001/jamainternmed.2013.12711. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. PAIN. 2013;154(11):2287–2296. doi: 10.1016/j.pain.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American College of Preventive Medicine. Use, abuse, misuse & disposal of prescription pain medication clinical reference. 2011 http://www.acpm.org/?UseAbuseRxClinRef. Accessed April 4, 2014.
- 39.MedlinePlus. Prescription drug abuse. 2014 http://www.nlm.nih.gov/medlineplus/prescriptiondrugabuse.html. Accessed April 4, 2014.


