Abstract
Multiple large‐scale neural networks orchestrate a wide range of cognitive processes. For example, interoceptive processes related to self‐referential thinking have been linked to the default‐mode network (DMN); whereas exteroceptive processes related to cognitive control have been linked to the executive‐control network (ECN). Although the DMN and ECN have been postulated to exert opposing effects on cognition, it remains unclear how connectivity with these spatially overlapping networks contribute to fluctuations in behavior. While previous work has suggested the medial‐prefrontal cortex (MPFC) is involved in behavioral change following feedback, these observations could be linked to interoceptive processes tied to DMN or exteroceptive processes tied to ECN because MPFC is positioned in both networks. To address this problem, we employed independent component analysis combined with dual‐regression functional connectivity analysis. Participants made a series of financial decisions framed as monetary gains or losses. In some sessions, participants received feedback from a peer observing their choices; in other sessions, feedback was not provided. Following feedback, framing susceptibility—indexed as the increase in gambling behavior in loss frames compared to gain frames—was heightened in some participants and diminished in others. We examined whether these individual differences were linked to differences in connectivity by contrasting sessions containing feedback against those that did not contain feedback. We found two key results. As framing susceptibility increased, the MPFC increased connectivity with DMN; in contrast, temporal‐parietal junction decreased connectivity with the ECN. Our results highlight how functional connectivity patterns with distinct neural networks contribute to idiosyncratic behavioral changes. Hum Brain Mapp 36:2743–2755, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: neuroimaging, behavioral economics, executive function, prefrontal cortex, multivariate analysis
INTRODUCTION
Our behavior is governed by the interaction of multiple brain regions [Park and Friston, 2013]. These interacting brain regions are organized into multiple distinct networks that orchestrate disparate cognitive processes [Smith et al., 2009]. For example, activation within the executive‐control network (ECN) has been generally associated with exteroceptive processes (though not exclusively [Critchley et al., 2004]) relating to cognitive control and goal‐directed attention [Dosenbach et al., 2007; Smith et al., 2009]. In contrast, the default‐mode network (DMN) has been generally linked to interoceptive processes based on its relative deactivation during tasks [Raichle et al., 2001; Shulman et al., 1997; Smith et al., 2009] and involvement in self‐referential processing [Gusnard et al., 2001]. Despite these broad conceptualizations, it remains unclear how DMN and ECN, as commonly considered in the literature [Laird et al., 2011; Smith et al., 2009], contribute to changes in behavior.
A common method for influencing behavior is the use of feedback, which can be perceived as validation for our choices. Indeed, the mere presence of feedback can alter cognitive performance [Pessoa, 2009; Ravizza et al., 2012]. Yet, the influence of feedback on performance is idiosyncratic [e.g., Jimura et al., 2010; Locke and Braver, 2008], aiding some individuals [Graham, 1984; Ravizza et al., 2012; Schonberg et al., 2007] while hindering others [Dweck, 1999; Kamins and Dweck, 1999; O'Brien et al., 2011]. Recent work has suggested that feedback from other individuals modulates activation within the medial‐prefrontal cortex (MPFC) [Somerville et al., 2006; Somerville et al., 2010]. The MPFC has been involved aspects of performance monitoring and feedback‐based adaptation in a variety of studies that include examinations of social feedback [Somerville et al., 2006] and outcome [Knutson et al., 2003] processing to promote behavioral change [Falk et al., 2011]. Unfortunately, parts of MPFC, including the pregenual cingulate and paracingulate, are situated in both the ECN and the DMN (Supporting Information Fig. 1). Thus, focusing on the MPFC without considering its broader role in distinct neural networks would necessarily conflate different functions, potentially those relating to interoceptive and exteroceptive processes, thereby, limiting our understanding of how MPFC contributes to changes in behavior.
We hypothesized that functional connectivity with distinct networks that contain the MPFC—namely ECN and DMN—would reflect an individual's propensity to change behavior based on fictitious feedback. To test this hypothesis, we adopted a paradigm that allowed us to examine how a specific behavioral trait—susceptibility to a framing manipulation [De Martino et al., 2006]—changes according to the presence or absence of feedback provided by a peer (Fig. 1). Although the presence of a peer creates a social context [Chein et al., 2011; Fareri and Delgado, 2014; Fareri et al., 2012; Sip et al., 2015], which may augment the salience of the feedback manipulation, we note that our core hypothesis is centered on the concept of feedback more generally and not necessarily the source (e.g., social or nonsocial) or type (e.g., positive or negative) of feedback. Indeed, the main goal was to quantify how the presence or absence of feedback influenced functional connectivity with ECN and DMN using independent component analysis (ICA) combined with dual‐regression analysis. Crucially, this approach dissociates responses from overlapping networks [Utevsky et al., 2014] while isolating the distributed computations that contribute to individual differences in behavior [Smith et al., 2014b]. Our analyses focused on two key questions. First, are fluctuations in framing susceptibility linked to changes in connectivity with ECN and DMN? Second, does MPFC connectivity with ECN or DMN increase in the presence of feedback?
Figure 1.
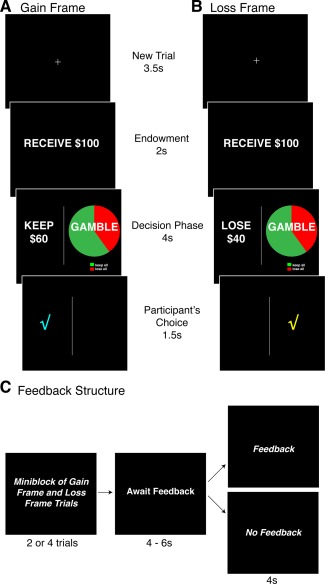
Experimental task. Participants engaged in a financial decision‐making task that has been previously used to study framing susceptibility. On each trial, participants were presented with a monetary endowment ($50 or $100) before choosing between sure and gamble options. The sure option was framed such that the participant could keep (Gain Frame; see panel A) or lose (Loss Frame; see panel B) a fixed proportion of the endowment. Notably, the Gain Frame and Loss Frames were mathematically identical (e.g., keeping $60 of $100 is the same as losing $40 of $100). The gamble option did not differ according to frame and was represented by a pie chart reflecting the probability (20%, 40%, 60%, and 80%) of winning or losing the entire endowment (with expected value matched to the sure option). In half of the sessions, participants received fictitious feedback from another person seated outside the scanner; remaining sessions utilized a similar trial structure but did not provide any feedback (see panel C). This design allowed us to examine fluctuations in behavior that arise from the presence of feedback. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
MATERIALS AND METHODS
Participants
We recruited 31 individuals (16 females) for the study (median age: 19 years, range: 18–32 years). Our prescreening process excluded individuals with a history of psychiatric or neurological illness. Prior to analysis, we also excluded four additional participants for excessive head motion (see Supplemental Methods), leaving a final sample of 27 participants (14 females; median age: 19 years). All participants provided written informed consent as part of a protocol approved by the Institutional Review Board of Rutgers University.
Framing Task
To study how changes in connectivity with spatially overlapping functional networks contribute to behavior, we reanalyzed a previous dataset from our laboratory [Sip et al., 2015]. Although there is no overlap in the goals or approaches of the original paper and the current investigation, we note that the paradigm afforded a unique opportunity to examine how distinct neural networks are associated with changes in a specific behavioral variable. In particular, participants performed a financial decision‐making task that allowed us to quantify individual differences in susceptibility to a framing manipulation.
During the imaging session, participants completed four 50‐trial runs of a financial decision‐making task that has been previously used to study framing effects [De Martino et al., 2006] (Fig. 1). On each trial, participants were shown an endowment ($50 or $100) before choosing between “sure” and “gamble” options. The sure option was framed such that the participant could keep (Gain Frame; Fig. 1A) or lose (Loss Frame; Fig. 1B) a fixed proportion of the endowment. The gamble option did not differ according to frame and was represented by a pie chart reflecting the probability (20%, 40%, 60%, and 80%) of winning (green portion) or losing (red portion) the entire endowment (with expected value matched to the sure option). Notably, the Gain Frame and Loss Frame were mathematically identical (e.g., keeping $60 of $100 is equivalent to losing $40 of $100); therefore, the likelihood of choosing the sure option or the gamble option should be identical in a perfectly rational participant (We note that the term “rational” here simply denotes consistency of choice). We quantified the susceptibility to the framing manipulation (i.e., the magnitude of the framing effect) as the signed difference between the proportion of gamble choices in the Loss Frame compared to the Gain Frame. Although most participants gamble more in the Loss Frame compared to the Gain Frame, using the signed difference between frames is critical for distinguishing the effects of feedback. Thus, a small framing effect indicates similar gambling behavior in both frames (i.e., low susceptibility to the framing manipulation) while a large framing effect indicates greater gambling behavior in the Loss Frame relative to the Gain Frame (i.e., high susceptibility to the framing manipulation). Importantly, the framing effect magnitude controls for overall changes in risk‐sensitive behavior and allows our analyses to isolate the effects of feedback on the susceptibility to the framing manipulation. Of course, we note that changes in framing susceptibility could arise from decreased risk‐sensitive behavior in the Gain Frame and/or increased risk‐sensitive behavior in the Loss Frame.
We coded the framing task using E‐prime 2.0 (Psychology Software Tools, Sharpsburg, PA). Stimuli were projected onto a screen at the back of the scanner bore and were viewed by the participants through mirrored glasses. Responses were recorded using a MRI‐compatible keypad. All participants received $65 for completing the study.
Procedure for Feedback Manipulation
The framing task allowed us to identify a specific behavioral variable quantifying susceptibility to the framing effect. Yet, our key goal was to examine whether changes in this behavior—susceptibility to the framing effect—were associated with distinct functional connectivity patterns. To do this, participants performed separate sessions of the framing task. In one session, they received feedback from a peer seated outside the scanner (see below); and in the other session, they did not receive feedback. On the day preceding the imaging session, participants were asked to come to the laboratory with a close friend of the same gender (neither a romantic partner nor a family member). Each friendship dyad completed the Inclusion of Other in Self scale [Aron et al., 1992], which provided an estimate of closeness between the two individuals. In addition, each dyad provided examples of five positive and five negative comments that they would normally offer to each other when engaging in shared activities where feedback is provided (e.g., playing video games) which were then used as feedback during the feedback session (interleaved runs with order counterbalanced across participants). Importantly, the feedback was the only source of knowledge the participants had available about their performance. While the feedback itself was not related to actual performance, the participants believed that it was performance‐based feedback meant to guide their decisions. This was confirmed by a debriefing questionnaire at the end of the experiment and a subjective measure suggesting that a majority of participants viewed the feedback as helpful (23 out of 27 participants).
Participants received randomly selected feedbacks from their friend in each feedback session (total 32 feedback through entire experiment). In the no feedback session, the words “No Feedback Provided” were used instead of giving explicit feedback. Thus, there were two conditions—feedback and no feedback—delivered in independent sessions [for further details on the experimental setup, please see Sip et al., 2015]. Crucially, we note that the peer was present throughout the entire experiment, thereby, reducing the likelihood that feedback and no feedback sessions differed due to social context.
Neuroimaging Data Acquisition
We collected neuroimaging data using a 3 Tesla Siemens MAGNETOM Trio scanner (equipped with 12 channels) at the Rutgers University Brain Imaging Center. Functional images sensitive to blood‐oxygenation‐level‐dependent contrast were acquired using a single‐shot T2*‐weighted echo‐planar imaging sequence with slices parallel to the axial plane [repetition time (TR): 2000 ms; echo time (TE): 30 ms; matrix 64 × 64; field of view (FOV): 192 mm; voxel size: 3.0 mm3; 32 slices; flip angle: 90°]. The first four volumes of each functional run were removed to allow for magnetic stabilization. To facilitate coregistration and normalization of functional images, we also collected high‐resolution anatomical scans covering the whole brain (TR: 1900 ms; TE: 2.52 ms; matrix 256 × 256; FOV: 256 mm; voxel size: 1.0 mm3; 176 slices; flip angle: 9°).
FMRI Preprocessing
Our preprocessing procedure utilized tools from the FMRIB Software Library (FSL Version 5.0.2; http://www.fmrib.ox.ac.uk/fsl/) package [Smith et al., 2004]. We first corrected for head motion by realigning the time series to the middle volume. Next, we corrected for intravolume slice‐timing differences using Fourier‐space phase shifting, aligning to the middle slice. Spatial smoothing employed a Gaussian isotropic kernel of full‐width‐half‐maximum 5 mm. The entire 4D dataset was then grand‐mean intensity normalized using a single multiplicative factor. To remove low frequency drift in the MR signal, we used a high‐pass temporal filter with a 100‐second cutoff. Finally, we spatially normalized our imaging data to the Montreal Neurological Institute (MNI) avg152 T1‐weighted template (4 mm isotropic resolution) using a 12‐parameter affine transformation implemented in FMRIB's Linear Image Registration Tool [Jenkinson and Smith, 2001]. Affine transformations were subsequently optimized with a nonlinear registration algorithm implemented in FSL. Our preprocessing also identified outlier volumes with excessive displacements [Power et al., 2015] and four participants with excessive head motion (see Supporting Information Methods).
FMRI Analyses
Our analytic procedures can be distilled into the following steps: (1) obtain networks using ICA; (2) identify networks matching DMN and ECN reported in prior work [Smith et al., 2009]; (3) quantify voxelwise connectivity with each network for Feedback and No Feedback conditions; (4) contrast connectivity maps between each condition for each network and identify regions in which the difference in connectivity is associated with the difference in framing susceptibility. These procedures are fleshed out in detail below and summarized in Figure 2.
Figure 2.
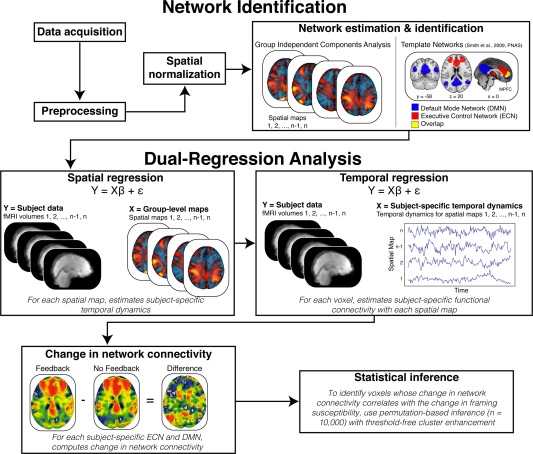
High‐level schematic of analytic approach. Our primary analyses proceeded in multiple steps. Data were first preprocessed and then spatially transformed into standard (MNI) space. Next, data from all participants and conditions were concatenated across time and submitted to a group independent component analysis (ICA). The group ICA produced a set of 25 spatial maps that were compared (using spatial correlation) with maps representing executive control network (ECN; red) and default mode network (DMN; blue) in prior work [Smith et al., 2009]. Notably, ECN and DMN overlapped in the medial prefrontal cortex (MPFC; yellow; maps thresholded at Z > 4). All maps were then entered into the dual‐regression analysis, which quantified, within each subject, each voxel's functional connectivity with each spatial map while controlling for the influence of other, potentially confounding, spatial maps. These functional connectivity measures were contrasted across feedback and no feedback sessions. Crucially, this procedure allowed us to isolate our key variable of interest—i.e., the presence of feedback—while formulating a between‐sessions psychophysiological interaction analysis that examines how connectivity changes according to the presence of feedback. The resulting connectivity maps were then subjected to permutation‐based statistical testing to evaluate whether differences in connectivity (Feedback minus No Feedback) reflect changes in framing susceptibility. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We used FSL's Multivariate Exploratory Linear Decomposition into Independent Components Version 3.10 [Beckmann and Smith, 2004] to identify 25 large‐scale neural networks (see Supporting Information Methods). To evaluate individual differences in connectivity with networks identified by the ICA, we employed a dual‐regression analytical approach [Filippini et al., 2009; Leech et al., 2011; Murty et al., 2014; Utevsky et al., 2014]. Dual‐regression analysis proceeds in two separate stages (Fig. 2). In a first spatial‐regression step, spatial maps are regressed onto each participant's functional data, resulting in a T (time points) × C (components) set of beta coefficients that characterize, in each participant and session, the temporal dynamics for each spatial map. Then, in the second temporal‐regression step, the resulting temporal dynamics that describe each network, in each participant and session, are regressed onto each participant's functional data. This step produces a set of spatial maps that quantify, within each participant and session, each voxel's connectivity with each map identified with the group ICA. Importantly, the temporal‐regression step estimates each voxel's connectivity with each spatial network while controlling for the influence of other networks—some of which may reflect artifacts, such as physiological noise and head motion. As an additional control for head motion, our temporal‐regression step also included six parameters describing motion (rotations and translations along the three principal axes) and volumes identified as outliers (see Supporting Information Methods). Removing outlier volumes via linear regression accounts for the nonlinear effects of motion (e.g., signal spikes, spin history effects, etc.) that cannot be described by motion parameters alone [Lemieux et al., 2007; Satterthwaite et al., 2013].
To identify maps in our ICA that correspond to the DMN and ECN reported in Smith et al. [2009], we conducted a spatial correlation analysis. A spatial correlation analysis quantifies the degree to which the values of one set of spatial points are correlated with the values of another set of spatial points; this approach is identical to a conventional correlation analysis (i.e., Pearson's R statistic) and has been employed in previous work [Smith et al., 2009]. We selected the components that best matched the DMN and ECN reported in Smith et al. [2009] [ECN: r max = 0.58 (other components: r mean = 0.02; r SD = 0.13); DMN: r max = 0.79 (other components: r mean = 0.01; r SD = 0.07)]. Other components in our dataset corresponded to other networks identified by Smith et al. [2009]; for example, see Supporting Information Table 1.
Using participant‐specific and condition‐specific connectivity maps corresponding to DMN and ECN, we then computed difference images between feedback conditions (i.e., Feedback minus No Feedback). Examining the change in connectivity (i.e., the difference) between sessions is considered a between‐sessions psychophysiological interaction (PPI) analysis [Friston, 2011; O'Reilly et al., 2012]. Unlike conventional PPI analysis where the key psychological variable of interest is often imbedded within a single session [Friston et al., 1997], our psychological variable of interest (i.e., the presence of feedback) differs across sessions and hence a simple subtraction is sufficient to isolate the change in connectivity. Thus, the between‐sessions PPI procedure allowed us to quantify how the presence of feedback influences connectivity with the DMN and ECN. Next, we constructed a group‐level general linear model to estimate whether fluctuations in framing susceptibility (i.e., Feedback Framing Effect minus No Feedback Framing Effect) correlated with changes in connectivity for each network. Our model also included covariates to control for gender [Smith et al., 2014b] and individual differences in average head motion between feedback sessions (i.e., difference in the proportion of outlier volumes).
Statistical significance of connectivity maps was assessed in a nonparametric fashion, using Monte Carlo permutation‐based statistical testing with 10,000 permutations [Nichols and Holmes, 2002]. We used an alpha of 0.05, which was corrected for multiple voxelwise comparisons across the whole brain and for multiple network comparisons across the ECN and DMN [Utevsky et al., 2014]. We estimated clusters of activation using threshold‐free cluster enhancement [Smith and Nichols, 2009], which retains a fundamentally voxel‐wise inference. Significant results are displayed using MRIcroGL (http://www.mccauslandcenter.sc.edu/mricrogl/). Finally, to evaluate the uncertainty associated with our results, we bootstrapped the effect sizes (N = 10,000) and identified the 99.9% confidence interval. These intervals are useful for depicting the likely magnitude of the true effect, which is potentially small, given the imperfect reliability of neural and behavioral measures [Kriegeskorte et al., 2010; Vul et al., 2009; Yarkoni, 2009].
RESULTS
Loss Framing Promotes Risky Decision Making
Our behavioral analyses focused on two key issues. First, we examined the influence of decision frame on risky choice. Several studies have shown that people take more risks when an outcome is framed as a potential loss [Tversky and Kahneman, 1981]. Consistent with this observation, we found that participants gambled more in the loss frame (M = 50%, SE = 3.7%) compared to the gain frame (M = 35%, SE = 2.9%; t (26) = 5.09, P < 0.001), indicating a robust framing effect across participants, regardless of whether feedback was present or absent (Fig. 3A).
Figure 3.
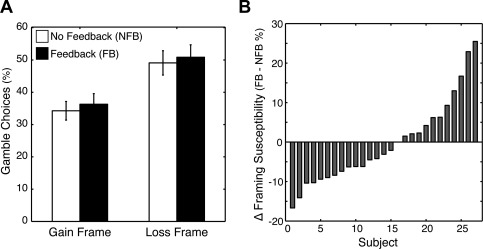
Behavioral effects of frame and feedback. (A) In both the Feedback (FB) and No Feedback (NFB) sessions, subjects gambled more when the decision was framed as a potential loss compared to a potential gain. The magnitude of this framing effect was similar across FB and NFB sessions. (B) Yet, we observed idiosyncratic changes in framing susceptibility (i.e., the magnitude of the framing effect in a subject) in the presence of feedback. In particular, we found that some subjects were less susceptible to the framing manipulation during the FB session compared to the NFB session. In contrast, other subjects were more susceptible to the framing manipulation during the FB session compared to the NFB session.
Second, given that psychologists have long recognized how feedback can shape subsequent behavior [Thorndike, 1898], we evaluated whether the framing effect was modulated by the presence of feedback. Although we did not find a main effect of feedback on framing susceptibility [Feedback (FB): M = 14.5%, SE = 3.1%; No Feedback (NFB): M = 14.8%, SE = 3.0%; t (26) = 0.15, P = 0.88], participants exhibited considerable individual differences in their framing susceptibility following feedback. In particular, framing susceptibility was heightened in some participants and diminished in others (FB minus NFB: range = −16.7:25.5%, SD = 10.6%). In a post hoc analysis, we found that the changes in framing susceptibility were trending toward a non‐Gaussian distribution (Jarque‐Bera test = 3.249, P = 0.07), suggesting that the observed changes are meaningful and not simply random fluctuations in behavior. Nevertheless, we note that such variability in behavior—whether random or induced by feedback—may have roots in the fluctuations of large‐scale neural networks [Fox et al., 2007].
Functional Connectivity Reflects Feedback Effects on Framing Susceptibility
Our behavioral results indicated that feedback had a wide range of effects on subsequent framing susceptibility, with some individuals becoming more susceptible and others individuals becoming less susceptible (Fig. 3B). We predicted that the idiosyncratic influence of feedback on subsequent behavior would be rooted, in part, in changes in functional connectivity with MPFC—a region whose activation increases in the presence of feedback [Somerville et al., 2006]. Unfortunately, MPFC sits at the intersection of two networks (see Supporting Information Fig. 1) that are hypothesized to have opposing effects on cognition [Fox et al., 2005; Greicius et al., 2003]. Activation within the executive control network (ECN) has been postulated to reflect exteroceptive processes related to cognitive control and goal‐directed attention [Dosenbach et al., 2007]. In contrast, the default mode network (DMN) has been linked to interoceptive processes due to its deactivation during cognitive tasks [Raichle et al., 2001; Shulman et al., 1997] and involvement in self‐referential processing [Gusnard et al., 2001]. Although interoceptive and exteroceptive processes may not be uniquely associated with DMN and ECN respectively [Critchley et al., 2004], it is clear that changes in connectivity with MPFC could reflect processes related to either network.
To address this problem, we used ICA to separate multiple overlapping spatial networks, including ECN and DMN. These networks were identified in our ICA output using a spatial correlation with resting‐state networks found in prior work [Smith et al., 2009]. Two of our ICA maps bared striking similarity to resting‐state networks associated with DMN (Fig. 4A; R max = 0.79) and ECN (Fig. 4B; R max = 0.58); in addition, our other non‐noise ICA maps resembled those identified in previous work (Supporting Information Table 1). We then submitted the time courses of DMN and ECN to a general linear model to estimate task‐dependent network responses (see Supporting Information Methods). We found that the decision phase of our task evoked significant activation within the ECN (NFB: t (26) = 2.0, P = 0.028; FB: t (26) = 1.78, P = 0.043) and deactivation within the DMN (NFB: t (26) = −10.94, P < 0.001; FB: t (26) = −8.16, P < 0.001). We also found that feedback (relative to no feedback) evoked increased activation within the DMN (t (26) = 3.61, P = 0.0013; Fig. 4C), which is consistent with prior work noting increased DMN responses during tasks requiring social and emotional processing [Mars et al., 2012a]. Next, these networks—and others derived from the ICA—were submitted to a dual‐regression analysis to examine whether changes in functional connectivity (as a function of the presence or absence of feedback) relate to subsequent changes in framing susceptibility. Our analysis revealed two key results. We found that increased connectivity between DMN and MPFC (MNIx,y,z = −10, 38, 12) during runs containing feedback reflected increased susceptibility to the framing manipulation (i.e., larger framing effect; R = 0.66, CI99.9% = 0.19 to 0.89) (Fig. 5). In contrast, decreased connectivity between ECN and temporal‐parietal juncture (TPJ) (MNIx,y,z = −30, −50, 32) during runs containing feedback was associated with increased susceptibility to the framing manipulation (i.e., larger framing effect; R = −0.81, CI99.9% = −0.94 to −0.30) (Fig. 5). Notably, these brain‐behavior relationships were not driven by the inclusion of confound regressors in our group‐level model (Fig. 5B); and we observed similar results when we removed the covariates for gender and head motion (DMN: P < 0.05, corrected; ECN: P < 0.001, uncorrected).
Figure 4.
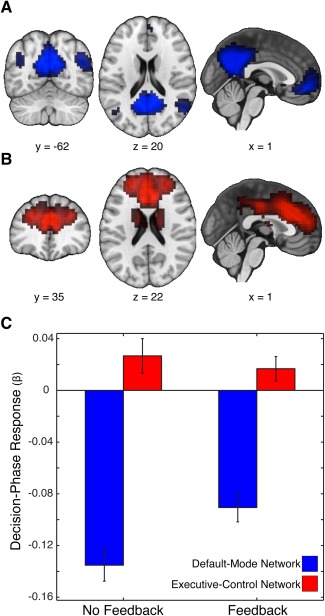
Networks identified by independent component analyses. We identified large‐scale neural networks using independent component analysis (ICA). This analysis resulted in 25 spatial maps, some reflecting artifacts (e.g., head motion) and others reflecting well‐characterized sensory and cognitive networks identified in prior work [e.g., Smith et al., 2009]. Our dual regression analyses focused on two networks identified in the ICA. (A) The default mode network (DMN) consisted of lateral parietal regions, posterior cingulate, and portions of the medial prefrontal cortex (MPFC). (B) The executive control network (ECN) consisted of the striatum, the anterior cingulate, and portions of the MPFC. For visualization purposes, maps are thresholded at Z > 4. (C) We also examined how the decision phase of the task modulates responses within each network. Consistent with prior work, we found that both networks were sensitive to the decision phase, with increased activation of the ECN and decreased activation of the DMN. The magnitude of activation in the DMN was greater in the feedback session, indicating that feedback modulates responses within DMN. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 5.
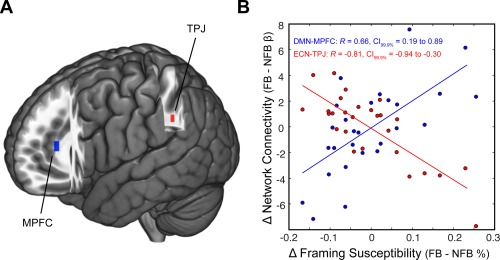
Fluctuations in functional connectivity reflect changes in framing susceptibility. We employed a between‐session psychophysiological interaction analysis to examine whether changes in functional connectivity with DMN and ECN correlated with changes in framing susceptibility. (A) Our analysis identified two regions—temporal‐parietal junction (TPJ) and medial‐prefrontal cortex (MPFC)—whose functional connectivity with DMN (blue) and ECN (red) changed as a function of the presence of feedback and changes in framing susceptibility. (B) In sessions containing feedback (relative to no feedback), increased connectivity between the DMN and MPFC was associated with increased framing susceptibility. In contrast, increased connectivity between the ECN and TPJ correlated with decreased framing susceptibility. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In a series of control analyses, we also examined whether our key results could be explained by other factors. We reasoned that idiosyncratic features within each dyad—particularly the dyad's social closeness [e.g., Fareri et al., 2012] and the perceived helpfulness of feedback—might influence the resulting behavioral change in framing susceptibility. We tested these alternative explanations using a rank‐order correlation. This analysis failed to reveal an association between the change in framing susceptibility and the social closeness within each dyad (ρ = −0.07, P = 0.73) or perceived helpfulness of feedback (ρ = −0.15, P = 0.46). Likewise, the absolute change in behavior was uncorrelated with social closeness (ρ = −0.05, P = 0.78) and perceived helpfulness of feedback (ρ = 0.03 P = 0.87). In addition, although we carefully controlled for head motion, we also checked whether individual differences in head motion contributed to our results—an important consideration given recent observations linking spurious functional connectivity results to head motion [Power et al., 2012]. Our analysis revealed that there was no association between the change in framing susceptibility and individual differences in average volume‐to‐volume head motion (r (25) = 0.12, P = 0.54). Taken together, these observations indicate that our reported changes in connectivity with DMN and ECN are linked to changes in framing susceptibility and not other factors measured within the constraints of our experiment.
Although our results clarify how changes in functional connectivity with ECN and DMN contribute to changes in behavior, other work has shown that functional connectivity between ECN and DMN may also be worth considering [Cole et al., 2010]. In a post hoc analysis, we therefore examined whether interactions between ECN and DMN contribute to feedback and changes in behavior using procedures established in prior work [Cole et al., 2010; Young et al., 2015]. Specifically, we performed a partial correlation analysis using the network time courses and motion confounds in each subject during sessions containing feedback and sessions containing no feedback. Our results indicated that the presence of feedback did not impact inter‐network connectivity between DMN and ECN (t (26) = −0.0636, P = 0.95). In addition, changes in inter‐network connectivity were unpredictive of changes in framing susceptibility (r (25) = 0.1817, P = 0.3643). These observations, though inconclusive due to limitations in statistical power, imply that inter‐network connectivity has less impact on behavior compared with functional connectivity with ECN and DMN.
DISCUSSION
A host of recent studies have highlighted the importance of considering how the interaction of multiple brain regions shapes behavior [Park and Friston, 2013]. These studies have led to the specific hypothesis that the brain is organized into a series of overlapping networks across multiple task states [Smith et al., 2009]. Yet, understanding the behavioral significance these networks has been challenging [Laird et al., 2011]. Here, we examined how two distinct networks—the DMN and the ECN—contribute to behavioral fluctuations following feedback from a peer.
The mere presence of feedback can influence cognitive performance [Ravizza et al., 2012], but can also lead to individual differences in performance based on how the feedback is interpreted [Dweck, 1999]. In this study, we investigated whether the idiosyncratic effects of feedback on decision making in particular would be reflected in the functional connectivity patterns with distinct neural networks that have opposing influences on cognition, namely the ECN and DMN. We adapted a gain/loss framing paradigm [De Martino et al., 2006], where participants chose to be conservative or risky with respect to an endowed amount of money. Consistent with prior observations [Tversky and Kahneman, 1981], participants tended to be riskier when a decision was framed as a loss rather than a gain. Critically, in half of the trials (split across runs), participants received occasional feedback from a peer observing outside the scanner.
The presence of feedback elicited a range of individual differences in participant's susceptibility to the framing effect, with some showing increased framing susceptibility while others demonstrating reduced framing susceptibility. These individual differences were reflected in changes in functional connectivity with the MPFC—a region involved in integrating external information such as feedback [Somerville et al., 2006] to influence future decisions [Boorman et al., 2013]. Specifically, increased susceptibility to the framing effect following feedback (relative to no feedback) was associated with both stronger connectivity between DMN and MPFC, and weaker connectivity between ECN and TPJ. Taken together, our results suggest that the presence of feedback can lead to individual differences in the expression of framing susceptibility and such effects of behavior may be due to the MPFC mediating between exteroceptive and interoceptive attentional processes.
The multifaceted MPFC has been involved in a variety of processes related to decision making [Rushworth et al., 2004], weighing costs and benefits of available stimuli [Cunningham and Zelazo, 2007], including personal goals [D'Argembeau et al., 2010] and social feedback from others [Somerville et al., 2006], to promote behavioral change [Falk et al., 2011]. In our experiment, participants received occasional feedback from peers sitting outside the scanner, which yielded individual differences in how one approached decisions framed as either gains or losses. Although the feedback in our task was presented randomly and was unrelated to any particular decision, it was tailored to the specific participant in the scanner. That is, each instance of feedback was one that the participant and the peer outside the scanner frequently used during social interactions. The personal nature of these messages could have been persuasive enough at an implicit level, even if the participants chose to disregard it. Indeed, self‐tailored messages have been found to recruit MPFC activity more so than generic ones [Chua et al., 2009], promoting adaptive changes in long‐term behaviors like use of sunscreen [Falk et al., 2010] and smoking [Falk et al., 2011]. Interestingly, in these studies, activation of MPFC correlated with changes in behavior but not self‐reported attitudes towards performing the behavior itself. In accordance with these findings, we also do not observe any changes in decision making based on the perceived helpfulness of the feedback. Rather, behavioral change is attributed to functional connectivity with networks that contain the MPFC, further highlighting an important role for the MPFC in integrating external information such as the presence of feedback to promote behavioral change.
While previous work has suggested that MPFC responds differently to evaluative feedback depending on self‐esteem [Somerville et al., 2010], our findings highlight the importance of considering such responses in the context of connectivity with the executive control network (ECN) and the default mode network (DMN). We found that both networks contributed to reductions in framing susceptibility through distinct functional connectivity patterns: in runs containing feedback compared to runs without feedback, DMN increased connectivity with MPFC while ECN decreased connectivity with the TPJ. Although the MPFC and TPJ are classically associated with the DMN [Buckner et al., 2008], our findings suggest that TPJ may participate in other networks depending on task demands [Utevsky et al., 2014]. Within the context of our paradigm, changes in connectivity with TPJ could reflect attentional and social functions because receiving feedback from a peer involves social interaction [Carter et al., 2012] and may be inherently salient, thus modulating attention [Kahnt and Tobler, 2013]. These functions need not be mutual exclusive, as TPJ may perform multiple functional roles, depending on the pattern of responses within TPJ [Woo et al., 2014] and/or its connectivity with other regions [Carter and Huettel, 2013; Mars et al., 2012b; Nelson et al., 2012; Smith et al., 2014a].
Unlike previous work, we employed a relatively novel approach—independent components analysis (ICA) combined with dual‐regression analysis—that allowed us to investigate how individual differences in one's ability to integrate external feedback into the decision process are governed by spatially overlapping networks containing the MPFC. Using this approach can be valuable in studies of functional connectivity. In addition to resolving spatially overlapping networks such as the ECN and DMN, ICA combined with dual regression can help account for artifacts that manifest as other components [Salimi‐Khorshidi et al., 2014]. These artifact components could be related to head motion, which has dramatic effects on estimates of functional connectivity [Power et al., 2015]. Removing noise components would not be possible if the ICA stage were bypassed by limiting the dual‐regression analysis to networks of interest [e.g., those found in Smith et al., 2009]. We note that ICA combined with dual‐regression is not restricted to ECN and DMN; other work has focused on alternative networks that are commonly found in studies employing ICA, including the frontoparietal networks [Leech et al., 2011; Smith et al., 2014b; Utevsky et al., 2014]. Nevertheless, one practical issue to consider with this approach is the extent to which a region of interest participates in one or more networks, potentially at different timescales [Smith et al., 2012]. Future work will therefore need to refine this general analytical approach and broaden its applicability.
We note that our results are accompanied by three caveats that merit further consideration. First, our results—like those reported elsewhere [Leech et al., 2011]—are derived from a between‐sessions PPI analysis and thus do not explicitly consider task‐specific changes in connectivity [Friston et al., 1997; O'Reilly et al., 2012]. In other words, changes in connectivity could, in part, be influenced by changes in responses to specific phases of the task (e.g., the decision phase and/or the receipt of feedback). Indeed, this observation is particularly relevant for DMN, which exhibited greater decision‐phase responses in sessions containing feedback. Although some studies have attempted to regress out task specific effects [He et al., 2007], it remains unclear whether such approaches are accurate, given the uncertainty in the hemodynamic response function [Woolrich et al., 2004], or whether such approaches would alter state‐dependent change in connectivity [Utevsky et al., 2014]. In any case, future studies could build on our findings by interspersing the presence or absence of feedback within the same imaging session. Although this approach would create an opportunity to examine task‐specific changes in connectivity, it may also conflate the psychological distinction between decisions for which the participant utilized external feedback and decisions for which feedback was unavailable. Given these potential problems, we therefore believe that our between‐sessions PPI approach is optimal for investigating feedback‐dependent connectivity within our design.
Second, the behavioral changes observed in our task could be related to factors that are unrelated to feedback. Although we controlled for this possibility by ruling out confounding factors measured within the constraints of our experiment, it remains conceivable that the fluctuations in behavior are due to unmeasured personality factors [Aminoff et al., 2012] or variation in brain structure [Hermundstad et al., 2013]. Alternatively, fluctuations in behavior could be random, potentially reflecting the possibility that feedback was viewed as a distracting external influence. Whether the fluctuations in framing susceptibility are due to feedback or other factors, understanding how large‐scale networks contribute to such variations in behavior has been longstanding goal in neuroscience [Fox et al., 2007].
Third, the feedback delivered in our task was linked to a social context: the participant believed that their peer was outside of the scanner providing feedback on their choices. This design choice, while enhancing the realism of the feedback, could introduce a social context whose effects may interact with feedback. However, we note that this potential social effect is partially mitigated by the fact that the peer was present throughout both sessions. Nevertheless, future work could build on our paradigm by incorporating a nonsocial control, such as a computer [Carter et al., 2012; Kätsyri et al., 2013], or parametrically manipulating social elements within the task [Fareri and Delgado, 2014; Fareri et al., 2012; Smith et al., 2014a; Utevsky and Platt, 2014]. In addition to examining the source of feedback (e.g., social vs. nonsocial), extensions of our work could also examine the type of feedback (e.g., positive vs. negative) presented. These extensions could clarify how both the source and type of feedback—which may involve various attentional, social, and affective functions [Roy et al., 2012]—contribute to changes in behavior and connectivity with DMN and ECN.
CONCLUSION
In sum, our findings highlight how distinct neural networks contribute to fluctuations in behavior. Specifically, we observed that the presence of feedback led to a wide range of individual differences in framing susceptibility. These changes are not explained by factors such as the perceived helpfulness of the feedback or closeness between the participants and their friend, but rather by changes in functional connectivity with the DMN and ECN—distinct neural networks containing the MPFC. Irrespective of whether these changes are adaptive [e.g., promoting healthier habits, Falk et al., 2010, 2011] or maladaptive [e.g., increasing risky behaviors, Chein et al., 2011; O'Brien et al., 2011], our results further underscore the importance of understanding how distinct neural networks contribute to individual differences in behavioral fluctuations.
AUTHOR CONTRIBUTIONS
D.V.S. conceived the research questions; K.E.S. and M.R.D. designed experimental task. K.E.S. collected data; D.V.S. analyzed data; D.V.S. and M.R.D. wrote the paper.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors are grateful for help from Ana Rigney, who assisted with data collection, and Kohitij Kar, who assisted with task programming and behavioral analyses. The authors also thank Amanda Utevsky for helpful comments on previous drafts of the manuscript.
REFERENCES
- Aminoff EM, Clewett D, Freeman S, Frithsen A, Tipper C, Johnson A, Grafton ST, Miller MB (2012): Individual differences in shifting decision criterion: A recognition memory study. Memory Cognit 40:1016–1030. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN, Smollan, D (1992): Inclusion of other in the self scale and the structure of interpersonal closeness. J Personality Social Psychol 63:596. [Google Scholar]
- Beckmann CF, Smith SM (2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23:137–152. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Rushworth MF, Behrens TE (2013): Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi‐alternative choice. J Neurosci 33, 2242–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Carter RM, Bowling DL, Reeck C, Huettel SA (2012): A distinct role of the temporal‐parietal junction in predicting socially guided decisions. Science 337:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Huettel SA (2013): A nexus model of the temporal‐parietal junction. Trends Cogn Sci 17:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L (2011): Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev Sci 14:F1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Liberzon I, Welsh RC, Strecher VJ (2009): Neural correlates of message tailoring and self‐relatedness in smoking cessation programming. Biol Psychiatry 65:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD (2010): Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage 52:590–599. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD (2007): Attitudes and evaluations: A social cognitive neuroscience perspective. Trends Cogn Sci 11:97–104. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Feyers D, Maquet P, Salmon E (2010): The neural basis of personal goal processing when envisioning future events. J Cogn Neurosci 22:1701–1713. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ (2006): Frames, biases, and rational decision‐making in the human brain. Science 313:684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck CS (1999): Self‐Theories: Their Role in Motivation, Personality, and Development. New York: Psychology Press. [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD (2010): Predicting persuasion‐induced behavior change from the brain. J Neurosci 30:8421–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Whalen D, Lieberman MD (2011): Neural activity during health messaging predicts reductions in smoking above and beyond self‐report. Health Psychol 30:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Delgado MR (2014): Differential reward responses during competition against in‐ and out‐of‐network others. Social Cogn Affective Neurosci 9:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR (2012): Social network modulation of reward‐related signals. J Neurosci 32:9045–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009): Distinct patterns of brain activity in young carriers of the Apoe‐Epsilon4 allele. Proc Natl Acad Sci USA 106, 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci 102:9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME (2007): Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56:171–184. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2011): Functional and effective connectivity: A review. Brain Connect 1:13–36. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Graham S (1984): Communicating sympathy and anger to black and white children: The cognitive (attributional) consequences of affective cues. J Personality Social Psychol 47:40. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M (2007): Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53:905–918. [DOI] [PubMed] [Google Scholar]
- Hermundstad AM, Bassett DS, Brown KS, Aminoff EM, Clewett D, Freeman S, Frithsen A, Johnson A, Tipper CM, Miller MB, Grafton ST, Carlson JM (2013): Structural foundations of resting‐state and task‐based functional connectivity in the human brain. Proc Natl Acad Sci USA 110:6169–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jimura K, Locke HS, Braver TS (2010): Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc Natl Acad Sci USA 107:8871–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Tobler PN (2013): Salience signals in the right temporoparietal junction facilitate value‐based decisions. J Neurosci 33:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamins ML, Dweck CS (1999): Person versus process praise and criticism: implications for contingent self‐worth and coping. Dev Psychol 35:835–847. [DOI] [PubMed] [Google Scholar]
- Kätsyri J, Hari R, Ravaja N, Nummenmaa L (2013): The opponent matters: Elevated fMRI reward responses to winning against a human versus a computer opponent during interactive video game playing. Cereb Cortex 23:2829–2839. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D (2003): A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event‐related fMRI. Neuroimage 18:263–272. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Lindquist MA, Nichols TE, Poldrack RA, Vul E (2010): Everything you never wanted to know about circular analysis, but were afraid to ask. J Cereb Blood Flow Metab 30:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. (2011): Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci 23:4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ (2011): Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci 31:3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L, Salek‐Haddadi A, Lund TE, Laufs H, Carmichael D (2007): Modelling large motion events in fMRI studies of patients with epilepsy. Magn Reson Imaging 25:894–901. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS (2008): Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cogn Affective Behav Neurosci 8:99–112. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF (2012a): On the relationship between the "Default Mode Network" and the "Social Brain". Frontiers Hum Neurosci 6:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MFS (2012b): Connectivity‐based subdivisions of the human right “temporoparietal junction area”: Evidence for different areas participating in different cortical networks. Cereb Cortex 22:1894–1903. [DOI] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA (2014): Resting state networks distinguish human ventral tegmental area from substantia nigra. Neuroimage 100:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, McDermott KB, Petersen SE (2012): In favor of a 'fractionation' view of ventral parietal cortex: Comment on Cabeza et al. Trends Cogn Sci 16:399–400; author reply 400–391. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L, Albert D, Chein J, Steinberg L (2011): Adolescents prefer more immediate rewards when in the presence of their peers. J Res Adolescence 21:747–753. [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen‐Berg H (2012): Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cogn Affective Neurosci 7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H‐J, Friston K (2013): Structural and functional brain networks: From connections to cognition. Science 342, doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Pessoa L (2009): How do emotion and motivation direct executive control? Trends Cogn Sci 13:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE (2015): Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105C:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Goudreau J, Delgado MR, Ruiz S (2012): Executive function in Parkinson's disease: Contributions of the dorsal frontostriatal pathways to action and motivation. Cogn Affective Behav Neurosci 12:193–206. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD (2012): Ventromedial prefrontal‐subcortical systems and the generation of affective meaning. Trends Cogn Sci 16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM (2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8:410–417. [DOI] [PubMed] [Google Scholar]
- Salimi‐Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM (2014): Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90:449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH (2013): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. Neuroimage 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Daw ND, Joel D, O'Doherty JP (2007): Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward‐based decision making. J Neurosci 27:12860–12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks .2. decreases in cerebral cortex. J Cogn Neurosci 9:648–663. [DOI] [PubMed] [Google Scholar]
- Sip KE, Smith DV, Porcelli AJ, Kar K, Delgado MR (2015): Social closeness and feedback modulate susceptibility to the framing effect. Social Neurosci 10:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Clithero JA, Boltuck SE, Huettel SA (2014a): Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Social Cogn Affective Neurosci 9:2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Utevsky AV, Bland AR, Clement N, Clithero JA, Harsch AE, Carter RM, Huettel SA (2014b): Characterizing individual differences in functional connectivity using dual‐regression and seed‐based approaches. Neuroimage 95:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Beckmann CF, Jenkinson M, Andersson J, Glasser MF, Van Essen DC, Feinberg DA, Yacoub ES, Ugurbil K (2012): Temporally‐independent functional modes of spontaneous brain activity. Proc Natl Acad Sci 109:3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM (2006): Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci 9:1007–1008. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF (2010): Self‐esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cereb Cortex 20:3005–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL (1898): Animal intelligence: An experimental study of the associative processes in animals. Psychological Monographs: General and Applied 2:i–109. [Google Scholar]
- Tversky A, Kahneman D (1981): The framing of decisions and the psychology of choice. Science 211:453–458. [DOI] [PubMed] [Google Scholar]
- Utevsky AV, Platt ML (2014): Status and the brain. PLoS Biol 12:e1001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA (2014): Precuneus is a functional core of the default‐mode network. J Neurosci 34:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H (2009): Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 4:274–290. [DOI] [PubMed] [Google Scholar]
- Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, Andrews‐Hanna JR, Wager TD (2014): Separate neural representations for physical pain and social rejection. Nat Commun 5:5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Smith SM (2004): Constrained linear basis sets for HRF modelling using variational bayes. Neuroimage 21:1748–1761. [DOI] [PubMed] [Google Scholar]
- Yarkoni T (2009): Big correlations in little studies: Inflated fMRI correlations reflect low statistical power‐commentary on Vul et al. (2009). Perspec Psychol Sci 4:294–298. [DOI] [PubMed] [Google Scholar]
- Young J, Smith DV, Coutlee C, Huettel SA (2015): Synchrony between sensory and cognitive networks is associated with subclinical variation in autistic traits. Frontiers Hum Neurosci 9, doi: 10.3389/fnhum.2015.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


