Key Clinical Message
Reported case of leukemic retinopathy mimicking common ischemic retinopathies in a young adult where ophthalmic visit was the first step to the diagnosis of chronic myeloid leukemia. It highlights the importance of routine eye exams and that clinicians should suspect leukemia in an otherwise healthy patient presenting with ischemic retinopathy.
Keywords: Anemia, flame shaped hemorrage, funduscopy, ocular manifestation, Roth's spot
Introduction
Leukemia, a cancerous disease of the white blood cells spreads throughout the bloodstream and may affect a number of organs 1. It begins in the bone marrow, where all blood cells are formed before being released into the bloodstream. Leukemia may present in different forms and are distinguishable per the form of abnormal leukocytes and by how quickly these cells leave the bone marrow and enter the bloodstream. Leukemia is essentially a malignant neoplasm involving the bone marrow and blood 1. The four main types of leukemia are acute myeloid leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, and chronic lymphocytic leukemia 2.
Acute myeloid leukemia (AML) is the most common type of leukemia that affects adults. The acute leukemia is a fast advancing disease that generates immature cells. These immature cells are unsuitable for normal physiological functions within the affected person. Chronic leukemia habitually develops gradually, and is characterized by reasonably higher proportions of mature cells. These reasonably high proportions of mature cells help sustain some of their customary functions 1.
The peak incidence of chronic myeloid leukemia is 3.1–10.1 per 100,000 people for those aged between 60–84, but decreases to 0.5 per 100,000 people for the 20–24 group 1. Chronic myeloid leukemia (CML) results from an acquired or a genetic insult to the DNA of a single bone marrow cell. The mutated cell divides uncontrollably into many cells (CML cells). Chronic myeloid leukemia does not fully hinder the formation of mature red cells, white cells, and platelets. As a consequence, chronic phase of myeloid leukemia is usually less fatal than acute leukemia, and more often than not patients diagnosed are asymptomatic. Most cases occur above 50 years with a median age of diagnosis of 64, but rarely occur in a young adult 1.
Clinically evident ocular involvement is common in patients with leukemia and has been described in up to 50% of patients at the time of diagnosis 3. Leukemic retinopathy is characterized by multiple preretinal and intraretinal hemorrhages that are most notably present in the posterior pole. Other clinical signs comprise: Roth's spots, cotton wool spots, exudates, retinal venous tortuosity, perivascular sheathing, and neovascularization. Roth's spot hemorrhages may point to a small area of retinal leukemic infiltration or platelet–fibrin depositions. Retinal lesions of peripheral neovasularization or “sea fan” neovascularization (typical of sickle cell retinopathy) may occur in patients with chronic leukemia and are thought to occur as a result of peripheral nonperfusion and ischemia from the hyperviscosity 3.
This is a case of leukemic retinopathy resulting from chronic myeloid leukemia, which mimics ischemic retinopathies in a young adult.
Case Report
A 20‐year‐old man presented to the University of Cape Coast Optometric Clinic for routine eye examination as part of the university's health screening program for fresh students. Snellen visual acuities recorded with his habitual correction was 6/5−2 in each eye, and his unaided visual acuities were 6/6−2 and 6/12 in the right and left eyes, respectively. His near acuity, both aided and unaided were N5 at 40 cm in each eye. Slit lamp assessment of the anterior segment showed no evidence of abnormality.
Dilated fundus examination was achieved using a drop of 1% Tropicamide. The lens and the vitreous humours of the right eye were clear. The cup to disc ratio was 0.2, but there were blot and dot hemorrhages on the perifoveal area approaching the posterior pole of the right eye, Roth's spots, and cotton wool spots. There was also the presence of venous dilation in the superior quadrant of the fundus. The cup to disc ratio for the left eye was 0.3 with similar hemorrhagic fundal presentation as found in the right eye except that more of the hemorrhages in the left eye were located at the mid‐periphery (Fig. 1).
Figure 1.
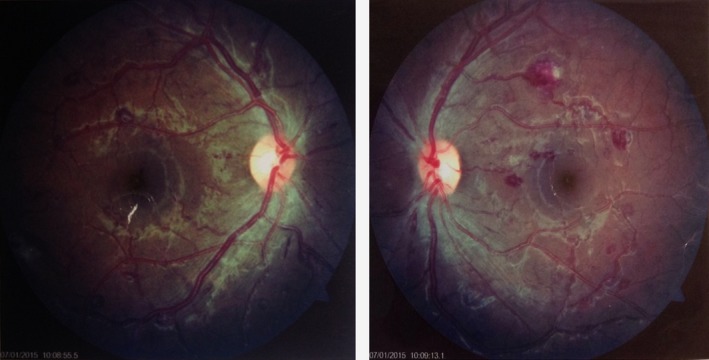
Fundus picture highlighting the degree of perifoveal hemorrhages in the right and left eye, respectively.
This ophthalmoscopic findings prompted further questioning which revealed that he had been experiencing excessive sweating especially at night, occasional bone pain, tiredness, and inability to tolerate aerobic exercise for the past 3 months prior to his visit. He attributed this to academic stress. His family medical history was remarkable for hypertension and diabetes.
Further assessment including blood pressure and pulse rate measurement were consistently normal when taken on four different occasions about the same time. Fasting blood sugar, FBS was also within normal range (5.1 mmol/L), however, glycosylated hemoglobin of 6.2% indicated prediabetic tendencies (normal: 4–6%, prediabetes 6.1–6.4%). An initial complete blood count showed a low hematocrit levels (37.1% as against an expected normal of 40–52%) and a high red blood cell distribution width of 15.9% (normal: 10–14%). Red blood cells levels were low (4.0 × 1012/L as against the expected normal of 4.40–5.90 × 1012/L) and white blood cells count was high 56.8 × 109/L (normal: 3–10.6 × 109/L). Lymphocytes count was 18.6 × 109/L (normal: 0.8–3.9 × 109/L) and granulocytes count was 35.5 × 109/L (normal: 1.1–7.8 × 109 L). MID also known as “mid‐range” cells count (including less frequently occurring cells correlating to monocytes, eosinophils, basophils, blast, and other precursor cells) was 2.7 × 109/L (normal range: 0.0–1.5 × 109/L). A repeat of complete blood count a week afterwards revealed a consistent decline in hematocrit levels (33.2%), but a high red blood cell distribution width of 19.8%, a low red blood cell count of 3.96 × 1012/L, white blood cell count of 160 × 109/L, lymphocytes count of 17.4 × 109/L, granulocyte count was 35.5 × 109/L. MID cells count was 47.0 × 109/L. The other formed blood elements were normal except mean corpuscular hemoglobin concentration that was elevated in the second complete blood count, 39.2 × 109/L (normal: 32–36 × 109/L). The platelets count were consistently within normal limits, but decreased from 234 × 109L to 157 × 109 L (normal: 150–400 × 109/L). Erythrocyte sedimentation rate (ESR) was high 16 mmfall/h (normal: 0–10), however, blood culture revealed no microbial growth after 5 days. Peripheral blood smear revealed mainly normocytic, normochromic cells along with numerous myeloid cells and neutrophilic band cells.
Hypertensive and diabetic retinopathies were among the differential diagnosis, but were ruled out because of the presence of Roth's spot, consistently normal blood pressure, pulse rate, fasting blood sugar and glycosylated hemoglobin 4. The Roth's spots are typical of retinopathy due to anemia, leukemia, and bacterial endocarditis. However, infective endocarditis was ruled out because blood culture result revealed no microbial growth. The hematological profile of low hematocrit levels, high red blood cell distribution width together with low red blood cell count amidst the fundus findings was suggestive of anisocytotic anemia. Nevertheless, some prospective studies of leukemic patients have established an association between acute leukemia and intraretinal hemorrhages, high white blood cell count, Roth's spots and declining, or low platelet count 5. Again, anemia could occur secondary to leukemia, hence, the existing ischemic retinopathy could not be solely attributed to anemia. The rapid increase in white blood cell count on two separate occasions with revealing high differentials and the presence of myeloid cells and neutrophilic band cells on peripheral blood smear amidst the fundus findings, barring any evidence of infection upon blood culture, led to a tentative diagnosis of leukemic retinopathy from a possible chronic myeloid leukemia.
At this stage, the patient was counseled and referred to a hematologist for further assessment in a bid to confirm the tentative diagnosis. The patient was noncompliant as he reported after a month with a complaint of blurred vision in the left eye. Assessment revealed a presenting visual acuity of counting fingers (CF) at 30 cm in the left eye and 6/5 in the right eye. Fundus examination revealed subretinal hemorrhage at the macula area leading to complete loss of foveal reflex in the left eye. Hematology visit was arranged immediately. Full blood count with differential at hematology visit revealed white blood cell count of 421.72 × 109/L (normal range:(2.50–8.50) × 109/L) along with neutrophil count of 352.32 × 109/L (normal range: 2.0–7.0 × 109/L), lymphocyte count of 11.81 × 109/L (normal range: 1.0–3.0 × 109/L), monocyte count of 13.08 × 109/L (normal range: 0.2–1.0 × 109), eosinophil count of 21.66 × 109/L (normal range :0.04–0.4 × 109/L), basophil count of 22.50 × 109/L (normal range: 0.02–0.1 × 109/L). Hematocrit level was 30.9% (normal range: 36–54%) indicative of anemia. Fluorescence in situ hybridization (FISH) was done on the peripheral blood cells and BCR‐ABL was detected. These findings along with positive FISH confirmed the diagnosis of CML.
The patient was immediately put on an oral hydroxyurea 1.5 g twice daily for a week and oral alluprinol 300 mg daily for 2 weeks. Patient was instructed to return for follow‐up care and ophthalmological monitoring of macular hemorrhage. After 2 months of treatment with hydroxyurea, patient's complete blood count was determined again and the result were as follows: white cell count, 1.78 × 109/L (normal: 2.50–8.5 × 109/L), neutrophil count, 0.69 × 109/L (normal: 2.00–7.00 × 109), lymphocyte count, 0.55 × 109/L (normal: 1.00–3.00 × 109/L), monocyte count, 0.11 × 109/L (normal: 0.20–1.00).This later findings was indicative of improved patient condition per the treatment given. The persistence of anemia as indicated by the red blood cell count of 3.23 × 1012/L (normal: 3.50–5.50) and hematocrit level of 28.9% (normal: 36.0–54.0) was expected with hydroxyurea treatment. The initial treatment was followed with imatinib (400 mg/day). The foveal reflex in the left eye was restored with aided visual acuity of 6/18 in the left eye and 6/5 in the right eye.
Discussion
Understanding of ocular association in leukemia is essential as the eye serves as the distinct site where leukemia's impact on nerves and blood vessels can be straightforwardly noticed. Ocular associated symptoms may sometimes serve as the initial mode of presentation of this systemic disease in 3.6% of patients with the disease conditions 6. Ocular complications of leukemia is known to be due to direct infiltration of the orbit and other tissues (iris, choroid, optic nerve), or vascular abnormalities affecting the retina (intraretinal hemorrhages, Roth's spots) 7, 8, 9, 10.
Several processes have been implicated in the ocular manifestations of CML since they epitomize the effects of this disease throughout the entire body system. In the present scenario, multiple mechanisms may have independently contributed to the observed manifestation. Such mechanisms include: reduced blood flow, vascular stagnation, retinal capillary dropout, and ischemia. Others associate the ocular findings to anemia; leukostasis; hyperviscosity syndrome; leukoembolization; endothelial lesion, and localized thrombosis secondary to toxic products released by the leukemic cells. Angiogenic factors caused by the ischemia resulting in increased serum levels of angiogenic growth factors, comprising elevated levels of vascular endothelial growth factor, fibroblast growth factor 2, hepatocyte growth factor, and matrix metalloproteinases have all been implicated 11, 12, 13, 14.
In this current case presentation, the high red blood cell distribution width was indicative of anisocytosis common in anemia 15. Furthermore, the increased glycosylated hemoglobin levels and the raised erythrocyte sedimentation rate could be attributed to anemia. However, the declining platelet levels indicated the likelihood of thrombocytopenia in due course. Meanwhile, both thrombocytopenia and anemia are almost always present in leukemia 1.
At birth, there is no chronic myeloid leukemia. It occurs when there is damage to the DNA of a solitary bone marrow cell. BCR‐ABL gene that causes chronic myeloid leukemia is found in some people, but not in others, though reasons for this observation remain unclear 1. On the other hand, in a few affected chronic myeloid leukemic persons, contact with extremely elevated measure of radiation have been observed, with marginal increases in risk for cancer patients undergoing radiotherapy. On the contrary, others suffer chronic myeloid leukemia without any history of radiation exposure.
The distinguishing factor for chronic myeloid leukemia is based on the genetic defect of chromosome 22 observed in the blood and marrow cells of patients with leukemia known to be shorter than in healthy people. This chromosome has consequently been termed the “Philadelphia chromosome” or “Ph chromosome” 1.
The ocular complications of leukemia may result directly from metastatic leukemic infiltrates or ensuing from the associated anemia or thrombocytopenia. Leukemic retinopathy is a common complication in both acute and chronic forms. In addition to the earlier mentioned complications of leukemic retinopathy, exudative retinal detachments and a variety of other retinal defects such as pallor and optic disc edema have been described in literature 3.
There is no direct treatment for leukemic retinopathy, however, chemotherapy, immunotherapy, and radiotherapy are usually employed to manage the underlining systemic cause. Chemotherapy that is best suited for intraocular leukemic infiltration, while application of external beam radiation is cut out for optic nerve or orbital lesions 16. The treatment of CML is informed by the specificity of the disease phase. That is, the chronic stable phase, accelerated phase, or the blast crisis. At the chronic phase, BCR‐ABL tyrosine kinase inhibitors (TKIs) for example, imatinib and interferon alpha, cytotoxic agents for example, hydroxyurea, and allogeneic hematopoietic cell transplantation (HCT) may be useful. Although allogeneic HCT remains the sole curative therapy, initial treatment with imatinib has been suggested 16. In most cases, initial imatinib treatment for chronic phase chronic myeloid leukemia halts the disease progression 17.
The patient's disease condition responded favorably to the initial treatment with hydroxyurea together with allopurinol. This case highlights the importance of routine eye exams and that clinicians should suspect leukemia in an otherwise healthy patient presenting with ischemic retinopathy.
Conflict of Interest
None declared.
Acknowledgment
The authors are thankful to staff of Optometry Department, University of Cape Coast.
Clinical Case Reports 2016; 4(2): 133–137
References
- 1. The Leukaemia and Lymphoma Society . 2014. Chronic myeloid leukaemia. Revised. Available at www.LLS.org. Cited on 2nd January, 2015.
- 2. Lanf, G. E. , Spraul C. W., and Lang G. K.. 1998. Ocular manifestation of hematological diseases. Klin Monbl. Augenheilkd. 212:419–427. [DOI] [PubMed] [Google Scholar]
- 3. Vardinnan, S. , and Hyjek E.. 2011. World health organization classification, evaluation and genetics of the mycloproliferative neoplasm variant. Hematology Am. Soc. Hematol. Educ. Program. 2011:250–256. [DOI] [PubMed] [Google Scholar]
- 4. Ling, R. , and James B.. 1998. White‐centred retinal haemorrhages (Roth spots). Postgrad. Med. J. 74:581–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reddy, S. C. , and Jackson N.. 2004. Retinopathy in acute leukaemia at initial diagnosis: correlation of fundus lesions and haematological parameters. Acta Ophthalmol. Scand. 82:81–85. [DOI] [PubMed] [Google Scholar]
- 6. Chaudhuri, T. , Roy S., and Roy P.. 2013. Ischaemic optic neuropathy induced sudden blindness as an initial presentation of acute lymphoblastic leukemia. Indian J. Med. Paediatr. Oncol. 34:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kincaid, M. C. , and Green W. R.. 1983. Ocular and orbital involvement in leukaemia. Surv. Ophthalmol. 27:211–232. [DOI] [PubMed] [Google Scholar]
- 8. Rosenthal, A. R. 1983. Ocular manifestations of leukaemia: a review. Ophthalmology 90:899–905. [DOI] [PubMed] [Google Scholar]
- 9. Murtha, T. J. 1994. Haematologic diseases: leukaemia, dysproteinemia and anaemia Pp 2986–3004 in Albert D. M., Jakobiec F. A., eds. Principles and practice of ophthalmology, Vol. 5. WB Saunders, Philadelphia. [Google Scholar]
- 10. Schachat, A. P . 1994. The leukaemias and lymphomas Pp 873–890 in Ryan S. J., ed.Retina. Basic science and inherited retinal diseases, Vol. 1, 2nd ed Mosby, St. Louis. [Google Scholar]
- 11. Nobacht, S. , Vandoninck K. F., Deutman A. F., and Klevering B. J.. 2003. Peripheral retinal nonperfusion associated with chronic myeloid leukemia. Am. J. Ophthalmol. 135:404–406. [DOI] [PubMed] [Google Scholar]
- 12. Jackson, N. , Reddy S. C., Hishamuddin M., and Low H. C.. 1996. Retinal findings in adult leukaemia: correlation with leukocytosis. Clin. Lab. Haematol. 18:105–109. [DOI] [PubMed] [Google Scholar]
- 13. Dobberstein, H. , Solbach U., Weinberger A., and Wolf S.. 1999. Correlation between retinal microcirculation and blood viscosity in patients with hyperviscosity syndrome. Clin. Hemorheol. Microcirc. 20:31–35. [PubMed] [Google Scholar]
- 14. Sillaber, C. , Mayerhofer M., Aichberger K. J., Krauth M. T., and Valent P.. 2004. Expression of angiogenic factors in chronic myeloid leukaemia: role of the bcr/abl oncogene, biochemical mechanisms, and potential clinical implications. Eur. J. Clin. Invest. 34:2–11. [DOI] [PubMed] [Google Scholar]
- 15. Baccarani, M. , Saglio G., Goldman J., Hochhaus A., Simonsson B., Appelbaum F., et al. 2006. Evolving concepts in the management of chronic myeloid leukaemia: recommendations from an expert panel on behalf of the European Leukaemia Net. Blood 108:1809–1820. [DOI] [PubMed] [Google Scholar]
- 16. Rafieetary, M. , Huddleston S., and Sigler E.. 2015. Differential diagnosis of retinal diseases‐many retinal conditions can be confused. Can you spot the masqueraders? Review of Optometry.
- 17. Hua, L. V. , and Salisa K. W.. 2010. Sudden unilateral visual loss as an initial presentation of chronic myelogenous leukaemia. Clin. Optom. 2:29–35. [Google Scholar]


