Abstract
The use of alternative donor transplants is increasing as the transplant eligible population ages and sibling donors are less available. We evaluated the impact of donor source on transplant outcomes for adults with acute myeloid leukemia undergoing myeloablative or reduced intensity conditioning transplant.
Between January 2000 and December 2010, 414 consecutive adult patients with acute myeloid leukemia in remission received myeloablative or reduced intensity conditioning allogeneic transplant from either a matched related donor (n=187), unrelated donor (n=76), or umbilical cord blood donor (n=151) at the University of Minnesota or Hôpital St. Louis in Paris.
We noted similar 6 year overall survival across donor types: matched related donor 47% (95% CI, 39–54%), umbilical cord blood 36% (95% CI, 28–44%), matched unrelated donor 54% (95% CI, 40–66%), mismatched unrelated donor 51% (95% CI, 28–70%) (p=0.11). Survival differed based on conditioning intensity and age with 6 year survival of 57% (95% CI 47–65%), 39% (95% CI, 28–49%), 23% (95% CI, 6–47%), 47% (95% CI, 36–57%) and 28% (95% CI, 17–41%) for myeloablative age 18–39, myeloablative age 40+, or reduced intensity conditioning ages 18–39, 40–56, and 57–74 respectively (p< 0.01). Relapse was increased with reduced intensity conditioning and lowest in younger patients receiving myeloablative conditioning (HR 1.0 versus 2.5 or above for all RIC age cohorts), p<0.01. Transplant related mortality was similar across donor types.
In summary, our data support the use of alternative donors as a graft source with MA or RIC for patients with acute myeloid leukemia when a sibling donor is unavailable.
Keywords: Allogeneic hematopoietic stem cell transplant, acute myeloid leukemia
Introduction
Allogeneic hematopoietic cell transplant (HCT) remains the only therapy that can provide extended disease free survival (DFS) for the majority of patients with acute myeloid leukemia (AML).1–3 However, post-transplant disease relapse remains a major therapeutic challenge. Efforts to identify patient, disease, and transplant features playing a role in post-HCT relapse risk continue with numerous reports documenting the role of cytogenetic risk, conditioning intensity, age, and disease status in transplant outcomes for AML.4–11
We analyzed the outcome of a large population of AML patients transplanted at two large centers, the University of Minnesota and Hôpital Saint Louis in Paris. We report the impact of specific patient, disease, and transplant variables on clinical outcomes in cohorts receiving similar myeloablative (MA) and reduced intensity conditioning (RIC) regimens. Our data highlight the interactions of age, conditioning intensity, and donor source on post-transplant outcomes and support the use of alternative donors when a sibling donor is not available.
Methods
Study Population
Between January 2000 and December 2010, 414 consecutive adult patients with AML in remission (complete remission (CR 1, 2 or 3) received MA or RIC allogeneic HCT from either an HLA-identical matched related donor (MRD) (n=187), unrelated donor (URD) (n=76), or umbilical cord blood (UCB) donor (n=151). Patients receiving more than one transplant for AML, those with French American British subtype M3, and those in relapse or with primary induction failure (PIF) were excluded.
Risk Stratification
Patients were risk stratified based on disease status at transplant (CR 1, 2 or 3) and by cytogenetic risk. Cytogenetic classification was limited by the differential availability of specific details between the two databases. The Paris data was available in ProMISe (Project Manager Internet Server), the European group for Blood and Marrow Transplantation (EBMT) web shared data base, in the following format: normal or abnormal chromosomes, presence or absence of complex karyotype, presence or absence of molecular markers with partial reporting of which molecular marker (NPM-1, FLT-3, BCR-ABL, WT-1, MLL, AML-ETO) was present. The availability of this data was confounded by the time period of the study since 2000. Complete cytogenetic data was available for the majority of University of Minnesota cases and FLT-3 or NMP-1 molecular data was available in more recent years. Merging these two data sets we classified risk using cytogenetic and molecular risk data as follows: Standard Risk included normal karyotype, favorable abnormalities including t(8;21) or inversion 16, CEBPA mutation, or NPM-1 mutation in the absence of FLT-3 ITD; Poor Risk included complex karyotype, monosomy 7, monosomy 5, monosomal karyotype, BCR-ABL, FLT-3 ITD, MLL (11q23), or all other known high risk abnormalities; Abnormal and uncertain significance included cases where an abnormality was documented without specifics or an abnormality of uncertain clinical significance was present (examples include CBF + c-kit + WT-1 or NPM-1 + WT-1).
HLA Typing, Matching, and Donor Selection
HLA related donors were primarily siblings based on family testing. URDs were defined as matched (8/8) if HLA-A, -C,-B, and DRB1 were identical at the allele level.12 Stem cells were harvested for sibling or URDs via marrow harvest (n=74) or filgrastim mobilized peripheral blood (n=189). UCB unit nucleated cell dose and matching have been described elsewhere;13 however, in brief were required to have a minimum of 4/6 antigen match between each cord and the recipient. In the absence of a sibling donor, UCB was the graft choice of preference for the University of Minnesota based on research priorities whereas Hôpital Saint-Louis utilized URDs in this situation. Preparative regimens were classified as either MA or RIC by established CIBMTR functional definitions.14–16
Treatment
Patients received either MA or RIC conditioning. MA conditioning from Paris included 120 mg/kg cyclophosphamide (60 mg/kg, on each of two consecutive days) and busulfan (3.2 mg/kg IV daily on four consecutive days), or 12 Gy Total Body Irradiation (TBI) in a fractionated regimen. For the University of Minnesota, the MA regimen for matched related donor (MRD) and unrelated donor (URD) was Cyclophosphomide (60mg/kg X day -6 and -5) plus TBI (165 cGy twice daily for 8 fractions days -4 through -1). UCB MA conditioning consisted of Fludarabine (25 mg/m2 daily days -8 through -6), cyclophosphamide (60 mg/kg IV daily days -7 and -6), and TBI (165 cGy twice daily for 8 fractions days -4 through -1). RIC at the Hôpital Saint-Louis consisted predominantly of fludarabine (30 mg/m2 IV daily from days -5 through -1), busulfan (3.2 mg/kg IV twice daily on days -4 and -3) plus rabbit anti-thymocyte globulin (ATG; 5mg/kg for siblings and 10 mg/kg for URDs on days -2 and -1). The University of Minnesota RIC regimen consisted of cyclophosphamide (50 mg/kg on day -6), Fludarabine (30–40 mg/m2 IV daily days -6 through -2), and total body irradiation (TBI; 200 cGy on day -1) for all donor sources. Equine ATG (15 mg/kg twice daily for six doses from day -6 through day -4) in the setting of RIC was used for those URDs who had only one cycle of multi-agent chemotherapy within three months or for related donors with only one cycle of multi-agent chemotherapy within six months prior to HCT. GVHD prophylaxis included cyclosporine (day -3 through +100–180) plus mycophenolate mofetil (MMF) (days -3 to +30) (56%) or cyclosporine (CSA) plus methotrexate (MTX) (40%).
Supportive care was similar in both institutions. Patients were hospitalized in single rooms utilizing high efficiency air filtration systems. Patients received prophylactic acyclovir for HSV or CMV prophylaxis plus anti-bacterial prophylaxis until day +21 or longer if on prednisone for GVHD, fungal prophylaxis with either fluconazole or voriconazole for 100 days, and pneumocystis juroveci prophylaxis typically with trimethoprim sulfamethoxazole for one year.
Data Collection
All patients were treated on protocols approved by the institutional review board of each hospital with prior informed consent for treatment and data analysis.
Data were prospectively collected. Data from Hôpital Saint-Louis in Paris was retrieved thru the European group for Blood and Marrow Transplantation (EBMT) and data from the University of Minnesota were prospectively collected in the institutional blood and marrow transplant (BMT) database. Data were merged for the combined analysis.
Statistical Analysis
The primary endpoint was overall survival (OS). Secondary endpoints included hematopoietic recovery, occurrence of aGVHD and CGVHD, transplant-related mortality (TRM), incidence of relapse, and disease-free survival (DFS). OS was defined as time to death from any cause and a 6 year time point was used due to the availability of extended follow-up. Hematopoietic recovery was defined as time to absolute neutrophil count ≥ 500 neutrophils/mcL for three consecutive days. Incidence and grade of aGVHD (acute graft versus host disease) at day +100 and absence or presence of chronic GVHD (cGVHD) at two years was recorded based on consensus criteria.17, 18 TRM was defined as any death in the first 28 days post-HCT or death after day 28 without evidence of relapsed leukemia. TRM results are reported at 1 year to capture later deaths due to transplant related toxicity. Relapse was defined as hematologic evidence of disease recurrence with those surviving without relapse censored at the date of last contact. Relapse was reported at 2 years as most post-transplant relapsed are evident within that time period. DFS was defined as survival without death or relapse censoring at the date of last contact.
Univariate probabilities of disease-free and overall survival were calculated using the Kaplan-Meier estimator with variance estimated by Greenwood’s formula.19 Probabilities of acute and chronic GVHD, TRM, and relapse were calculated using cumulative incidence curves to accommodate competing risks.20 Ninety-five percent confidence intervals for all probabilities and p-values of pairwise comparisons were derived from pointwise estimates and calculated. Single variable comparisons were made using log-rank tests with standard weights.
Multivariable regression models were fit for each outcome: Cox regression21 for OS and DFS, and Fine and Gray22 competing risks regression for all other outcomes reporting as hazard ratios (HR). TRM was analyzed with a competing risk of relapse, and relapse, GVHD, and hematopoietic recovery were analyzed with a competing risk of mortality. All models were pre-specified and included categorical factors for cytogenetic (standard, poor, abnormal but unknown significance), donor type (MRD, UCB, matched URD, mismatched URD), disease status (CR1, CR2 or CR3), and age-conditioning combinations (MA 18–39, MA 40–56, RIC 18–39, RIC 40–56, RIC 57–74) due to their association. Subgroup analysis investigation showed no significant association between donor source and conditioning and thus was not included in final modeling. Treatment center had minimal influence, thus was not included in the final models. SAS software (SAS Institute, Cary, NC) was used to perform statistical analyses.
Results
Patient Characteristics
Patient characteristics (Table 1) were similar across donor types (MRD, UCB, URD) with respect to gender, KPS, and age. MRD had fewer with poor risk cytogenetic/molecular profile compared to UCB or to matched and mismatched URD (38% versus 53%, 51%, and 52%, respectively). There were many MRD treated in CR1 (78% versus 58% in UCB, 73% in matched URD and 48% in mismatched URD). UCB (64%) transplant recipients were more likely to receive RIC compared to MRD (40%) and compared to matched (25%) or mismatched URDs (10%). Those receiving URD stem cell sources were more likely to be exposed to ATG in their conditioning compared to MRD and UCB (45–48% matched and mismatched URD versus 11% MRD and 15% UCB). GVHD prophylaxis associated with conditioning intensity with a higher percentage of CSA/methotrexate in the MRD and URD cohorts.
Table 1.
Patient Characteristics by Donor Type
| MRD* n (%) |
UCB n (%) |
Matched URD n (%) |
Mismatched URD n (%) |
Total n (%) |
|
|---|---|---|---|---|---|
| N | 187 | 151 | 55 | 21 | 414 |
| Cytogenetic classification | |||||
| Poor | 71 (38) | 80 (53) | 28 (51) | 11 (52) | 190 (46) |
| Standard | 74 (40) | 62 (41) | 15 (27) | 2 (10) | 153 (37) |
| Abnormal of unknown significance | 42 (22) | 9 (6) | 12 (22) | 8 (38) | 71 (17) |
| Age | |||||
| 18–39 | 57 (30) | 49 (32) | 21 (38) | 13 (62) | 140 (34) |
| 40–56 | 91 (49) | 62 (41) | 24 (44) | 7 (33) | 184 (44) |
| 57–74 | 39 (21) | 40 (26) | 10 (18) | 1 (5) | 90 (22) |
| Disease Status at HCT | |||||
| CR1 | 145 (78) | 88 (58) | 40 (73) | 10 (48) | 283 (68) |
| CR2 or CR3 | 42 (22) | 63 (42) | 15 (27) | 11 (52) | 131 (32) |
| Gender | |||||
| Female | 86 (46) | 73 (48) | 25 (45) | 7 (33) | 191 (46) |
| Male | 101 (54) | 78 (52) | 30 (55) | 14 (67) | 223 (54) |
| Conditioning | |||||
| Myeloablative | 112 (60) | 55 (36) | 36 (65) | 19 (90) | 222 (54) |
| Reduced intensity | 75 (40) | 96 (64) | 19 (35) | 2 (10) | 192 (46) |
| Graft source | |||||
| Cord blood | 0 (0) | 151 (100) | 0 (0) | 0 (0) | 151 (36) |
| Marrow | 45 (24) | 0 (0) | 20 (36) | 9 (43) | 74 (18) |
| PBSC | 142 (76) | 0 (0) | 35 (64) | 12 (57) | 189 (46) |
| GVHD prophylaxis | |||||
| CSA/MMF | 71 (38) | 144 (95) | 16 (29) | 2 (10) | 233 (56) |
| CSA/MTX | 110 (59) | 0 (0) | 35 (64) | 19 (90) | 164 (40) |
| Other | 6 (3) | 7 (5) | 4 (7) | 0 (0) | 17 (4) |
| ATG Use | |||||
| Yes | 20 (11) | 22 (15) | 25 (45) | 10 (48) | 77 (19) |
| No | 167 (89) | 129 (85) | 30 (55) | 11 (52) | 337 (81) |
| CMV | |||||
| R+ | 116 (62) | 88 (58) | 25 (45) | 15 (71) | 244 (59) |
| R−/D− | 50 (27) | 63 (42) | 20 (36) | 3 (14) | 136 (33) |
| R−/D+ | 21 (11) | 0 (0) | 10 (18) | 3 (14) | 34 (8) |
| Karnofsky score | |||||
| ≤ 80 | 49 (26) | 27 (18) | 11 (20) | 8 (38) | 95 (23) |
| 90–100 | 134 (72) | 121 (80) | 44 (80) | 13 (62) | 312 (75) |
| Unknown | 4 (2) | 3 (2) | 0 (0) | 0 (0) | 7 (2) |
| Years from diagnosis to HCT | |||||
| Median | 0.4 | 0.5 | 0.5 | 1.2 | 0.5 |
| Range | 0.2–7.8 | 0.1–7.8 | 0.4–3.3 | 0.3–13.7 | 0.1 – 13.7 |
MRD= matched related donor; UCB=umbilical cord blood; URD=unrelated donor; HCT=hematopoietic cell transplant; ATG= Anti-Thymocyte Globulin; GVHD=graft versus host disease; CSA=cyclosporine; MMF=mycophenylate mofetil; MTX=methotrexate; CMV=cytomegalovirus
includes 8 non-sibling related donors
Engraftment
The median time to engraftment of ANC was 16 days with 96% recovering by day +50. Twelve events of primary graft failure were noted; 10 in the UCB group. In multivariate analysis the only factors predictive of time to neutrophil recovery were conditioning intensity and donor type with quicker ANC recovery in the RIC groups (Age 18–39: HR 0.5, 95% CI, 0.3–0.9; Age 40–56: HR 0.5, 95% CI, 0.3–0.6; and Age 57–74: HR 0.6. 95% CI, 0.4–0.8; p<0.01) and slower, less frequent recovery in UCB donors (HR 2.8, 95% CI 2.1–3.6) p<0.01.
Survival
After a median follow-up of 5.2 years, 184 patients survive. The six year probability of survival was 44% (95% CI, 38–49%).
We observed similar six year overall survival (OS) across donor types: MRD 47% (95% CI, 39–54%), UCB 36% (95% CI, 28–44%), matched URD 54% (95% CI, 40–66%), mismatched URD 51% (95% CI, 28–70%) (p=0.11). (Figure 1, Table 2) We observed a non-significant finding of somewhat poorer survival in patients undergoing RIC UCB at 28% (95% CI, 19–38%) compared with RIC MRD of 46% (95% CI, 33–58%) or matched URD at 52% (95% CI, 29–72%) (p=0.23). However, the patients undergoing RIC UCB were more often transplanted in CR2/3 (39% versus 28% in MRD and 21% in matched URD) and a greater proportion had poor risk cytogenetics (55% versus 44% in MRD).
Figure 1.
Survival Based on Donor Source
Table 2.
Univariate Analysis of HCT Outcomes
| N (%) |
6 year OS (95% CI) |
6 year DFS (95% CI) |
2 year Relapse (95% CI) |
1 year TRM (95% CI) |
|
|---|---|---|---|---|---|
| Total | 414 (100) |
44% (38–49) |
41% (36–46) |
29% (24–34) |
20% (16–24) |
| Cytogenetic classification | P=0.07 | P=0.02 | P=0.04 | P=0.33 | |
| Poor | 190 (46) |
40% (33–47) |
35% (28–42) |
34% (27–41) |
23% (17–28) |
| Standard | 153 (37) |
46% (37–54) |
44% (35–52) |
26% (18–33) |
18% (12–24) |
| Abnormal of unknown significance | 71 (17) |
50% (37–62) |
50% (38–62) |
24% (14–34) |
18% (9–27) |
| Donor type/HLA matching | P=0.11 | P=0.11 | P=0.05 | P=0.53 | |
| MRD | 187 (45) |
47% (39–54) |
44% (37–52) |
26% (19–32) |
20% (14–25) |
| UCB | 151 (36) |
36% (28–44) |
34% (27–42) |
36% (28–45) |
20% (14–26) |
| URD matched | 55 (13) |
54% (40–66) |
50% (36–63) |
20% (9–31) |
25% (14–37) |
| URD mismatched | 21 (5) |
51% (28–70) |
39% (18–60) |
33% (13–53) |
14% (4–29) |
| Disease Status at HCT | P=0.09 | P=0.10 | P=0.04 | P=0.80 | |
| CR1 | 283 (68) |
46% (40–52) |
44% (38–50) |
26% (21–31) |
20% (15–25) |
| CR2 or CR3 | 131 (32) |
38% (29–46) |
35% (26–43) |
36% (27–45) |
21% (14–27) |
| Gender | P=0.25 | P=0.17 | P=0.57 | P=0.84 | |
| Female | 191 (46) |
49% (42–57) |
47% (40–54) |
28% (21–34) |
21% (15–27) |
| Male | 223 (54) |
39% (32–46) |
36% (29–43) |
30% (24–37) |
20% (14–25) |
| Conditioning/Age | P<0.01 | P<0.01 | P<0.01 | P=0.10 | |
| MA/18–39 | 127 (31) |
57% (47–65) |
55% (45–63) |
18% (11–25) |
18% (11–25) |
| MA/40–56 | 95 (23) |
39% (28–49) |
39% (29–49) |
25% (16–34) |
31% (21–40) |
| RIC/18–39 | 13 (3) |
23% (6–47) |
12% (1–38) |
38% (12–64) |
31% (10–55) |
| RIC/40–56 | 90 (22) |
47% (36–57) |
38% (27–48) |
39% (28–50) |
16% (8–23) |
| RIC/57–74 | 89 (22) |
28% (17–41) |
28% (16–40) |
38% (28–49) |
16% (8–23) |
OS=Overall Survival; DFS=Disease Free Survival; TRM=Transplant Related Mortality; CI=Confidence Interval; HLA=human leukocyte antigen; MRD= matched related donor; UCB=umbilical cord blood; URD=unrelated donor; CR=complete remission; MA=myeloablative; RIC= reduced intensity conditioning
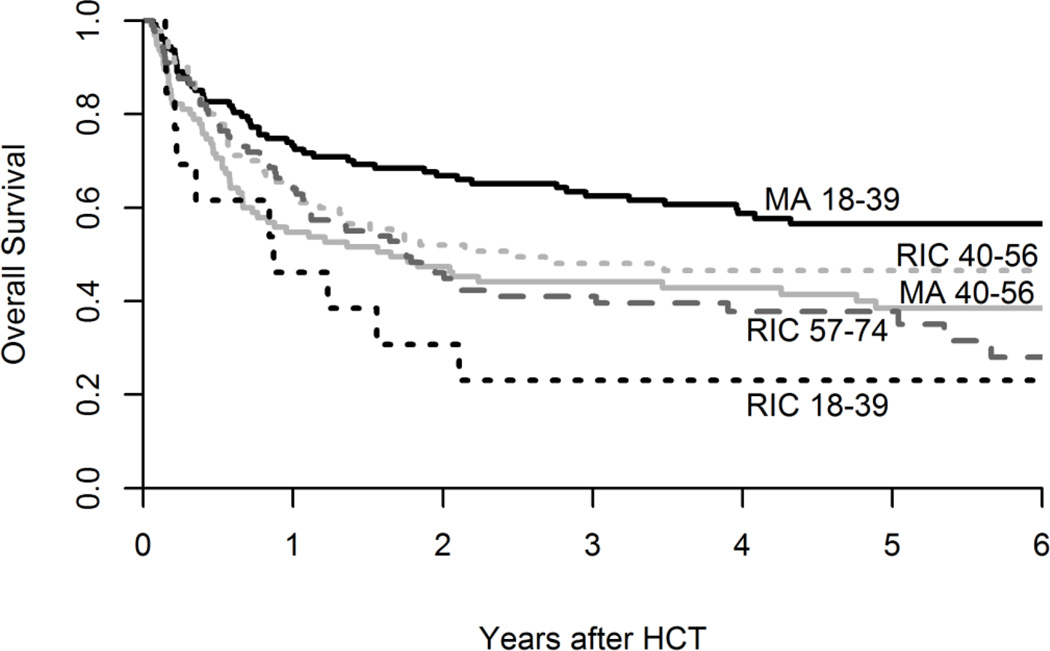
Six year OS differed in significance based on age and conditioning intensity were: MA age 18–39; 57% (95% CI 47–65%); MA age 40+, 39% (95% CI, 28–49%), RIC age 18–39; 23% (95% CI, 6–47%); RIC age 40–56 47% (95% CI, 36–57%) and RIC age 57–74; 28% (95% CI, 17–41%) (p< 0.01). (Table 2, Figure 1) The interaction of age and conditioning remained important in multivariate analysis as younger patients (age 18–39) undergoing MA had superior survival compared to a small cohort of similar aged patients undergoing RIC HCT (HR 2.3, 95% CI 1.1–4.6, p< 0.01). (Table 3) This small cohort (RIC, Age 18–39; n=13), in comparison to the MA (age 18–39) cohort, had more with KPS ≤ 80 (46% vs. 20%), advanced remission status CR2/3 (46% vs. 34%), and poor risk cytogenetics (54% vs. 43%). Notably, TRM and relapse risks were higher in this group and account for their poor survival.
Table 3.
Multivariate Analysis
| 6 year OS |
2 year Relapse |
1 year TRM |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Cytogenetic classification | P=0.11 | P=0.10 | P=0.44 | |||
| Standard | 1.0 | - | 1.0 | - | 1.0 | - |
| Abnormal of unknown significance | 0.9 | 0.6 – 1.4 | 1.3 | 0.7 – 2.5 | 0.8 | 0.4 – 1.5 |
| Poor | 1.3 | 1.0 – 1.8 | 1.6 | 1.0 – 2.4 | 1.2 | 0.7 – 1.9 |
| Donor type | P=0.28 | P=0.34 | P=0.4 | |||
| MRD | 1.0 | - | 1.0 | - | 1.0 | - |
| UCB | 1.3 | 1.0 – 1.8 | 1.3 | 0.9 – 1.9 | 1.4 | 0.8 – 2.3 |
| URD matched | 1.0 | 0.6 – 1.6 | 0.8 | 0.4 – 1.5 | 1.4 | 0.8 – 2.7 |
| URD mismatched | 1.0 | 0.5 – 1.9 | 1.5 | 0.6 – 3.4 | 0.7 | 0.2 – 2.4 |
| Gender | P=0.60 | P=0.71 | P=0.98 | |||
| Female | 1.0 | - | 1.0 | - | 1.0 | - |
| Male | 1.1 | 0.8 – 1.4 | 1.1 | 0.7 – 1.5 | 1.0 | 0.6 – 1.5 |
| Disease Status | P=0.05 | P=0.06 | P=0.94 | |||
| CR1 | 1.0 | - | 1.0 | - | 1.0 | - |
| CR2 or CR3 | 1.3 | 1.0 – 1.8 | 1.5 | 1.0 – 2.2 | 1.0 | 0.6 – 1.6 |
| Conditioning/Age | P<0.01 | P<0.01 | P=0.04 | |||
| MA/18–39 years | 1.0 | - | 1.0 | - | 1.0 | - |
| MA/40–56 | 1.9 | 1.3 – 2.8 | 1.6 | 0.9 – 2.9 | 1.8 | 1.0 – 3.0 |
| RIC/18–39 | 2.3 | 1.1 – 4.6 | 2.6 | 1.0 – 7.0 | 1.5 | 0.5 – 4.3 |
| RIC/40–56 | 1.3 | 0.9 – 2.0 | 2.6 | 1.5 – 4.5 | 0.6 | 0.3 – 1.3 |
| RIC/57–74 | 1.7 | 1.2 – 2.5 | 2.5 | 1.4 – 4.3 | 0.9 | 0.5 – 1.6 |
HR=Hazard Ratio; CI=Confidence Interval; OS=Overall Survival; TRM=Transplant related mortality; HLA=human leukocyte antigen; MRD= matched related donor; UCB=umbilical cord blood; URD=unrelated donor; CR=complete remission; MA=Myeloablative; RIC= Reduced intensity conditioning
The majority of deaths were due to disease recurrence (n=119, 52%) with GVHD (n=32, 14%), infection (n=28, 12%), other miscellaneous HCT-related complications (n=46, 20%) (septic shock, organ failure, graft failure, hemorrhage, acute respiratory distress syndrome, secondary malignancy, graft failure), and unknown (n=5,2%) accounting for the remainder.
Disease free survival was 41% (95% CI, 36–46%); however, as the majority of relapsing patients died, DFS was similar to overall survival.
Relapse and Transplant related mortality (TRM)
The overall incidence of relapse at two years was 29% (95% CI, 24–34%). Relapse was more frequent in those with higher risk disease as defined by cytogenetics or remission status at transplant. Two year relapse for those with poor risk cytogenetic/molecular profiles was 34% (95% CI, 27–41%) versus 26% for standard risk (95% CI 18–33%) or 24% for abnormal/unknown significance (95% CI 14–34%) (p=0.04). For those transplanted in CR2 or CR3, relapse was increased at 36% (95% CI, 27–45%) versus 26% (95% CI, 21–31%) for those in CR1 (p=0.04). Lastly, conditioning intensity influenced relapse with those receiving RIC showing an incidence of 38–39% across all age cohorts compared to 18% and 25% in the MA age 18–39 and 40–56 age groups, respectively (p<0.01).
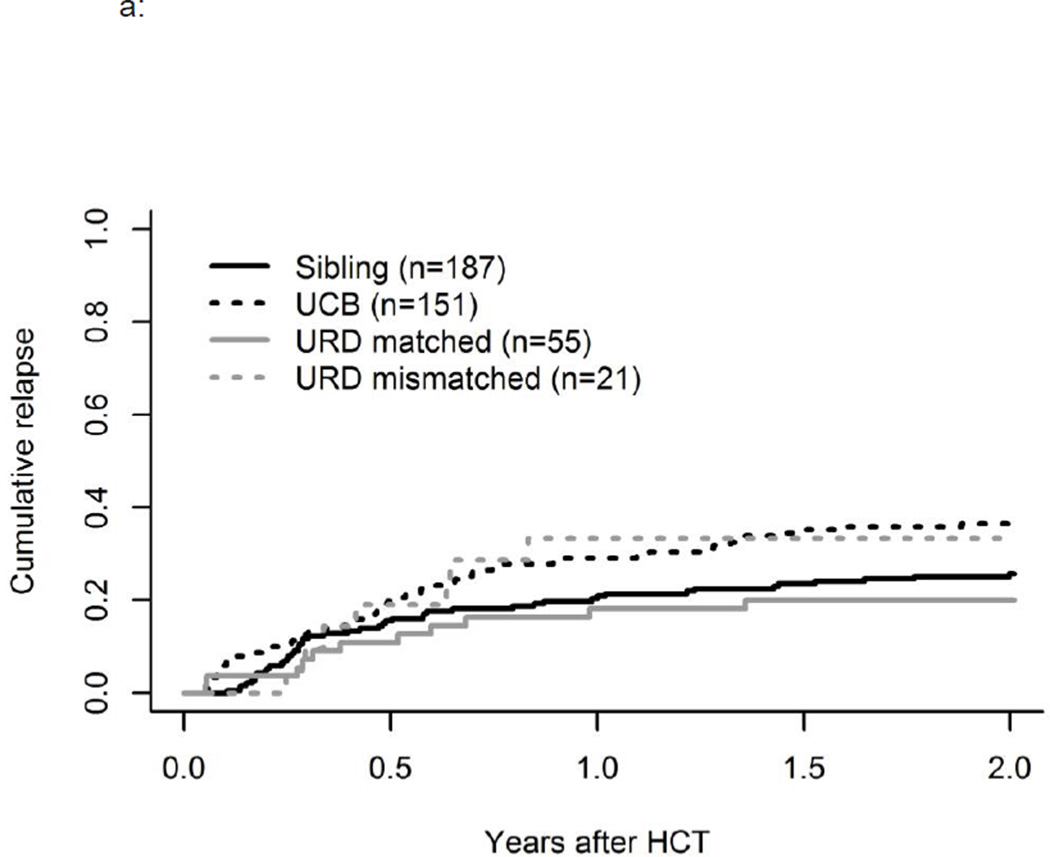
In univariate analysis relapse was slightly increased in those receiving UCB transplants at 36% (95% CI, 28–45%) and mismatched URD at 33% (95% CI, 13–53) compared to matched URD at 20% (95% CI, 9–31%) and MRD at 26% (95% CI, 19–32%) (p=0.05) (Figure 3a). However, UCB recipients were more likely to receive RIC, and in multivariate analysis, conditioning/age remained significantly associated with relapse (p<0.01) but donor type did not. (p=0.34, Table 3) Subgroup analysis showed that the combination of UCB and RIC had particularly high relapse of 46% (95% CI, 35–57%), compared to 20% (95% CI, 9–31%) in those receiving MA UCB (p=0.01). As noted, these RIC UCB recipients had more advanced disease and more poor risk cytogenetics., and in multivariate analysis of relapse, significantly higher relapse rates were observed across all age cohorts of RIC HCT (p<0.001). (Table 3)
Figure 3.
a: Influence of Donor Source on Relapse
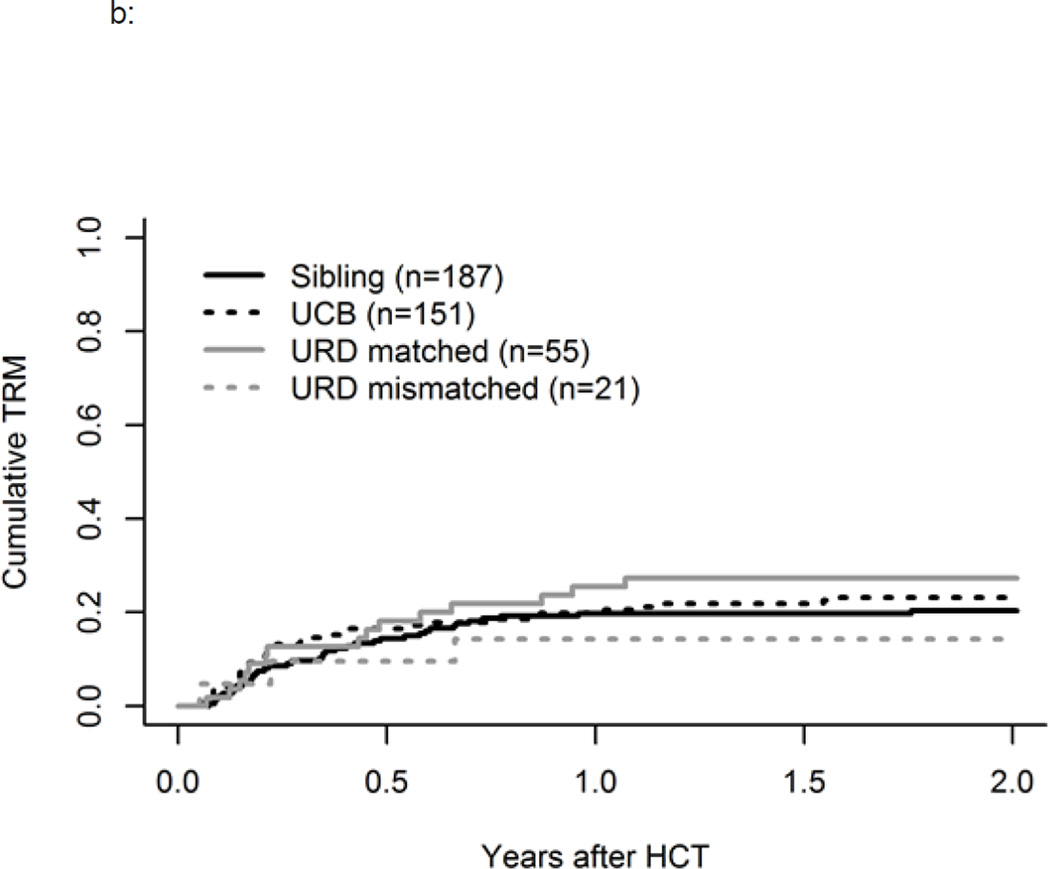
b: Influence of Donor Source on TRM
TRM at one year was 20% (95% CI, 16–24%). We observed no significant differences based on remission status, donor type (Figure 3b), cytogenetic/molecular risk group, or gender. As compared to the younger MA group, TRM was highest in older MA patients, age 40–56, compared to the younger MA group (HR 1.8, 95% CI, 1–3) and was also higher in the youngest RIC cohort age 18–39 as explained by their higher risk disease and poorer performance status (HR 1.5, 95% CI, 0.5–4.3), p=0.04.
Acute and Chronic GVHD
The incidence of day +100 aGVHD grade II-IV for the entire cohort was 62% (95% CI, 56–67%) with only 16% (95% CI, 12–19) severe grade III-IV aGVHD. Severe aGVHD was lowest in MRD 9% (95% CI, 5–13%), compared to URD 15% (95% CI, 5–24%), UCB 24% (95% CI, 17–31%), and mismatched URD 24% (95% CI, 6–42%) p<0.01. In multivariate analysis, a higher risk of severe aGHVD was observed in those receiving an UCB graft source (HR 3.2, 95% CI 1.6–6.2) or mismatched URD (HR 2.6, 95% CI 0.9–7.8)(p=0.01) and in males (HR 1.7, 95% CI, 1–2.7, p=0.05).
The overall incidence of cGVHD at two years was 34% (95% CI, 29–39%). In multivariate analysis, risks of cGVHD were higher in males (HR 1.8, 95% CI 1.3–2.6) (p<0.01) and HCTs during CR2/CR3 (HR 1.5, 95% CI 1.0–2.1, p=0.03). There was no significant influence of donor type, age or conditioning intensity on the incidence of cGVHD.
Discussion
We investigated patient, disease, and transplant factors impacting survival, relapse, and TRM risk in a well characterized population of 414 adult AML patients receiving consistent MA and RIC HCT regimens. Our analyses revealed similar outcomes across donor source receiving either MA or RIC. These findings build upon earlier reports in the older RIC setting,23 and provide solid evidence for use of alternative donors when a sibling donor is unavailable. Our data also highlight the interaction of conditioning intensity and age, the impact of remission status at transplant and cytogenetic risk, and the increased relapse risks in those receiving RIC conditioning. Thus, our data support the use of MA conditioning to limit relapse risk when feasible.
Of notable importance, our study evaluates a heterogeneous cohort of patients treated with three different donor sources across both MA and RIC conditioning approaches. As may occur in any retrospective review the groups are not evenly balanced with respect to disease or transplant characteristics that could impact outcome. Extensive subset analysis when patient numbers within subsets are small can potentially lead to incorrect conclusions. While we analyze important subsets in our manuscript, we attempted to minimize overgeneralizations based upon statistically insignificant analyses. Overall we demonstrated that OS was similar across donor types and that high risk disease features including poor risk cytogenetics, advanced disease stage CR2/3 and RIC contributed more to risk of relapse than did donor source. While we observed a trend to inferior OS in those patients undergoing RIC, paired interactions of donor source and conditioning intensity were not statistically significant supporting our main conclusions..
Prior comparisons of HCT using alternative donor sources have had mixed results. Some reports have suggested increased TRM using mismatched URDs and UCB donor sources and consequently lower survival.24, 25 Others have reported improved outcomes for older patients with sibling donors vs. URDs26 yet comparable results with URDs and UCBT.27 We recently reported a collaborative analysis investigating donor source in an older (age 50+) cohort of AML patients, all receiving RIC and revealed similar outcomes using MRDs, URDs, and UCB donors.23 The current study, a larger group of adults with AML receiving RIC and MA conditioning at our two centers, highlights similar survival, DFS, relapse, and TRM across donor sources with either conditioning intensity. Our study did not include haploidentical donor sources. However, as this alternative donor options is studied further and is currently the basis of an ongoing BMT CTN randomized trial using RIC (protocol 1101), forthcoming data will allow for comparisons including this alternative donor source.
Clear data on the value of greater conditioning regimen intensity for AML and MDS is still lacking though the use of RIC has increased in order to offer allogeneic HCT to older patients. Many retrospective studies have highlighted lower risks of TRM offset by increased rates of relapse in RIC with similar OS.28–31 More recent studies note similar outcomes for those in complete remission.32–33 This ongoing conditioning intensity debate prompted a national randomized trial within the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901 to prospectively answer this question. While closed to accrual, future reports of their data will be helpful in answering this question. In the interim, our data strongly suggest lower relapse in patients receiving MA conditioning.
Rates of advanced aGVHD were highest in those receiving UCB or mismatched URD stem cell sources, but were in line with other published reports.27 Our UCB and mismatched URD cohorts included a higher proportion of advanced disease status (CR2/3) and poor risk cytogenetics and thus may have been more heavily pre-treated entering transplant thus increasing their risk of severe aGVHD. cGVHD rates were associated with more advanced disease.
Our data highlights long follow-up of a sizeable population treated uniformly in two experienced centers and support the use of alternative donors as a graft source for patients with non-favorable risk AML when a sibling donor is unavailable. Clinically suitable patients should be considered for MA conditioning to reduce relapse risk.
Figure 2.
Survival Based on Age and Conditioning
Acknowledgments
The authors would like to thank Connie Blasing for manuscript submission preparation.
This work was supported in part by grants from the National Cancer Institute P01 CA65493 (C.G.B) and Leukemia and Lymphoma Society Scholar in Clinical Research Award, grant R6029-07 (C.G.B.).
This work was also supported in part by NIH grant P30 CA77598 utilizing Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions:
EW, RP, DW, and GS generated the study concept. EW and RP collected and prepared data for analysis. RS completed data analysis and generated figures and tables. EW, RP, DW, and GS interpreted the data. EW prepared the manuscript. DW, RP, GS contributed significant edits and input into final manuscript. NB, MR, AX, CB, CU, FS reviewed and approved the manuscript
Disclosures:
The authors have no relevant conflicts of interest to disclose.
References
- 1.Stelljes M, Krug U, Beelen DW, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol. 2014;32(4):288–296. doi: 10.1200/JCO.2013.50.5768. [DOI] [PubMed] [Google Scholar]
- 2.Kurosawa S, Yamaguchi T, Miyawaki S, et al. A Markov decision analysis of allogeneic hematopoietic cell transplantation versus chemotherapy in patients with acute myeloid leukemia in first remission. Blood. 2011;117(7):2113–2120. doi: 10.1182/blood-2010-05-285502. [DOI] [PubMed] [Google Scholar]
- 3.Kurosawa S, Yamaguchi T, Uchida N, et al. Comparison of allogeneic hematopoietic cell transplantation and chemotherapy in elderly patients with non-M3 acute myelogenous leukemia in first complete remission. Biol Blood Marrow Transplant. 2011;17(3):401–411. doi: 10.1016/j.bbmt.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale RE, Hills R, Kottaridis PD, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–3665. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 7.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo RJ, Atenafu EG, Craddock K, Chang H. Allogeneic hematopoietic cell transplantation may alleviate the negative prognostic impact of monosomal and complex karyotypes on patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(5):690–695. doi: 10.1016/j.bbmt.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Fang M, Storer B, Estey E, et al. Outcome of patients with acute myeloid leukemia with monosomal karyotype who undergo hematopoietic cell transplantation. Blood. 2011;118(6):1490–1494. doi: 10.1182/blood-2011-02-339721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estey EH. Acute myeloid leukemia, 2013 update on risk-stratification and management. Am J Hematol. 2013;88(4):318–327. doi: 10.1002/ajh.23404. [DOI] [PubMed] [Google Scholar]
- 11.Ustun C, Wiseman AC, Defor TE, et al. Achieving stringent CR is essential before reduced-intensity conditioning allogeneic hematopoietic cell transplantation in AML. Bone Marrow Transplant. 2013;48(11):1415–1420. doi: 10.1038/bmt.2013.124. [DOI] [PubMed] [Google Scholar]
- 12.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunstein CG, Baker KS, Wagner JE. Umbilical cord blood transplantation for myeloid malignancies. Curr Opin Hematol. 2007;14(2):162–169. doi: 10.1097/MOH.0b013e32802f7da4. [DOI] [PubMed] [Google Scholar]
- 14.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champlin R, Khouri I, Shimoni A, et al. Harnessing graft-versus-malignancy: non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol. 2000;111(1):18–29. doi: 10.1046/j.1365-2141.2000.02196.x. [DOI] [PubMed] [Google Scholar]
- 17.Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011 Oct 13;118(15):4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Cox D. Regression models and life tables. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Peffault de Latour R, Brunstein CG, Porcher R, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013;19(9):1355–1360. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisdorf D, Eapen M, Ruggeri A, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biol Blood Marrow Transplant. 2014;20(6):816–822. doi: 10.1016/j.bbmt.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alousi AM, Le-Rademacher J, Saliba RM, et al. Who is the better donor for older hematopoietic transplant recipients: an older-aged sibling or a young, matched unrelated volunteer? Blood. 2013;121(13):2567–2573. doi: 10.1182/blood-2012-08-453860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119(23):5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19(12):2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 29.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108(3):836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 30.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12(10):1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: long-term follow-up. Leukemia. 2010;24(5):1050–1052. doi: 10.1038/leu.2010.12. [DOI] [PubMed] [Google Scholar]
- 32.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martino R, Valcarcel D, Brunet S, Sureda A, Sierra J. Comparable non-relapse mortality and survival after HLA-identical sibling blood stem cell transplantation with reduced or conventional-intensity preparative regimens for high-risk myelodysplasia or acute myeloid leukemia in first remission. Bone Marrow Transplant. 2008;41(1):33–38. doi: 10.1038/sj.bmt.1705879. [DOI] [PubMed] [Google Scholar]