Abstract
OBJECTIVES/INTRODUCTION
The incidence of chronic lung disease (CLD) varies among groups defined by their early pattern of respiratory disease. Although CLD is common among infants with continuous exposure to increased ambient oxygen throughout the first two postnatal weeks the antecedents of CLD among preterm infants without this exposure are not well understood.
PATIENTS AND METHODS
We examined data collected prospectively on the 1204 (out of 1506) infants born in 2002 to 2004 at 23 to 27 completed weeks of gestation who survived to 36 weeks post-menstrual age (PMA). Based on their initial respiratory presentation and need for supplemental oxygen during the first two weeks, infants were classified as having early and persistent pulmonary dysfunction (EPPD), early recovery of pulmonary function followed by deterioration (PD), or consistently good pulmonary function characterized by low FiO2 (Low FiO2).
RESULTS
CLD was diagnosed in 69% of infants with EPPD, in 52% with PD, and 17% in the Low FiO2 group. Risk factors for CLD varied among these groups. Birth weight z-score < -1 conveyed information about CLD risk in all three groups and was the major risk factor for infants in the Low FiO2 group (Odds Ratio [OR] 27; 95% confidence interval [CI] 7–95). Mechanical ventilation at 7 days was associated with increased risk in the pulmonary deterioration group (OR 4.2, 95% CI 2.5–6.9) and the early and persistent pulmonary dysfunction group (OR 2.7, 95% CI 1.5–4.7), but not the Low FiO2 group (OR 1.5, 95% CI 0.5–3.9).
CONCLUSION
Both the likelihood of a very preterm infant developing CLD and the profile of risk factors linked with CLD are related to the infant’s pattern of respiratory disease during the first two postnatal weeks. Among infants with little exposure to oxygen during this period, fetal growth restriction, not mechanical ventilation, is the factor with the strongest association with CLD.
Keywords: lung disease, prematurity, preterm infant
OBJECTIVES/INTRODUCTION
Early pulmonary dysfunction in extremely low gestational age newborns (ELGANs) can be characterized by three distinct patterns, based on the fraction of inspired oxygen they require in the first two postnatal weeks.1 A minority of ELGANs have relatively normal pulmonary function throughout the first two postnatal weeks. Another group has pulmonary deterioration (PD), characterized by resolving lung disease during the first postnatal week, and followed in the second week by a requirement for increased supplemental oxygen and, in some cases, mechanical ventilation. A third group has early and persistent pulmonary dysfunction (EPPD) requiring mechanical ventilation and high concentrations of supplemental oxygen throughout this time period.
The incidence of chronic lung disease (CLD), also known as bronchopulmonary dysplasia, varies among groups defined by their early respiratory function. Among infants with EPPD, approximately two-thirds develop CLD, and the oxygen and ventilation exposures in this group most resemble historical antecedents of BPD.1 Infants with PD are at moderate risk of CLD and have less exposure to oxygen and ventilation than EPPD infants. Although almost one-fifth of infants with relatively normal lung function throughout the first two postnatal weeks develop CLD, they have virtually no exposure to supplemental oxygen and ventilation during that period. These observations suggest that several pathophysiologic pathways play a role in the development of CLD. Understanding the relative contribution of CLD antecedents within these groups might provide clues to the mechanisms of injury that lead to CLD.
The objective of this study was to identify clinical and demographic antecedents and modifiers of CLD risk in three groups of infants defined by their pattern of early postnatal respiratory function.
METHODS
The ELGAN Study
The infants included in this analysis are a subset of infants enrolled in a multi-center epidemiologic study to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in ELGANs (the ELGAN Study).1,2 From March 2002 to August 2004, women delivering before the 28th week of gestation at one of 14 participating institutions were asked to enroll in the study. Individual institutional review boards at each of the institutions approved the enrollment and consent processes (see Acknowledgements for the list of institutions that approved the study). Of 1506 infants enrolled, 1204 had both the information necessary to make an early respiratory status assignment and survived to 36 weeks post-menstrual age (PMA) when a CLD diagnosis was made.
Patterns of Early Respiratory Function and Chronic Lung Disease
ELGANs were classified into three mutually exclusive groups: those with relatively normal pulmonary function (Low FiO2 group: FiO2 consistently < 0.23 on all days between 3 and 7 postnatal days and receiving FiO2 ≤ 0.25 on Day 14), those with pulmonary deterioration during the second week of life after a period of normal lung function (PD group: FiO2 < 0.23 on any days between 3 and 7 days and receiving FiO2 > 0.25 on day 14), and those with early and persistent pulmonary dysfunction (EPPD group: FiO2 consistently ≥ 0.23 on all days between 3 and 7 postnatal days and receiving FiO2 > 0.25 on Day 14).1 There were no patients with an FiO2 > 0.23 on any day between 3 and 7 postnatal days and receiving < 0.25 on Day 14, so this group was not included. The diagnosis of CLD was based on whether or not the child was receiving supplemental oxygenation at 36 weeks PMA.
Demographic, Pregnancy and Neonatal Variables
Pregnancy characteristics and data describing newborns at the time of delivery included maternal race, receipt of antenatal steroids, chorioamnionitis, pregnancy complications, multi-fetal pregnancy, gender, gestational age, and birth weight. The birth weight Z-score is the number of standard deviations the infant’s birth weight is above or below the median weight of infants at the same gestational age in a standard data set.3
The SNAP-IITM (Score for Neonatal Acute Physiology-II)4 was calculated from measures taken during the first 12 hours of life. Mode of ventilation was defined as the highest level of support on each day and ranged from no support, supplemental oxygen by hood or nasal cannula, nasal continuous positive airway pressure, and conventional mechanical ventilation to high frequency ventilation and was recorded on days 0–7, 14, 21, and 28, and at 36 weeks PMA. We also recorded the number of days each infant received supplemental oxygen, CPAP, and conventional mechanical ventilation (including high frequency ventilation). Diagnoses of pneumothorax, pulmonary interstitial emphysema, and pulmonary hemorrhage were those made by the clinicians caring for the infant.
Confirmed early bacteremia was defined as recovery of an organism from blood drawn during the first postnatal week, and confirmed late bacteremia as recovery of an organism from blood drawn during the second, third or fourth week. Confirmed tracheal colonization required the recovery of a pathogen from tracheal aspirate.
The diagnosis of PDA was made by clinicians using their own operational definitions, which might or might not have included echocardiographic findings. We did not record the day of diagnosis. We recorded whether the PDA was ligated and whether indomethacin was offered as medical therapy. If a child had surgical ligation of the PDA, the infant was assigned to the surgical therapy group only, even if the infant first received medical therapy. Infants classified as having received medical therapy received indomethacin and did not have the PDA ligated.
The presence of chorioamnionitis and funisitis was determined by an ELGAN study pathologist at each institution who first engaged in training procedures to minimize inter-observer variability, was masked to maternal history, and used pre-defined operational definitions; 1126 placentas were examined.5,6
Medications were recorded if given on any day during the first week through fourth weeks, and included surfactant, analgesics (i.e., morphine, fentanyl, or methadone), sedatives (i.e., lorazepam, midazolam, or chloral hydrate), vitamin A, and steroids (i.e., hydrocortisone and dexamethasone). Indications were not recorded.
Data Analysis
We evaluated whether groups of antecedents of CLD differed among the three early respiratory pattern groups. First we calculated risks of CLD among infants classified by their early respiratory pattern and the presence or absence of other characteristics and exposures. The characteristics that most clearly distinguished infants at highest risk of CLD from their peers were then included in logistic regression models to assess the strength of association of each characteristic/exposure to the risk of CLD within each early respiratory pattern group, while adjusting for other factors included in the regression. Gestational age categories (23–24, 25–26, 27 weeks) and birth weight Z-score groups (<-2, ≥ -2 but <-1, ≥ -1) 7,8 were included in every multivariable model.
Because postnatal phenomena, such as the need for ventilatory assistance, can be influenced by antepartum phenomena, we created logistic regression models in which risk factors are ordered in a temporal pattern, so that the earliest occurring predictors/covariates of an outcome (e.g., CLD) are entered first and are not displaced by later occurring covariates work.9–14 For these time-oriented risk models (TORMs), we categorize sets of antecedents/covariates by the time they occur or are identified. We grouped prenatal and birth characteristics and exposures into the antenatal epoch, all exposures and characteristics during the first week into the early neonatal epoch, and exposures and characteristics occurring or reported between weeks 2–4 into the late neonatal epoch. We included in the antenatal epoch a hospital stratum (group) term to account for the possibility that infants born at a particular hospital are more like each other than like infants born at other hospitals.15 Because the risk of CLD among infants born with a sibling did not differ from that of singletons, we did not adjust for number of fetuses.
We used a step down procedure within each epoch, seeking a parsimonious solution without interaction terms. After the antenatal epoch variables were identified, the early neonatal variables were added. We then dropped non-significant neonatal variables but did not permit displacement of antenatal variables. Finally, the late neonatal epoch variables were added to the reduced antenatal/early neonatal set and non-significant late neonatal variables dropped. The contributions of relevant variables in the final model are presented as odds ratios with 95% confidence intervals. We created three TORMs of CLD, one for each pattern of early respiratory function.
RESULTS
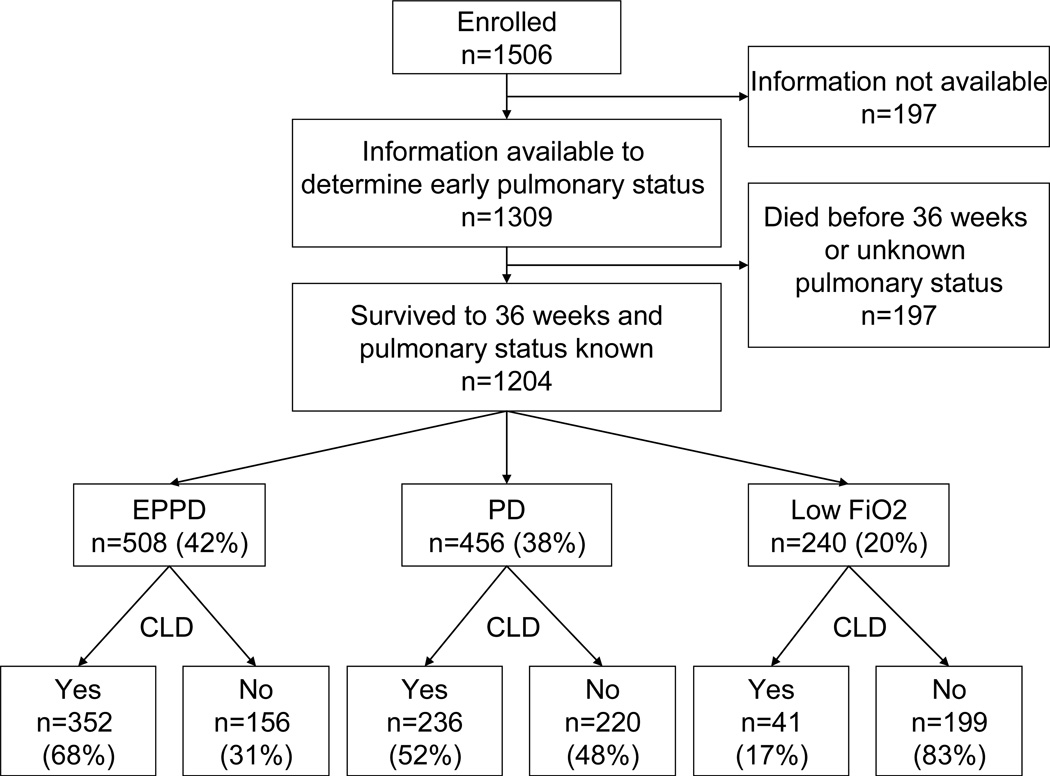
Among 1506 infants enrolled in the ELGAN Study, 1204 survived to 36 weeks PMA and constitute the cohort for this study (Figure). The early respiratory function was categorized as EPPD in 42% (n=508) of the infants, as PD in 38% (n=456), and as Low FiO2 in 20% (n=240). The incidence of CLD varied among groups defined by these respiratory patterns during the first two postnatal weeks. CLD was diagnosed in 69% of infants in the EPPD group, 52% in the PD group, and 17% in the Low FiO2 group.
Figure 1.
Derivation of cohort. EPPD=early and persistent pulmonary dysfunction (an FiO2 consistently ≥ 0.23 on all days between 3 and 7 postnatal days and receiving FiO2 > 0.25 on Day 14); PD=Pulmonary Deterioration (an FiO2 < 0.23 on any days between 3 and 7 days and receiving FiO2 > 0.25 on day 14); Low FiO2=consistently low FiO2 (an FiO2 consistently < 0.23 on all days between 3 and 7 postnatal days and receiving FiO2 ≤ 0.25 on Day 14). CLD=chronic lung disease among survivors.
Univariate Analyses
Antenatal factors [Table 1]
Table 1.
Univariate associations between antenatal factors and the risk of chronic lung disease (CLD). The right-most column provides the maximum number of infants with the attribute listed in each row; any differences are due to missing data. All other data are the percentage of infants with CLD among those infants with the attribute listed on the two left columns and the respiratory patterns listed as column headings. For example, among infants with a gestational age of 23–24 weeks who had the Low FiO2 respiratory pattern, the prevalence of CLD was 30%.
| Antenatal factors | Respiratory Pattern | Row N |
|||
|---|---|---|---|---|---|
| Low FiO2 | PD* | EPPD* | |||
| Gestational age (weeks) |
23–24 | 30 | 76 | 85 | 250 |
| 25–26 | 19 | 57 | 64 | 560 | |
| 27 | 15 | 33 | 62 | 394 | |
| Birth weight (g) |
≤ 750 | 41 | 74 | 81 | 461 |
| 751–1000 | 14 | 43 | 58 | 512 | |
| > 1000 | 11 | 29 | 51 | 231 | |
| Birth weight Z-score |
< −2 | 70 | 68 | 86 | 75 |
| ≥ −2 to −1 | 38 | 70 | 80 | 163 | |
| ≥ −1 | 13 | 47 | 65 | 966 | |
| Sex | Male | 18 | 56 | 69 | 638 |
| Female | 16 | 47 | 69 | 566 | |
| Antenatal steroid | Complete | 17 | 50 | 71 | 767 |
| Partial | 16 | 56 | 67 | 316 | |
| None | 21 | 53 | 64 | 117 | |
| Number of fetuses | Single | 18 | 53 | 69 | 819 |
| Multiple | 16 | 50 | 69 | 385 | |
| Cesarean delivery | Yes | 16 | 54 | 70 | 804 |
| No | 24 | 48 | 68 | 400 | |
| Delivery indication | PTL | 15 | 49 | 67 | 526 |
| pPROM* | 12 | 52 | 67 | 265 | |
| Preeclampsia | 40 | 64 | 74 | 163 | |
| Abruption | 19 | 48 | 73 | 126 | |
| Cervical Insufficiency | 13 | 45 | 57 | 72 | |
| Fetal indication | 10 | 67 | 85 | 52 | |
| Percent CLD | 17 | 52 | 69 | 52 | |
| Maximum Column N | 240 | 456 | 508 | 1204 | |
PD=pulmonary deterioration, EPPD=early and persistent lung dysfunction, pPROM=prolonged, premature rupture of membranes
Lower gestational age and lower birth weight were associated with an increased risk of CLD in all respiratory-pattern groups. Lower birth weight z-scores were associated with an increased risk of CLD in all groups, particularly in the Low FiO2 group. In this group, 38% of infants with a birth weight Z-score between -2 and -1 and 70% of infants with a birth weight Z-score of < -2 developed CLD, compared to 13% among appropriate for gestational age birth weight infants. Among infants in the Low FiO2 group, those delivered for maternal indications (preeclampsia) were at an increased risk of CLD (40%, compared to 10–19% among other indications for delivery). In the PD group, male infants were more likely to develop CLD (56% vs. 47% among female infants). Aside from gestational age, birth weight and birth weight z-score, no antenatal characteristic or exposure among infants in the EPPD group conveyed information about the risk of CLD.
Early neonatal factors [Table 2]
Table 2.
Univariate associations between early neonatal factors and the risk of chronic lung disease (CLD). The right-most column provides the maximum number of infants with the attribute listed in each row; any differences are due to missing data. All other data are the percentage of infants with CLD among those infants with the attribute listed on the two left columns and the respiratory patterns listed as column headings. For example, among infants with a SNAP-II score ≤ 19 who had the Low FiO2 respiratory pattern, the prevalence of CLD was 16%.
| Early neonatal factors | Respiratory Pattern | Row N |
|||
|---|---|---|---|---|---|
| Low FiO2 | PD | EPPD | |||
| SNAP-II™* | ≤ 19 | 16 | 42 | 61 | 622 |
| 20–29 | 17 | 57 | 72 | 303 | |
| ≥ 30 | 31 | 74 | 76 | 260 | |
| Surfactant | Yes | 17 | 55 | 70 | 1083 |
| No | 18 | 28 | 63 | 121 | |
| Hydrocortisone | Yes | 20 | 70 | 69 | 89 |
| No | 17 | 50 | 69 | 1115 | |
| Dexamethasone | Yes | 33 | 75 | 61 | 12 |
| No | 17 | 52 | 70 | 1192 | |
| Analgesic | Yes | 23 | 61 | 69 | 664 |
| No | 13 | 43 | 69 | 540 | |
| Sedation | Yes | 26 | 54 | 59 | 174 |
| No | 16 | 51 | 72 | 1030 | |
| Vitamin A | Yes | 26 | 48 | 66 | 342 |
| No | 15 | 53 | 71 | 862 | |
| Confirmed bacteremia |
Yes | 20 | 66 | 65 | 73 |
| No | 17 | 51 | 70 | 1130 | |
| Confirmed tracheal infection |
Yes | 0 | 62 | 68 | 48 |
| No | 18 | 52 | 70 | 1147 | |
| Mechanical ventilation** (day 7) |
Yes | 29 | 68 | 75 | 726 |
| No | 14 | 30 | 42 | 477 | |
| Percent CLD* | 17 | 52 | 69 | 52 | |
| Maximum Column N | 240 | 456 | 508 | 1204 | |
SNAP-II™=Score for Neonatal Acute Physiology, PD=pulmonary deterioration, EPPD=early and persistent pulmonary dysfunction, CLD=chronic lung disease
Mechanical ventilation includes conventional mechanical ventilation and high frequency ventilation
A SNAP-II™ greater than 30 and receipt of mechanical ventilation on postnatal day 7 were associated with an increased risk of CLD in all groups. In the Low FiO2 group, receipt of dexamethasone, vitamin A, analgesics, and sedation were associated with an increased risk of CLD. In the PD group, receipt of surfactant, hydrocortisone, dexamethasone, and analgesics, as well as confirmed bacteremia and tracheal infection, were associated with an increased risk of CLD. In the EPPD group, receipt of surfactant was associated with an increased risk of CLD and receipt of dexamethasone, sedation, and vitamin A were associated with a decreased risk of CLD.
Late neonatal factors [Table 3]
Table 3.
Univariate associations between late neonatal factors and the risk of chronic lung disease (CLD). The right-most column provides the maximum number of infants with the attribute listed in each row; any differences are due to missing data. All other data are the percentage of infants with CLD among those infants with the attribute listed on the two left columns and the respiratory patterns listed as column headings. For example, among infants who received hydrocortisone that had the Low FiO2 respiratory pattern, the prevalence of CLD was 40%.
| Late neonatal factors | Respiratory Pattern | Row N |
|||
|---|---|---|---|---|---|
| Low FiO2 | PD* | EPPD* | |||
| Hydrocortisone | Yes | 40 | 76 | 69 | 142 |
| No | 17 | 40 | 69 | 1062 | |
| Dexamethasone | Yes | 50 | 74 | 86 | 78 |
| No | 17 | 51 | 67 | 1126 | |
| Analgesic | Yes | 27 | 66 | 75 | 578 |
| No | 14 | 39 | 60 | 626 | |
| Sedation | Yes | 32 | 63 | 74 | 264 |
| No | 16 | 49 | 67 | 940 | |
| Vitamin A | Yes | 21 | 50 | 65 | 354 |
| No | 16 | 52 | 72 | 850 | |
| Confirmed bacteremia |
Yes | 16 | 63 | 73 | 298 |
| No | 17 | 48 | 68 | 905 | |
| Confirmed tracheal infection |
Yes | 28 | 63 | 74 | 230 |
| No | 17 | 49 | 68 | 964 | |
| Mechanical ventilation(day 14)** |
Yes | 31 | 72 | 77 | 717 |
| No | 14 | 26 | 36 | 486 | |
| Mechanical ventilation(day 21)** |
Yes | 43 | 72 | 77 | 696 |
| No | 12 | 27 | 39 | 504 | |
| Patent ductus arteriosus (PDA) |
Yes | 20 | 58 | 71 | 798 |
| No | 14 | 40 | 64 | 406 | |
| Pneumothorax (PTX) |
Yes | 25 | 73 | 81 | 91 |
| No | 17 | 50 | 68 | 1113 | |
| Pulmonary interstitial emphysema (PIE) |
Yes | 50 | 79 | 85 | 189 |
| No | 16 | 49 | 63 | 1015 | |
| Necrotizing Enterocolitis*** |
No/Stage I, II | 15 | 50 | 69 | 1009 |
| Stage IIIa | 100 | 67 | 50 | 13 | |
| Stage IIIb | 38 | 92 | 68 | 48 | |
| Isolated perf | 50 | 71 | 88 | 34 | |
| Percent CLD | 17 | 52 | 69 | 52 | |
| Maximum Column N | 240 | 456 | 508 | 1204 | |
PD=pulmonary deterioration, EPPD=early and persistent pulmonary dysfunction
Includes conventional mechanical ventilation and high frequency ventilation
Bell's staging
In all groups, infants who received dexamethasone, analgesics, or sedation, as well as those who were ventilated on day 14 or 21, had confirmed tracheal infection, developed pneumothorax or isolated intestinal perforation, were at increased risk of CLD. In the Low FiO2 group, receipt of hydrocortisone and vitamin A, or required surgery for necrotizing enterocolitis were associated with an increased risk of CLD. Infants in the PD group were at increased risk of CLD if they received hydrocortisone, had confirmed bacteremia, or required surgery for necrotizing enterocolitis. In the EPPD group, confirmed bacteremia and PDA were associated with an increased risk, and receipt of vitamin A was associated with a decreased risk of CLD.
Placenta histology and microbiology [Table 4]
Table 4.
Univariate associations between placenta histologic characteristics and the risk of chronic lung disease (CLD). The right-most column provides the maximum number of infants with the attribute listed in each row; any differences are due to missing data. All other data are the percentage of infants with CLD among those infants with the attribute listed on the two left columns and the respiratory patterns listed as column headings. For example, among infants who had inflammation of the chorionic plate that had the Low FiO2 respiratory pattern, the prevalence of CLD was 12%.
| Placenta histology | Respiratory pattern | Row N |
|||
|---|---|---|---|---|---|
| Low FiO2 | PD* | EPPD* | |||
| Inflammation chorionic plate** | Yes | 12 | 48 | 75 | 212 |
| No | 19 | 53 | 69 | 895 | |
| Inflammation chorion/deciduas*** | Yes | 8 | 53 | 75 | 411 |
| No | 25 | 51 | 67 | 696 | |
| Neutrophilic infiltration fetal stem vessels |
Yes | 15 | 57 | 81 | 274 |
| No | 19 | 50 | 67 | 814 | |
| Umbilical cord vasculitis**** | Yes | 11 | 53 | 73 | 181 |
| No | 20 | 50 | 70 | 901 | |
| Thrombosis of fetal stem vessels |
Yes | 40 | 62 | 77 | 58 |
| No | 17 | 52 | 70 | 1039 | |
| Infarct | Yes | 32 | 56 | 75 | 190 |
| No | 15 | 51 | 69 | 927 | |
| Increased syncytial knots | Yes | 40 | 59 | 76 | 223 |
| No | 13 | 50 | 69 | 898 | |
| Decidual hemorrhage/fibrin deposition |
Yes | 25 | 48 | 72 | 182 |
| No | 17 | 52 | 70 | 923 | |
| Percent CLD* | 18 | 52 | 70 | 52 | |
| Maximum Column N | 227 | 429 | 470 | 1026 | |
PD=pulmonary deterioration, EPPD=early and persistent pulmonary dysfunction, CLD=chronic lung disease
stage 3 and severity 3
grades 3 and 4
grades 3, 4 and 5
Thrombosis of fetal stem vessels, infarcts, and increased syncytial knots were associated with an increased risk of CLD, and this was most pronounced in the Low FiO2 group. In all groups, no organism or group of organisms recovered from the placenta conveyed information about CLD risk (data not shown).
Time Oriented Risk Models [Table 5]
Table 5.
Odds ratios and 95% confidence intervals obtained with time-oriented risk models of chronic lung disease for three patterns of respiratory disease (Low FiO2, Pulmonary Deterioration [PD], or Early and Persistent Pulmonary Dysfunction [EPPD]) during the first four postnatal weeks.
| 1. Antenatal | Low FiO2 | PD | EPPD |
|---|---|---|---|
| GA 23–24 wks | 3.2 (2.0, 8.8) | 2.5 (1.5, 4.3) | |
| GA 25–26 wks | 1.9 (1.1, 3.1) | ||
| BW Z-score < −1 | 26 (7.0, 95) | 4.4 (2.3, 8.2) | 2.0 (1.1, 3.9) |
| Male | 1.9 (1.2, 3.1) | ||
| Cesarean delivery | 0.5 (0.2, 1.2) | ||
| Indication is PE or FI | 1.9 (0.98, 3.8) | ||
| 2. Early neonatal (week 1) | |||
| SNAP-IITM 30+ | 3.3 (1.02, 11) | 2.0 (1.1, 3.9) | |
| Definite bacteremia | 2.6 (1.01, 6.9) | ||
| Surfactant | 0.2 (0.1, 0.7) | ||
| Mechanical ventilation on day 7 | 1.5 (0.5, 3.9) | 4.2 (2.5, 6.9) | 2.7 (1.5, 4.7) |
| 3. Late neonatal (weeks 2–4) | |||
| Dexamethasone | 3.0 (1.2, 7.2) | ||
| Analgesic | 3.4 (1.2, 9.5) | ||
| PTX | 1.9 (1.1, 3.2) | ||
| PIE | 17 (2.1, 140) | 2.6 (1.5, 4.6) |
Variables offered:
Antenatal epoch: gestational age 23–24 weeks, gestational age 25–26 weeks, birth weight Z-score, sex, complete course of antenatal steroids, multiple birth, cesarean delivery, delivery for preeclampsia or fetal indication, thrombosis of the fetal stem vessels in the placenta
Early neonatal epoch (week 1): SNAP-IITM ≥ 30, confirmed early bacteremia, confirmed tracheal infection, receipt of surfactant, hydrocortisone, dexamethasone, analgesic, sedation, or vitamin A, mechanical ventilation
Late neonatal epoch (weeks 2–4): confirmed bacteremia, confirmed tracheal infection, receipt of hydrocortisone, dexamethasone, analgesics, sedation, or vitamin A, patent ductus arteriosus, pneumothorax, pulmonary interstitial emphysema
Low FiO2 group
In the Low FiO2 group, birth weight z-score < -1 was associated with an increased risk of CLD. In the early neonatal epoch, only SNAP-IITM >30 was associated with an increased risk of CLD, while receipt of surfactant was associated with a reduced risk. In the late neonatal epoch, receipt of analgesics was associated with an increased risk of CLD. The presence of pulmonary interstitial emphysema was also associated with increased risk; however, this diagnosis was assigned to only six infants.
PD group
Infants in the PD group were at increased risk of CLD if their gestational age was < 27 weeks, their birth weight Z-score was < -1, and they were male. In the early neonatal epoch, SNAP-IITM >30, definite bacteremia, and mechanical ventilation on day 7 were associated with an increased risk of CLD, while a diagnosis of pneumothorax in the late neonatal epoch was associated with an increased risk of CLD.
EPPD group
Among infants in the EPPD group, those whose gestational age was 23–24 weeks, had a birth weight Z-score of < -1 were at increased risk of CLD. Delivery for preeclampsia or a fetal indication approached nominal statistical significance. Only mechanical ventilation on day 7 entered in the early neonatal epoch, while two late neonatal variables, pulmonary interstitial emphysema and receipt of hydrocortisone, provided additional information about an increased risk of CLD.
DISCUSSION
In this study, we evaluated CLD risk factors in three groups of infants characterized by their pulmonary function during the first two weeks after birth. These groups differed in their likelihood of developing CLD. Differences in early exposure to oxygen might not only have defined these three groups, but also influenced CLD risk. However, we hypothesized that each group had its own risk profile for CLD that included factors not directly related to pulmonary function or therapies. We were particularly interested in antecedents of CLD among infants with little exposure to increased concentrations of oxygen during early life (the Low FiO2 group). Although multiple factors were significant in univariate analysis (e.g., vitamin A), once adjustments were made in multivariate analysis, many of these were no longer significant.
In a previous report, we demonstrated that fetal growth restriction was the antenatal factor that best predicted CLD.16 In the current study, fetal growth restriction was associated with increased CLD risk in all groups defined by early pulmonary function, even after adjustment for early and late postnatal neonatal exposures and other morbidities. The observation that this effect was most pronounced in the Low FiO2 group suggests that processes that limit fetal growth might predispose to abnormal lung growth before and after birth, ultimately resulting in pulmonary dysfunction. This cascade of events appears to occur even in the absence of exposures likely to result in lung injury.
Severe growth restriction might contribute to CLD risk in several ways. First, factors that impair fetal somatic growth might also impair lung development, resulting in abnormal development of terminal air sacs and alveoli, or abnormal pulmonary angiogenesis.17 This might be similar to the changes characteristic of the "new BPD".18,19
Second, an imbalance between angiogeneic and anti-angiogeneic factors might disrupt normal placental angiogenesis and abnormal fetal angiogenesis, including the vasculature of the fetal lung. Preeclampsia is the disorder most closely associated with fetal growth restriction and is also associated with disturbed angiogenesis.20 Our observation of an increased risk of CLD among those whose placenta had increased syncytial knots, a histologic abnormality characteristic of preeclampsia, supports this possibility.
Third, chronic fetal hypoxia, sometimes identified as a factor that impairs growth21, might be accompanied by impaired lung development. This possibility is supported by observations from animal studies. Impaired alveolar and pulmonary artery development occurs in neonatal mice exposed to chronic hypoxia during the first two weeks of life, a period of lung development that corresponds to human fetal lung development during the third trimester.22 Regardless of the mechanism, the interaction between factors that control fetal somatic growth and lung maturation is complicated.
Fetal growth restriction also was associated with CLD in the PD group and to a lesser extent in the EPPD group. In the PD group, characteristics associated with gestational age and male gender appear to have influenced CLD risk. In addition, among neonatal exposures, mechanical ventilation was most influential. Although gestational age and FGR were important in the EPPD group, mechanical ventilation at 7 days and dexamethasone treatment in the late neonatal period were the factors most strongly associated with CLD. The latter association probably reflects selective use of this therapy in infants likely to develop CLD. PIE was also associated with increased risk. These observations suggest that even in the absence of vulnerability imparted by growth restriction, infants with PD are particularly vulnerable to CLD due to extreme immaturity or exposures that injure the lung (e.g. high oxygen concentrations and mechanical ventilation).
In the Low FiO2 group and EPPD group, exposure to analgesics and dexamethasone, respectively, during the late neonatal period was associated with an increased risk of CLD. For the Low FiO2 group, one possibility is that analgesic use itself renders an infant more likely to develop CLD, directly or through secondary effects. Narcotics, such as morphine, depress the respiratory drive and might therefore prolong the need for mechanical ventilation and, thus, increase the risk of ventilator-induced lung injury. In a randomized controlled trial 23, infants treated with mechanical ventilation who were allocated to receive continuous morphine infusions were ventilated for one week longer than infants allocated to placebo.24 Another explanation for this observation is that exposure to analgesics or dexamethasone is indicative of greater illness severity or longer duration of mechanical ventilation. A more valid conclusion about dexamethasone is available from meta-analyses of randomized, placebo controlled trials,25,26 which indicate that this treatment reduces the risk of CLD. The decision to treat an infant with an analgesic or to ventilate an infant might reflect the physician’s perception that the infant is sufficiently ill to require these therapies, rather than analgesics or ventilation contributing to CLD risk. Alternatively, many infants treated with mechanical ventilation receive analgesics, for sedation. This is an example of confounding by indication, a problem common to many epidemiologic studies incorporating clinical decisions as variables, outcomes or exposures.27,28 For example, later-occurring conditions or events, such as necrotizing enterocolitis or PDA ligation, might both require treatment with narcotic analgesics and increase the risk of CLD through pathogenic mechanisms not involving the medication.
Intra-amniotic inflammation has been implicated in the development of so-called atypical CLD (i.e. CLD not preceded by respiratory distress syndrome).29 All infants in our Low FiO2 group and many infants in our PD group would have been classified as having atypical CLD using this definition. We found no association between CLD and either histologic markers of placental inflammation or microbiologic evidence of placental infection and CLD in groups defined by their early lung function. Our data suggest that intrauterine infection is unlikely to be a risk factor for CLD, regardless of the presence or absence or early respiratory disease. Chorioamnionitis has been inconsistently reported as affecting respiratory outcomes (respiratory distress syndrome and CLD)30,31, most likely because the diagnosis of chorioamnionitis is challenging and does not provide information about the organism, duration, or extent of fetal involvement. One possibility that we did not find an association is that respiratory care practices in ELGAN centers might have attenuated the putative pathway to CLD that involves intrauterine inflammation followed by postnatal ventilator-induced lung injury.31
We did not observe a relationship between severe NEC and CLD risk, as has been reported previously.32,33 One explanation for the difference in these findings is that our sample size permitted adjustment for a large number of confounders and antecedents. Another is that antenatal phenomena contributed to both CLD and NEC, but by our use of time oriented regression modeling we reduced the probability of perceiving an epiphenomenon (such as NEC) as a risk factor.
Conclusion
The risk of CLD varies among infant groups defined by their pulmonary function during the first two postnatal weeks. Among infants with little exposure to oxygen during this period, FGR is the factor most strongly associated with CLD. Among infants with PD or EPPD, other factors, such as gestational age, male gender, and mechanical ventilation also convey nearly as much or more information about CLD risk. Therefore, CLD among infants with little exposure to oxygen early in life might result almost exclusively from fetal phenomena related to lung growth. These observations have the potential to inform future investigations of the biological mechanisms underlying the association between patterns of early pulmonary function and the development of CLD.
"What is already known on this topic" –Pulmonary dysfunction in the first two postnatal weeks in preterm infants can be characterized into three groups by the fraction of inspired oxygen they require. The risk of chronic lung disease varies among infant groups defined by their pulmonary function during the first two postnatal weeks.
"What this study adds" –Among infants with little exposure to oxygen during this period, fetal growth restriction is the factor most strongly associated with chronic lung disease. Among infants with pulmonary deterioration or early and persistent pulmonary dysfunction, other factors, such as gestational age, male gender, and mechanical ventilation convey nearly as much or more information about chronic lung disease risk as fetal growth restriction.
Acknowledgments
The authors wish to acknowledge our ELGAN study colleagues:
Olaf Dammann, Tufts Medical Center, Boston MA; Bhavesh L. Shah, Baystate Medical Center, Springfield MA; Camilia Martin, Beth Israel Deaconess Medical Center, Boston, MA; Robert Insoft, Brigham & Women's Hospital, Boston, MA; Karl Kuban, Boston Medical Center, Boston, MA; Francis Bednarek, U Mass Memorial Health Center, Worcester, MA; John Fiascone, Tufts Medical Center, Boston MA; Richard A. Ehrenkranz, Yale University School of Medicine, New Haven, CT; T. Michael O’Shea, Wake Forest University/Baptist Medical Center, Winston-Salem, NC; Stephen C. Engelke, University Health Systems of Eastern Carolina, Greenville, NC; Carl Bose, The University of North Carolina at Chapel Hill, Chapel Hill NC; Mariel Poortenga, Ed Beaumont, DeVos Children's Hospital, Grand Rapids, MI; Nigel Paneth, Sparrow Hospital, Lansing MI; Michael D. Schreiber, University of Chicago Hospital, Chicago, IL; Daniel Batton, William Beaumont Hospital, Royal Oak, MI; Greg Pavlov, Frontier Science and Technology Research Foundation, Amherst, NY, and our project officer, Deborah Hirtz.
Funding: This study was supported by a cooperative agreement with NINDS 5U01NS040069-04. Dr. Carl Bose was supported by the Thrasher Research Fund.
Copyright Licence statement: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Group and co-owners or contracting owning societies (where published by the BMJ Group on their behalf), and its Licensees to permit this article (if accepted) to be published in Archives of Disease in Childhood editions and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence.
Abbreviations
- PD
pulmonary deterioration
- ELGAN
extremely low gestational age newborn
- CLD
chronic lung disease
- PDA
patent ductus arteriosus
- EPPD
early and persistent pulmonary dysfunction
- SNAP
score for Neonatal Acute Physiology
- FGR
fetal growth restriction
- PTX
pneumothorax
- PMA
post-menstrual age
- PE
preeclampsia
- pPROM
prolonged, premature rupture of membranes
- FI
fetal indication
- PTL
preterm labor
- BW
birth weight
Footnotes
Financial Disclosure: The authors have no financial issues to disclose.
Competing Interests: The authors have no competing interests to disclose.
References
- 1.Laughon M, Allred EN, Bose C, O'Shea TM, Van Marter LJ, Ehrenkranz RA, et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics. 2009;123(4):1124–1131. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laughon M, Bose C, Allred E, O'Shea TM, Van Marter LJ, Bednarek F, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119(2):273–280. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 4.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 5.Hecht J, Onderdonk A, Delaney M, Allred E, Kliman H, Zambrano E, et al. Characterization of chorioamnionitis in second trimester c-section placentas and correlation with microorganism recovery from sub-amniotic tissues. Pediatr Dev Pathol. 2007:1. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 6.Hecht JL, Kliman HJ, Allred EN, Pflueger SM, Chang CH, Doss BJ, et al. Reference weights for placentas delivered before the 28th week of gestation. Placenta. 2007;28(10):987–990. doi: 10.1016/j.placenta.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995;126(4):605–610. doi: 10.1016/s0022-3476(95)70362-4. [DOI] [PubMed] [Google Scholar]
- 8.Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O'Shea TM. Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics. 1999;104(6):1345–1350. doi: 10.1542/peds.104.6.1345. [DOI] [PubMed] [Google Scholar]
- 9.Leviton A, Pagano M, Kuban KC, Krishnamoorthy KS, Sullivan KF, Allred EN. The epidemiology of germinal matrix hemorrhage during the first half- day of life. Dev Med Child Neurol. 1991;33(2):138–145. doi: 10.1111/j.1469-8749.1991.tb05092.x. [DOI] [PubMed] [Google Scholar]
- 10.Leviton A, Kuban KC, Pagano M, Allred EN, Van Marter L. Antenatal corticosteroids appear to reduce the risk of postnatal germinal matrix hemorrhage in intubated low birth weight newborns. Pediatrics. 1993;91(6):1083–1088. [PubMed] [Google Scholar]
- 11.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Hypothyroxinemia of prematurity and the risk of cerebral white matter damage. J Pediatr. 1999;134:706–711. doi: 10.1016/s0022-3476(99)70285-4. [DOI] [PubMed] [Google Scholar]
- 12.Leviton A, Dammann O, Allred EN, Kuban KCK, Pagano M, Van Marter LJ, et al. Antenatal corticosteroids and cranial ultrasound abnormalities. Am J Obstet Gynecol. 1999;181:1007–1017. doi: 10.1016/s0002-9378(99)70344-3. [DOI] [PubMed] [Google Scholar]
- 13.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birthweight infants. Pediatric Research. 1999;46(5):566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Laptook AR, O'Shea TM, Shankaran S, Bhaskar B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115(3):673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 15.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22(16):2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- 16.Bose C, Van Marter LJ, Laughon M, O'Shea TM, Allred EN, Karna P, et al. Fetal Growth Restriction and Chronic Lung Disease Among Infants Born Before the 28th Week of Gestation. Pediatrics. 2009 doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998;53(1):81–94. doi: 10.1016/s0378-3782(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 18.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46(6):641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160(4):1333–1346. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- 20.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21(5):279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neerhof MG, Thaete LG. The fetal response to chronic placental insufficiency. Semin Perinatol. 2008;32(3):201–205. doi: 10.1053/j.semperi.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, et al. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L86–L95. doi: 10.1152/ajplung.00534.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363(9422):1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 24.Bhandari V, Bergqvist LL, Kronsberg SS, Barton BA, Anand KJ. Morphine administration and short-term pulmonary outcomes among ventilated preterm infants. Pediatrics. 2005;116(2):352–359. doi: 10.1542/peds.2004-2123. [DOI] [PubMed] [Google Scholar]
- 25.Halliday HL, Ehrenkranz RA, Doyle LW. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2009;(1):CD001146. doi: 10.1002/14651858.CD001146.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Halliday HL, Ehrenkranz RA, Doyle LW. Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2009;(1):CD001145. doi: 10.1002/14651858.CD001145.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sorensen HT, Blot WJ. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther. 2002;9(3):199–205. doi: 10.1097/00045391-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Walker AM. Confounding by indication. Epidemiology. 1996;7(4):335–336. [PubMed] [Google Scholar]
- 29.Lee J, Oh KJ, Yang HJ, Park JS, Romero R, Yoon BH. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med. 2009:1–7. doi: 10.1080/14767050902994705. [DOI] [PubMed] [Google Scholar]
- 30.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97(2):210–215. [PubMed] [Google Scholar]
- 31.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140(2):171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 32.Schulzke SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med. 2007;161(6):583–590. doi: 10.1001/archpedi.161.6.583. [DOI] [PubMed] [Google Scholar]
- 33.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]