Abstract
Background
Sugarcane is an economically important crop contributing to about 80 % of the world sugar production. Increasing efforts in molecular biological studies have been performed for improving the sugar yield and other relevant important agronomic traits. However, due to sugarcane’s complicated genomes, it is still challenging to study the genetic basis of traits, such as sucrose accumulation. Sucrose transporters (SUTs) are critical for both phloem loading in source tissue and sucrose uptaking in sink tissue, and are considered to be the control points for regulating sucrose storage. However, no genomic study for sugarcane sucrose transporter (SsSUT) families has been reported up to date.
Results
By using comparative genomics and bacterial artificial chromosomes (BACs), six SUT genes were identified and characterized in S. spontaenum. Phylogenetic analyses revealed that the two pairs SsSUTs (SsSUT1/SsSUT3 and SsSUT5/SsSUT6) could be clustered together into two separate monocot specific SUT groups, while SsSUT2 and SsSUT4 were separated into the other two groups, with members from both dicot and monocot species. Gene structure comparison demonstrated that the number and position of exons/introns in SUTs were highly conserved among the close orthologs; in contrast, there were variations among the paralogous SUTs in Sacchuarm. Though with the high polyploidy level, gene allelic haplotype comparative analysis showed that the examined four SsSUT members exhibited conservations of gene structures and amino acid sequences among the allelic haplotypes accompanied by variations of intron sizes. Gene expression analyses were performed for tissues from seedlings under drought stress and mature plants of three Saccharum species (S.officinarnum, S.spotaneum and S.robustum). Both SUT1 and SUT4 expressed abundantly at different conditions. SUT2 had similar expression level in all of the examined tissues, but SUT3 was undetectable. Both of SUT5 and SUT6 had lower expression level than other gene member, and expressed stronger in source leaves and are likely to play roles in phloem loading. In the seeding plant leave under water stress, four genes SUT1, SUT2, SUT4 and SUT5 were detectable. In these detectable genes, SUT1 and SUT4 were down regulated, while, SUT2 and SUT5 were up regulated.
Conclusions
In this study, we presented the first comprehensive genomic study for a whole gene family, the SUT family, in Saccharum. We speculated that there were six SUT members in the S. spotaneum genome. Out of the six members, SsSUTs, SsSUT5 and SsSUT6 were recent duplication genes accompanied by rapid evolution, while, SsSUT2 and SsSUT4 were the ancient members in the families. Despite the high polypoidy genome, functional redundancy may not exist among the SUTs allelic haplotypes supported by the evidence of strong purifying selection of the gene allele. SUT3 could be a low active member in the family because it is undetectable in our study, but it might not be a pseudogene because it harbored integrated gene structure. SUT1 and SUT4 were the main members for the sucrose transporter, while, these SUTs had sub-functional divergence in response to sucrose accumulation and plant development in Saccharum.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-016-2419-6) contains supplementary material, which is available to authorized users.
Keywords: Saccharum species, Sucrose transporter, Gene family, Allelic haplotype, Polyploidy
Background
Sucrose transporters (SUTs) are important for both phloem loading in source tissue and sucrose uptake into some sink cells [1]. SUTs are considered to be the control points for sucrose storage in plant because they can carry sucrose across cell membranes and play an important role in loading sucrose into phloem systems in a series of steps [1]. Plant SUTs belong to the glycoside-pentoside-hexuronide (GPH) cation symporter family (TC2.A.2) that is part of the major facilitator super-family (MFS) [2]. Transporters in the GPH family have the primary characteristics of MFS proteins that contain 12 transmembrane spanning domains with N- and C-termini in the cytoplasm [3]. Since the first plant sucrose transporter SoSUT1 was functionally identified using an elegant yeast complementation strategy from spinach (Spinacea Oleracea) [4], SUTs have been demonstrated to effect on the multiple aspects of plant development such as biomass partitioning, pollen germination, restraining plant growth, fruit size reduction and ethylene biosynthesis [5–9].
Comprehensive understanding of the molecular structure and evolution of a gene family in plant species is the first step towards understanding their physiological roles and metabolic mechanism involved in different growth phases. Recent studies have revealed that SUT was a small gene family that consisted of at least four SUT genes in the most plant species. The SUT family members have been identified from a number of plant species such as Arabidopsis [10], rice (Oryza sativa) [11], wheat (Triticum aestivum) [12], populus [8], sorghum (Sorghum bicolor) [13] and pineapple (Ananas comosus) (Unpublished, Zhang and Ming) as well. Phylogenetic analysis of SUT family suggested that the plant SUT could be divided into five subgroups including two monocot specific subgroups, one dicot specific and two monocot-dicot subgroups. SUT family members in many plant species are divergent in function and deferentially expressed in different tissues types or at different plant developmental stages. For instance, in rice, OsSUT1 expression has been detected in germinated seeds, leaf blades, leaf sheaths and panicles [14–16]; the expressions of both OsSUT3 and OsSUT5 are dramatically lower in embryos than those of OsSUT1, OsSUT2, and OsSUT4 [17]; OsSUT4 has been detected in most tissues such as roots, leaves, and panicles [11], and could play a role in sucrose loading into the sheath phloem of the upper leaves during the post-heading period for sucrose transport to developing grains [18]. In Arabidopsis [10], AtSUC2 has been detected in phloem and companion cells in source leaves [19, 20] and functions in loading sucrose into the phloem sieve elements (SEs), correspond with the result identified by tissue-specific complementation of different promoters [7, 21] and by 14C labeling studies [22]; AtSUC3 and AtSUC4 are expressed in minor veins of source leaves of mature plants [23–25].
Sugarcane (Saccharum spp.) is one of the world’s most produced crops (FAOSTAT, 2015), and contributes to about 80 % of the world sugar and about 40 % of ethanol production worldwide. Modern sugarcane cultivar has one of the most complex genome among all the crops; by being both aneupoid and autopolypoid with an extreme ploidy level that can range from octoploidy (x = 8) to dodecaploidy (x = 12). Approximately 80 % of cultivars’ chromosomes are derived from S. officinarum and 10–20 % is derived from S.spontaneum with the remained from interspecific recombination [26–28]. Sugarcane is not only an economically important crop species, but also serves as an important model crop for studying sucrose transporters because its remarkable ability to accumulate vast amounts of sucrose in its stems that can reach close to 700 mM or in excess of 50 % of the dry weight (DW) (DW) [29]. But to date, limited works in characterizing these SUT genes have been reported, except for SUT1 (transcripts accession:AY780256.1, GU812864.1 and BU925792). In an earlier study based on sugarcane Expressed Sequence Tag (EST) database survey, SUT1 was revealed to be more abundant in the mature internodes than the other tissues [30]. This result was confirmed by a study for Hawaiian sugarcane cultivar, in which the SUT1 transcript levels increased during maturation and sucrose storage, whereas, SUT1 expression was observed to be not affected by sugarcane yellow leaf virus (SCYLV) infection in sugarcane [31]. Biochemical analysis of sugarcane SUT1 suggested that SUT1 was highly selective for sucrose, but had a relatively low affinity for sucrose, inhibited by sucralose and played key role in sucrose loading from the vascular tissues into the storage sites in parenchyma cells of sugarcane stems [32, 33]. Besides SUT1, the gene family of SUTs is not understood in sugarcane due to the formidable challenge caused by its high degree of polyploidy and heterozygosity genome.
In this study, to gain comprehensive understandings of the molecular and evolutionary characterization as well as the possible functions of SUT family in sugarcane, based on combination of comparative genomics strategies and high genome coverage of bacterial artificial chromosomes (BACs) libraries resources, we identified and characterized SUT gene families in Saccharum species and investigated their transcriptional expression patterns. The analysis in this study mainly focused on: (1) identifying the gene members and allele haplotypes of the SUT gene family in sugarcane; (2) analyzing evolutionary relationship, exon/intron organization of the SUT gene family; and (3) characterizing the expression patterns of the SUT gene family in three progenitor Saccharum species.
Methods
Plant materials
Three varieties of Saccharum species were used in the study: LA-Purple (S. officinarum, 2n = 8x = 80), Molokai6081 (S. robustum, 2n = 8x = 80) and SES208 (S. spontaneum, 2n = 8x = 64) [34]. Plants were grown in plastic pots under greenhouse conditions and standard growing practices. Tissue samples were obtained from 10-month old plants (as replicates) for leaf roll, leaf, top immature internode (i.e. internode number 3), premature internode (i.e. internode number 9 for ‘LA Purple’ and Molokai6081 due to short internode, and internode number 6 for SES208 due to long internode) and mature internode (i.e. internode number 15 for ‘LA Purple’ and Molokai6081, and internode number 9 for SES208 due to long internode – most SES208 plants have about 12 internodes). The internodes were numbered from top to bottom according to the method of Moore [35]. Stem and leaf tissues from seedlings of the three species were collected at 35 days after planting. For drought stress treatment, the 35 day-old seedlings were treated with PEG6000 (30 %) for 48 h, and stem and leaf tissues were collected. The tissues were immediately frozen using liquid nitrogen and stored at −80 °C prior to RNA isolation.
BAC libraries
The haploid of S. Spontaneum SES208, Ap85-441 (2n = 4x = 32), was used to construct the BAC library. Nuclei were isolated from the young leaf tissues of AP85-441 following the method described by Ming et al. [36]. The high molecular weight DNA embedded in agarose was partially digested using HindIII. Fractions at approximately 100 kb were recovered and cloned into pSMART BAC vector (Lucigen, LA). 38,400 clones from AP85-441 BAC libraries were picked and stored in 100 384-well plates with freezing medium. BAC clones were grided onto Performa II Nylon Filters (Genetix) using Q-Pix 2 (Genetix).
Database search for the SUT gene family and phylogenetic analyses
The sequence data used in this study were collected using the keyword “sucrose transporter” and a query search in the GenBank using the known SUT gene sequences from sorghum [13], rice [11] and Arabidopsis [24]. Matches achieved similarity scores of 50.0 and probability scores >50.0 and e-value <10−4 were collected.
The amino acid sequences of sucrose transporter gene family members in 6 monocotyledons (Zea mays, Sorghum bicolor, Oryza sativa, Brachypodium distachyon, Setaria italica and Saccharum spontaneum) and 5 dicotyledons (Arabidopsis thaliana, Citrus sinensis, Glycine max, Solanum tuberosum and Vitis vinifera,) identified by searching public databases available at various resources. The phylogenetic trees were constructed with the MEGA5.2.1 program with ClustalW alignment using default parameter.
Identification and sequencing of SUT families from BAC library
BAC library screening was carried out as described by Yu et al.[37]. The BAC clones representing different haplotypes were selected. The insert size of BAC clones was estimated by comparing with standard size markers using CHEF gel electrophoresis. The BAC DNAs were isolated using PhasePrepTM TMBAC DNA kit (Sigma-Aldrich, NA0100-1KT) and the sequencing libraries were prepared individually with unique barcode for each clone. The sequencing libraries were then pooled and sequenced with 150 bp, pair-end reads on Illumina Hiseq2500 at Center for Genomics and Biotechnology in Fujian Agriculture and Forestry University. The raw reads were then assembled using SPAdes Genome Assember v. 3.1.1 (http://bioinf.spbau.ru/en/spades).
Genomic sequence annotation and functional prediction
The genomic sequences of SUT genes were annotated by DNA subway (http://dnasubway.iplantcollaborative.org/), and the corresponding CDS sequences were translated into protein by the EXPASy-translate tool (http://web.expasy.org/translate/). The exon-intron structures were graphed using online tool GSDS (http://gsds.cbi.pku.edu.cn/). The putative conserved domains of sucrose transporter protein were detected by using BLASTp (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and InterPro (http://www.ebi.ac.uk/interpro/scan.html). The protein transmembrane helices domain was predicted using TMPRED (http://www.ch.embnet.org/software/TMPRED_form.html). The isoelectric point and relative molecular mass of the protein were predicted using ExPASy (http://web.expasy.org/compute_pi/).
Analysis of sucrose transporter gene co-expression profiling
The sorghum gene models were used as reference to align the Saccharum RNA-seq database by using NOVOALIGN (http://www.novocraft.com/) with default parameter. The normalization and statistical evaluation of differential gene expression has been performed using EDGE-R with a p-value cut-off of 0.05 and using the Benjamini-Hochberg (1995)[38] method for multiple testing corrections. The raw data was normalized according to the default procedure of the differential expression analysis package used. The dispersion was estimated using the pooled setting. The expression values were log-transformed, and cluster analyses were performed using a software cluster with Euclidean distances and the hierarchical cluster method of “complete linkage clustering”. The clustering tree was constructed and viewed in JAVA Treeview.
Experimental validation of expression levels of SUT gene by qRT-PCR
The expression levels of six SUT genes in three tissues (internode 9, 15 and leaf roll in LA-Purple, internode 8,13 and leaf roll in Molokai6081, and internode 6, 9 and leaf roll in SES208) of three Saccharum species were validated by qRT-PCR. Gene-specific primer pairs were designed by using Integrated DNA Technologies (IDT) (http://www.idtdna.com/Primerquest/Home/Index). After treated with DNase I (Tiangen, China), two microgram of RNA was used in reverse transcription with the SuperScript VILO cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s guidelines. The real-time qPCR was performed by using Multicolor Real-Time PCR Detection System (Bio-Rad) with conditions for all reactions were 95 °C for 30s, 40 cycles of 95 °C for 5 s, followed by 60 °C for 30s, and 95 °C for 10s. Melting curve analysis were performed to confirm the PCR specificity. The glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) and Eukaryotic elongation factor 1a (eEF-1a) were selected as internal standard for normalization [39], and three replicates were completed for each sample. The relative expression level for each SUT gene in different tissues of three Saccharum species were calculated by using the 2-ΔΔCt method. The correlation coefficient was calculated between the transcript accumulation levels obtained by RNAseq and qRT-PCR using Excel.
Results
Identification of six SUT genes in Sugarcane
20 positive BAC clones from AP85-441 were identified using 6 probes (Additional file 1) designed from six well-annotated SUTs genomic regions in Sorghum bicolor (Table 1). To determine haplotypes, the PCR fragments of the six SUTs were cloned by using these probe primers and sequenced, which confirmed 14 of these 20 BAC clones contains different paralogous and homologous haplotypes. The six S.spontaneum SUTs were referred to SsSUT1-SsSUT6 according to sequence similarity with sorghum SUTs [13]. In the 14 SUT sequences, both SsSUT1 and SsSUT5, both SsSUT3 and SsSUT6 and remaining two SUTs have 2, 4 and 1 allelic haplotypes, respectively. The allelic haplotypes of each SUTs were indicated additional“-h1” to “-h4” to the gene name end. Using the gene model sequences of the annotated SUT genes as queries, both the in-house EST and Genbank database were extensively searched. The results showed that all the SsSUTs had the corresponding ESTs in the Genbank database except SsSUT3 (Additional file 2).
Table 1.
Information of the putative SUT genes in sorghum
| Gene name | Gene ID | Location of the gene |
|---|---|---|
| SbSUT1 | Sb01g045720 | NC_012870.1|:68807992-68813945 chromosome 1 |
| SbSUT2 | Sb04g038030 | NC_012873.1|:67544380-67548965 chromosome 4 |
| SbSUT3 | Sb01g022430 | NC_012870.1|:c28297080-28293801 chromosome 1 |
| SbSUT4 | Sb08g023310 | NC_012877.1|:c55444565-55438275 chromosome 8 |
| SbSUT5 | Sb04g023860 | NC_012873.1|:c53548702-53545522 chromosome 4 |
| SbSUT6 | Sb07g028120 | NC_012876.1|:c63108611-63106213 chromosome 7 |
The six SsSUTs containing complete ORFs (open reading frames) with the predicted molecular weights ranged from 51.84 to 63.41 kDa in sugarcane (Table 2). Comparing with the SUT family from sorghum, SsSUT5 showed a lower molecular weight, and the remaining gene pairs between these two species were consistent. SsSUT5 and SsSUT6 shared a higher similarity of protein sequences (82 %) in contrast to the other 14 pairwise sequences between the remaining four genes in the SUT families (39–69 %) (Table 3). The analyses of the deducted protein sequences of the SUT genes in sugarcane revealed that all these gene families had highly conserved MFS domains and contained 12 membrane-spanning helices (Additional file 3). A conserved histidine residue was presented in the first loop domain corresponding to His-65 [40] and amino acids which corresponded to the G-X-X-X-D/E-R/K-X-G-[X]-R/K-R/K motif reside in the second and eighth loop domains [41, 42]. Additionally, SsSUT4 contained a LXXLL motif in the N-terminal domain, indicating that it might be targeted to the tonoplast [13, 43].
Table 2.
Comparison of the characterization of the SUTs between sugarcane and sorghum
| Sorghum | Sugarcane | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene name | Amino acids size | Molecular weights (kDa) | Domains | Isoelectric point (pI) | Transmembrane helices | Gene name | Amino acids size | Molecular weights (kDa) | Domains | Isoelectric point (pI) | Transmembrane helices | Identity |
| SbSUT1 | 519 | 54.99 | MFS domain | 8.86 | 12 | SsSUT1 | 521 | 55.08 | MFS domain | 8.79 | 12 | 96 % |
| SbSUT2 | 594 | 63.33 | MFS domain | 6.00 | 12 | SsSUT2 | 598 | 63.41 | MFS domain | 5.94 | 12 | 96 % |
| SbSUT3 | 507 | 53.20 | MFS domain | 6.58 | 12 | SsSUT3 | 508 | 53.47 | MFS domain | 7.46 | 12 | 96 % |
| SbSUT4 | 501 | 53.44 | MFS domain | 8.60 | 12 | SsSUT4 | 501 | 53.44 | MFS domain | 8.60 | 12 | 98 % |
| SbSUT5 | 534 | 56.38 | MFS domain | 8.72 | 12 | SsSUT5 | 495 | 51.84 | MFS domain | 7.97 | 12 | 83 % |
| SbSUT6 | 536 | 56.36 | MFS domain | 8.45 | 12 | SsSUT6 | 554 | 58.86 | MFS domain | 8.46 | 12 | 88 % |
Table 3.
Amino acid sequences pairwise comparisons (% similarity) between SUT gene members in sugarcane
| SsSUT1 | SsSUT2 | SsSUT3 | SsSUT4 | SsSUT5 | SsSUT6 | |
|---|---|---|---|---|---|---|
| SsSUT2 | 55 % | - | - | - | - | - |
| SsSUT3 | 69 % | 53 % | - | - | - | - |
| SsSUT4 | 47 % | 43 % | 45 % | - | - | - |
| SsSUT5 | 50 % | 43 % | 49 % | 39 % | - | - |
| SsSUT6 | 53 % | 47 % | 53 % | 41 % | 82 % | - |
Allelic haplotype analysis of SsSUTs
Genomic sequence comparisons within the allelic haplotypes from the four SUTs revealed that these allelic haplotypes shared very high similarity above 99 %. Slight variations were observed within the allelic haplotypes of SsSUTs. The deducted protein sequences were compared within the four SsSUTs. The results showed that, for the protein sequence of allelic haplotypes, both SsSUT3 and SsSUT5 shared identities of 98.38 %, while, SsSUT5 and SsSUT6 had 98.38 and 97.79 % sequence similarity, respectively. The specific amino acids variations were discovered through the alignment of the allelic haplotypes from each SUTs. 2, 5, 8 and 5 amino acids within SsSUT1, SsSUT3, SsSUT5, SsSUT6 respectively were observed to be vary among the allelic haplotypes (Table 4). In addition, in SsSUT6, compared with h3 and h4, h1 and h2 had shorter peptides with 20 amino acids deletion caused by the shift of exon structure (Fig. 1). Furthermore, in SsSUT5, 7 of the variant amino acids were located at the sixth loop domain (T280I; G301S; K333R and T337K) and transmembrane helixes (F44L; N348S and I433V), indicating that potential functional variation existed among these allelic haplotypes (Fig. 1).
Table 4.
The variation of deducted amino acid sequences among allelic haplotypes within the four SsSUT
| SUT | No. of variations (SsSUT-h1 vs other haplotype) | Amino acid variations |
|---|---|---|
| SsSUT1 | 2 | S21A,T329S |
| SsSUT2 | N/A | - |
| SsSUT3 | 5 | A10S, G18 insertion, G28E, I109T, E200D |
| SsSUT4 | N/A | - |
| SsSUT5 | 8 | F44L, T280I, G301S, K333R, T337K, N348S, I433V, G482 insertion |
| SsSUT6 | 6 | V5 deletion, S28 insertion, G39S, Q78…A97 deletion, D345N, I404M |
Fig. 1.
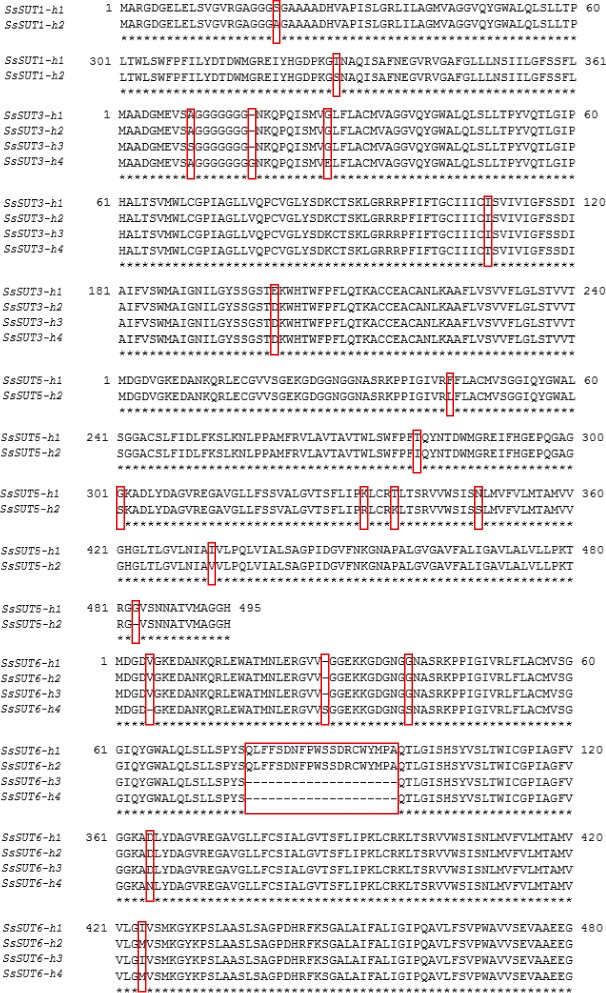
Alignment of the amino acid sequences of SsSUT haplotypes. Amino acid sequences of haplotypes were aligned using the DNAMAN program. Similarity in amino acids across all the sequences is indicated by stars. The difference between haplotypes was shown in a red box
In general, gene structures of the SsSUTs allelic haplotypes presented high conservation for exon/intron numbers and exon sizes, while, for intron size, in SsSUT3, SsSUT3-h3’s first intron was larger than other allelic haplotypes; and in SsSUT6, both SsSUT6-h3 and SsSUT6-h4 contained a smaller second exon than SsSUT6-h1 and SsSUT6-h2 (Fig. 2).
Fig. 2.
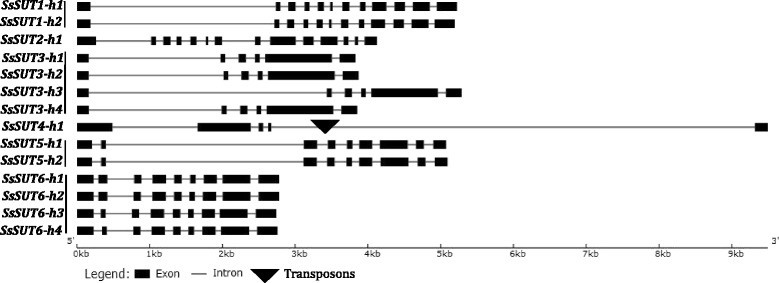
Comparison of the allelic gene structures of SsSUTs. Boxes represent exons, triangles represent transposons
Nonsynonymous to synonymous substitution ratio (Ka/Ks) was analyzed to investigate evolutionary function constraint in S.spontaneum. To identify the evolutionary forces acting on four SUT genes having alleles (SsSUT1, SsSUT3, SsSUT5, SsSUT6) (Fig. 3), the Ka/Ks was calculated. Within the coding regions, the Ka/Ks ratio was much less than 1, indicating that purifying selection was the dominant force driving the evolution of SsSUT genes.
Fig. 3.
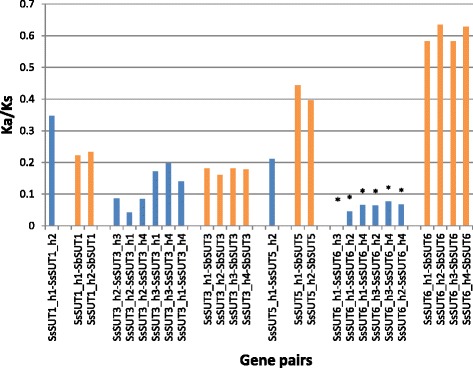
The Ka/Ks of SsSUT haplotypes and SbSUT-SsSUT. The lower value of Ka/Ks was indicated by stars
Comparative analysis of gene structure between SsSUT and other plant SUT
The gene structures of SsSUT family has a great variation with exon numbers ranging from five to fourteen, and their introns were aligned accordance with the GT-AG rule for splicing sites. SsSUT1, SsSUT3 and SsSUT4 had larger first introns than the other SUT genes. Both SsSUT3 and SsSUT4 only have five exons that were lesser than the other SsSUTs (Fig. 4). In addition, comparative analysis of SsSUT families suggested that, the fifth exon in SsSUT3 were presumed to split into 3–4 exons, and the first, second, and fifth exons in SsSUT4 were presumed to evolve into 2–4 exons (Fig. 4). SsSUT5 and SsSUT6 shared highest similarity of exon/intron pattern in spite of the great size variation between the second introns, which correlated to their amino acids similarities (Table 3).
Fig. 4.
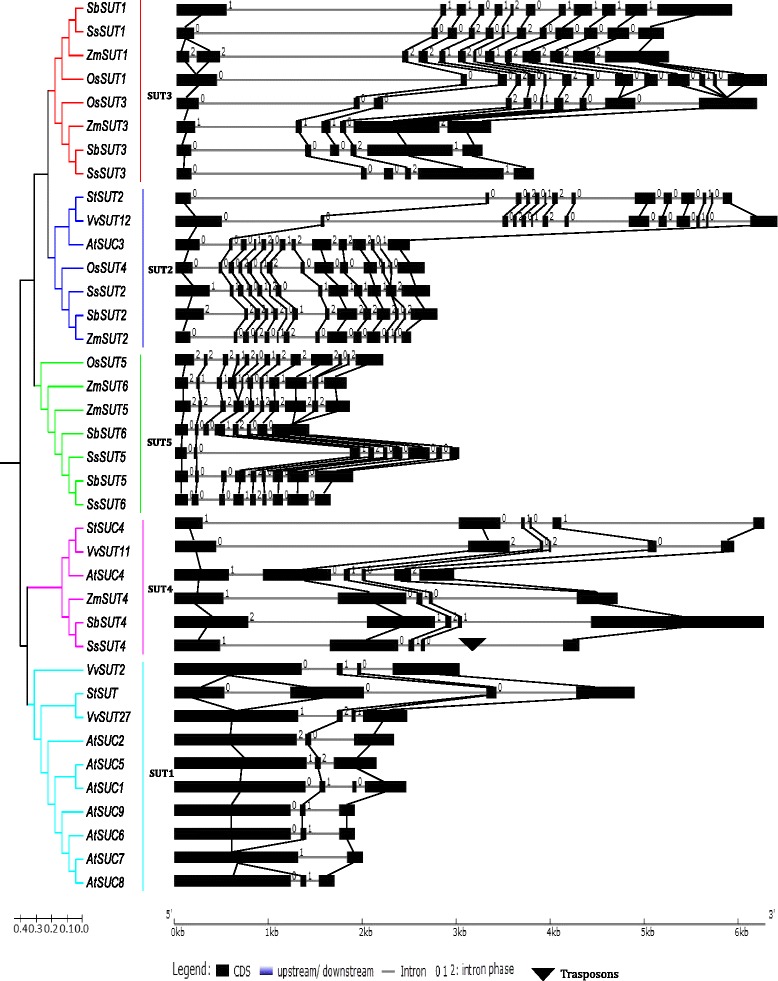
Comparison of the gene structure of the six members of the SsSUT gene family. Three monocotyledons (Zea mays, Sorghum bicolor, Oryza sativa) and dicotyledons (Arabidopsis thaliana, Solanum tuberosum, Vitis vinifera) SUT gene family are also shown for comparison. Boxes represent exons, triangles represent transposons
To investigate the evolutionary mechanisms underlying the genesis of gene families, we performed comparative analyses of the SsSUT structures with the SUT families from sorghum, rice, Arabidopsis (Arabidopsis thaliana), maize, grape (Vitis vinifera) and potato (Solanum tuberosum) (Fig. 4). The SUTs from these species could be divided into five groups SUT1 to SUT5, which were consistent with previous studies [13, 44]. The results showed that SUTs in the same group had the similar gene structures. In the dicot specific group SUT1, most of the genes had two to four exons, in which the first exons were large and the second ones were small. In this group, the only exception was StSUT2, which had a smaller first exon, due to the possible exon splitting comparing with the other dicot species. In group SUT4 that was closely related to group SUT1, all the genes had first two large exons that were likely originated from SUT1 first exon splitting based on sequence comparison. It was interesting that in SUT4 group, the dicot genes and the monocot genes had siminar number of exons, 5 and 6, respectively, and the sequences of the fifth and sixth exons in dicot were presumed to originated from the monocot fifth exon splitting. Furthermore, based on the genomic and amino acids sequence comparisons among the 16 sequences from the seven plant species in group SUT1 and SUT4, the common ancestral gene of monocots and dicots were suggested to have two exons and the exon members have differentiated in a later period of evolution caused by exon splits and partial exon fusions.
The genes in group containing SUT2 had similar exon number of 13 or 14 for both dicot and monocot species. Gene size expansion due to intron size stretching in dicot plant grape and potato were observed in this group. In the SUT3 group, besides ZmSUT1 with an additional small intron caused by the first exon splitting, the remaining SUT3 group genes harbor large first introns, which included wheat TaSUT1D [12] and tomato LeSUT2 [24]. In contrast to SUT2 group, the SUT3 group had varied number of exons ranging from 6 to 14; among them, SsSUT3, SbSUT3 and ZmSUT3 had six exons, the other genes in the subfamilies contained 10–14 exons; both of OsSUTs in this group had more exons number than their orthologous genes. In monocot specific group SUT5, similar to the high identities shown by the alignment of amino acid sequence of SsSUT5 and SsSUT6, high conservation of exon/intron structures were observed from the schematic representation; SsSUT5 had larger second exon than the other genes; similar to SUT3 group, OsSUT had more exon number than their orthologous genes. Scrutiny of the exon/intron structure of the 22 genes in branch with SUT3/SUT2/SUT5 revealed that the exons could be corresponding to six exons, in which the first and the last two exons were observed to be fused/spited. These results suggested that the genes in this branch might originate from common ancestral gene containing six exons for both monocots and dicots.
Phylogenetic analysis of SsSUT and other plant SUT homologs
To comprehensively analyze the evolutionary relationships of SUT families between S. spontaneum and other plant species, we aligned 62 plant amino acid sequences from 5 dicots and 6 monocots including S.spontaneum, using ClustalX to construct an unrooted tree with Neighbor-Joining method (Fig. 5). Same as the distribution above, the SUTs were phylogenetically distributed into five groups, SUT1, SUT2, SUT3, SUT4 and SUT5, respectively. Among these five groups, SUT1 genes are only found in dicotyledonous, in contract, genes in SUT3 and SUT5 groups are only from monocotyledonous. Whereas, the remaining two groups, SUT2 and SUT4, are found in both dicot and monocot, and could be well classified into two distinct subclades. These results strongly suggested that plant SUTs were diverged from a recent evolutionary event after the common ancestor of dicots and monocots. In addition, the SUT families could be divided into two branches in the phylogenetic tree, with SUT1 and SUT4 groups in one branch and the other three groups in another branch, indicting two ancestral genes were the origins of SUTs in both dicot and monocot.
Fig. 5.
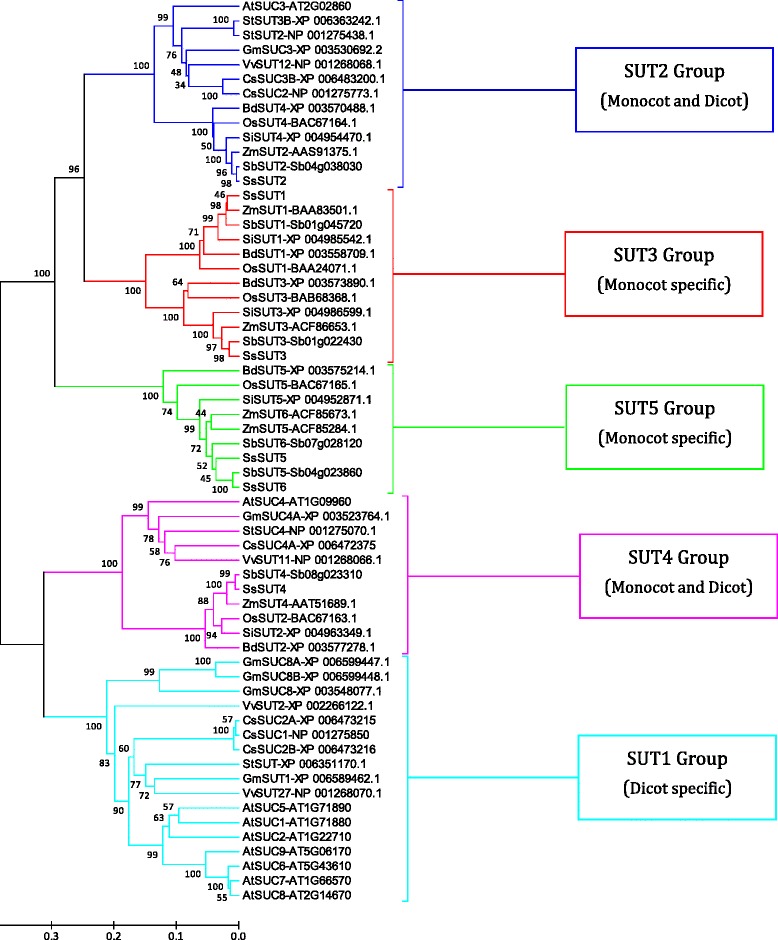
Phylogenetic analysis of SsSUT and other plant SUT homologs. Unrooted phylogenetic tree of plant SUT proteins constructed using the neighbour-joining method with MEGA 5.2.1 program. ZmSUT: Zea mays, SbSUT: Sorghum bicolor, BdSUT: Brachypodium distachyon, OsSUT: Oryza sativa, AtSUT: Arabidopsis thaliana, CsSUT: Citrus sinensis, GmSUT: Glycine max, StSUT: Solanum tuberosum,VvSUT: Vitis vinifera, SiSUT: Setaria italica, SsSUT:Saccharum spontaneum
In dicot specific SUT1 group, the paralogous genes from each of the dicot species were observed to be closely related, indicting recent gene duplications after the divergence of dicotyledons (Fig. 5). In addition, both SUT gene number and sequences had great variations among the dicot plant species, suggesting rapid evolutionary dynamics exist in the dicotyledonous SUT families (Figs. 5 and 6). In SUT4 group, all dicots and monocots had only one gene member, which the phylogenetic distribution were generally consistent with the plant species taxonomy (Figs. 5 and 6). In SUT2, as description above, the genes from dicot and monocot species could be further classified into two subclades, respectively. In this group, dicot plants have d 1-2 gene members, while, all the monocot plants only hadone gene member, which suggestted that dicot plants SUT2 group were undergoing expansion. In the monocot specific SUT3, the examined monocot species consisted of two paralogous genes from two separated clades, suggesting the gene duplication event occurred before the divergence of these dicot plants. In contract to SUT3 group, in SUT5 group, recent gene duplication events were observed in the Andropogoneae plants (S. sponteneum, Sorghum bicolor and Zea Mays) and Bambusa oldhamiias shown by the closest phylogenetic distribution of genes within these plant species.
Fig. 6.
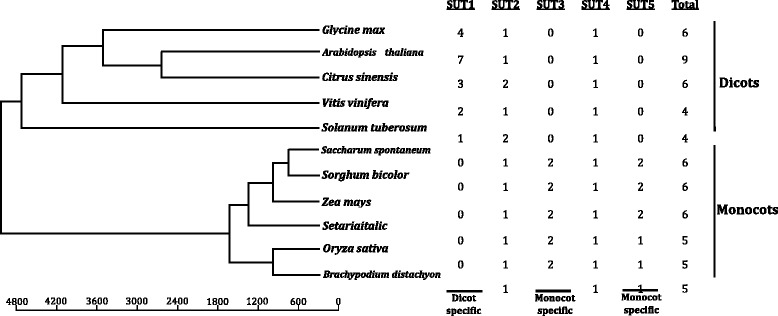
The distribution of SUT family member in monocotyledon (6 species) and dicotyledon (5 species)
In the SsSUT family, SsSUT4 was the solo member in a branch, but SsSUT1, SsSUT2, SsSUT3, SsSUT5 and SsSUT6 were clustered together and shared a more recent common ancestral gene. Thus, SsSUT4 was suggested to be the oldest gene, while, both of SsSUT5 and SsSUT6 should be younger than the splitting of Trib, Andropogoneae Dumort and Zea Mays. In addition, SsSUT5 and SsSUT6 were observed to be undergoing rapid evolution as shown by lower amino acids sequences similarity than other orthologous genes between the sorghum and Saccharum (Table 2).
Gene expression of SUTs among three Saccharum species
To investigate the possible physiological functions for SUTs, we performed comparative transcriptome profiling among three Saccharum species at different developmental stages of seedling and five different tissues from the mature leaf (mature and leaf roll) and stalks (mature, maturing and immature) by using RNA-seq method. The RNA-seq results were verified by qRT-PCR in three tissues (leaf roll, mature stalk and maturing stalk) from each of the three Saccharum speices (Additional file 4 and Additional file 5). There is a significant positive relationship ( R2 = 0.711 and p < 0.001) between the Reads Per Kilobases per Million reads (RPKM) based on RNA-seq and the relative expression level based on qRT-PCR (Fig. 7a).
Fig. 7.
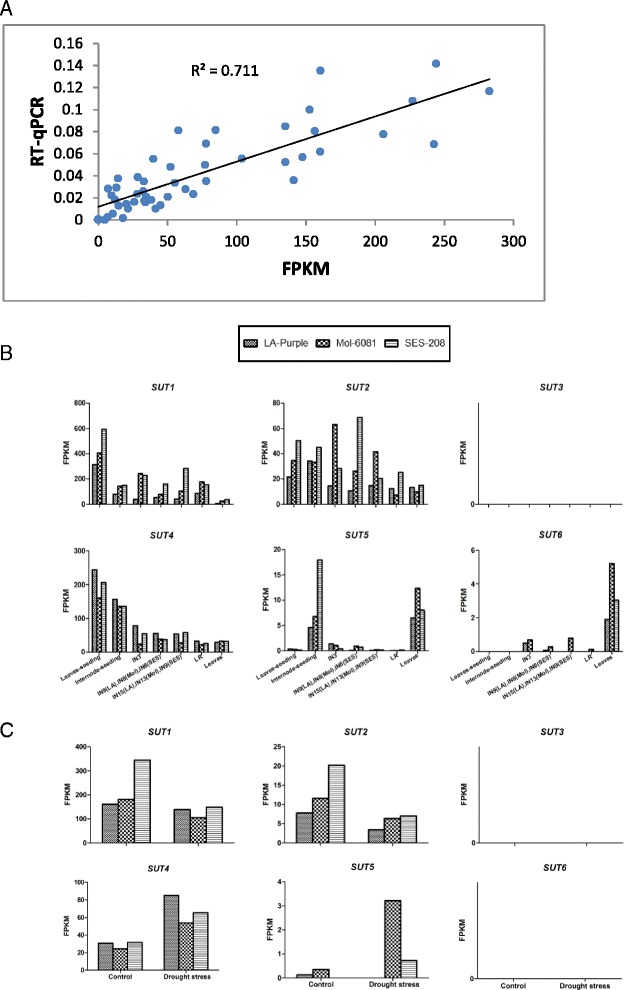
The SUT gene family expression based on RPKM in different tissues of different stage in three Saccharum species. The expression level of 6 SUT genes in seeding stage and mature stage of nature condition. a correlation coefficient between RNAseq (X-axe) and qRT-PCR (Y-axe) of six SUT genes. b, and in seeding stage of drought stress condition (c). IN, internode; LR, leaf roll. Internnodes 3, 9, 15, internodes 3, 8, 13 and internodes 3, 6, 9 were from Saccharum officinarum (LA-Purple), Saccharum robustum (Molokai6081) and Saccharum spontaneum (SES208), respectively
In SUT families, the transcription of SUT3 was undetectable in all the examined tissues from Sacchaurm plants, which was consistent with that of SUT3 in sorghum [13]. The other gene expression levels had significant variations with a clear trend of transcript levels from higher to lower SUT1, SUT4, SUT2, SUT5 and SUT6. Of them, SUT1 and SUT4 had predominant expression levels among the gene families, indicating that the two genes were the fundamental members in SUT families.
SUT1 transcripts were abundant in both source and sink organs, and were the most expressed gene in all tissues except for the mature leaf, indicating SUT1 was the key member. The gene displayed a higher expression level in sink tissues (stem) than the source tissue (leaf) in mature plant. However, it was the opposite in seedling plants, suggesting the gene was more important in phloem loading for seedlings than the mature plant. Comparing with both S. sponteanum and S. robustum, SUT1 displayed a lower expression level in the high sucrose S. officinarum (LA-Purple), in all examined tissues. In addition, under drought stress, SUT1 was observed to be down regulated in S. robustum.
SUT2 was expressed in all the organs examined for the three Saccharum species. In the seedling plants, similar expressions were observed in both stem and leaves among these Saccharum species. In the mature plants, SUT2 displayed a lower expression level in the source tissue of leave and leafroll for two lower sucrose accumulating Saccahurm species, S. sponteneum and S. robustum, while, high sugar S. officinarum had a more uniform SUT2 expression level in all of the examined tissues (Fig. 7b). Under drought stress, twofold lower levels of expressions were observed in the three Saccharum species than their control (Fig. 7c).
SUT4 had higher expression level in the seedling than the mature plants (Fig. 7b), suggesting it might contribute more for sucrose loading at the early age of plant development; whereas, SUT4 displayed similar expression level in the examined tissues from both seedling and mature plants. Obviously, SUT4 was not correlated to the sucrose content differential among the Saccharum species. Under drought stress, in contrast to SUT1 and SUT2, SUT4 was up regulated in the leaf of three Saccharum species.
SUT5 displayed dramatically higher expression level in sink tissues (stem) than in source (leave) in the seeding plant; in contract, in the mature plants, the expression level were lower in leave than the other tissues. Similar to SUT4, SUT5 showed up-regulation under water stress. SUT6 was undetectable in the seeding plants and had similar expression pattern as SUT5 in the mature plants that gene expression exhibited higher in leave than the other tissues.
Discussions
Genomic study for the gene families is the first step toward the gene functional study. However, the identifications of gene family in sugarcane is still a formidable challenge caused by its complex genomes. Recently, the whole genome sequencing of sorghum and other relative species of Saccharum provides the references for comparative genomics to identify the gene families in Saccharum species. In previous studies, based on comparative genomics, the gene families of phosphoenol pyruvate carboxylase gene [45], sucrose synthase [34], sucrose phosphate synthase [46], ATP-dependent phosphofructokinase [37] were identified by using the EST database, DNA fragment, and cDNA cloning. However, none of these studies has investigated on the genomics of whole gene families because of the lack of genomic sequences for sugarcane. Our study through comparative genomics and BACs sequencing is the first report for the structure of a gene family and their gene allelic haplotypes in Saccharum.
Evolutionary conservation and divergence of SsSUT
Plant SUTs had been well documented in previous studies for gene phylogenetic analyses two classifications [11, 13, 44, 47, 48]. One classification was to divide plant SUTs into three types, type I, type II and type III, with the reference of Arabidopsis [11, 48], in which, type I and type II SUTs were localized to the plasma membrane, while type III SUTs were associated with vacuolar membrane [48]. Another classification was to group the SUTs into five groups SUT1, SUT2, SUT3, SUT4 and SUT5 [13, 44, 49]. Comparing these two classifications, SUT1 was included in type I, SUT2 was in type II, and SUT3, SUT4 and SUT5 were in type III. The former classification is likely associated with dicot plants studies, while, the latter is used for both dicot and monocot plants, especially for gene evolution studies. In this study, we used large amount of plant SUTs for phylogenetic analysis, and the results confirmed the later classification, by revealing the existence of one dicot specific and two monocots specific groups and the independence evolution process in plant SUT families (Fig. 5). Furthermore, the comparative analysis of these plant SUTs showed that genes in SUT3 group were more conservative than the genes in SUT5 group, and further provided the direct evidence of the recent duplication event occurred after monocot/dicot divergence. The evolution history of SsSUTs, which was sorted by age in duplicated descending order, SsSUT4, SsSUT2, SsSUT2/SsSUT3, and SsSUT5/SsSUT6.
The comparative analyses could be used to predict the number of SUT gene family members in Saccharum. Without the whole genome sequences for Saccharum, it could be debatable to conclude that we have discovered all SUTs in Sacchaurm, in spite of our high coverage BACs libraries. In this study, both phylogenetic analysis and sequences comparison revealed that Saccharum spontaneum and Sorghum bicolor were both composed of six SUTs members and each gene pairs were orthologous between the two species (Figs. 5 and 6). Beside SUT1 group, which was dicot specific, monocot plants have the same gene numbers in SUT2, SUT3 and SUT4 groups, the remaining group SUT5 had the same gene number in Andropogoneae plants, which were found to be the result of a recent duplication (Fig. 6). Therefore, it is less likely that any SUT gene duplication events had occurred after the diverging of sorghum and Saccharum, knowing no additional gene in this study. Hence, we concluded that the six SsSUTs comprise the SUT family in the Saccarhum spontanum genome. Further experiments such as Southern Blot could be used to verify the conclusion.
The exon–intron structure differences were demonstrated to be accomplished by three main types of mechanisms, exon/intron gain/loss, exonization/pseudoexonization, and insertion/deletion [50]. In this study, comparative analyses of gene structures for SsSUT made it possible to evaluate the SUT gene structure evolution in plant (Fig. 4). All SUTs genes, including SsSUTs, had a great variation of exon numbers, ranging from 2 to 14. Comparing with the gene structure variation, the protein sequences were more conserved among the paralogous genes in Sacchaurm, as shown by all SUT members containing 12 membrane-spanning helices and similar protein sizes. A common feature of plant SUTs was that the 12 membrane-spanning helices were distributed roughly uniformly in the deducted peptides of each gene in conserved position. Thus, the gene structure evolution after these plant divergences did not cause significant coding region variations. Therefore, the SUT structures variations were mainly evolved from intron gain/lost and insertion/deletion but not from exonization/pseudoexonization.
Sorghum is one of the closest relative diploid genera of Sacchaurm. Comparative analysis of the orthologous between SsSUTs and SbSUTs made it possible to investigate the specific evolutionary events after the polyploidzation of Saccharum. In SsSUTs, SsSUT5 harbored a much larger second intron than its closest paralogous SsSUT6 and the other orthologs (Fig. 4). Similarly, SsSUT3 contained a larger first intron than its orthologous gene SbSUT2. In addition, SsSUT4 were observed to have a putative TE (Transposable elements) insertion in the last intron (Fig. 4). Our results suggested that the SsSUT families were undergoing gene extension following polyploidization in S.spontaneum. The estimated monoploid genome size of S. spontaneum (843 Mb) and S. Officinarum (985 Mb) were both larger than the monoploid genome size of sorghum at 760 Mb [34], supporting the conclusion that the genome of Saccharum expanded in general after polyploidization. Our results demonstrated the first case that expansion in intron regions of a gene families contribute to genome expansion in Sacchaurm.
In this study, allelic haplotype sequences for SsSUT1, 3, 5 and 6 were comparatively analyzed for gene structures and Ka/Ks. Relative conservative gene structures were observed among allelic haplotypes within each of the four SsSUTs, whereas, SsSUT3-h3 contained a larger first intron than the other three haplotypes, both SsSUT6-h1 and SsSUT6-h2 had larger second exons (Fig. 2). In addition, the Ka/Ks ratios, which are all under 0.4, revealed that all the SUTs allelic haplotypes were under strong purifying selection (Fig. 3). Multiple alleles in polypoidy are considered to be functional redundant at the time of origin [51–53]; the conservation and constraint purification within the allelic haplotypes of SsSUTs were likely due to the key function of SUT in Saccharum. It would be worthy to note that the transcription of SsSUT3 was undetectable in the examined tissues while its haplotypes were under constrain selection, indicating that SsSUT3 might have a necessary function for Sacchaurm. In paleopolyploids [54–57], and recent allopolyploid species, such as wheat [58, 59] and Tragopogon [60, 61], eliminations and pseudogenizations of key functional genes after polyploidzation have been well documented. The allelic gene variations supposed to the key topics for studying the genome dosage of Sacchaurm species and further investigation for them would provide the foundation to understand the molecular basis of sugarcane genetics.
Gene expression and functions of SUTs in Saccharum
Examination of SUT gene expressions in Saccharum species in source and sink tissues of seedling and mature plants proves an insightful indication regarding the roles of gene functions. Previous studies of gene expression were mostly done on Saccharum hybrids with combined genetic background of S.officinarum and S.sponteneum. To simplify genetic backgrounds, in this study, three Saccharum species, high-sucrose S.offcinarum, low-sucrose S.robustum (the potential domestic progenitor of S.officinarum) and stress-tolerant S.spontaneum were used for studying the SUTs expression profiles.
SUT1 was the only gene in SUT family, which had been well documented in Saccharum hybrid [30, 31, 33, 62]. In these previous studies, ShSUT1 had been shown to have high expression level in premature stem tissue, and decreased expression level in mature internodes [33]. ShSUT1 was demonstrated to be highly selective for sucrose, inhibited by sucralose and had the function in loading sucrose from the vascular tissue into the stem parenchyma cells [30, 33]. Consistent with previous study [30], our results suggested that the expression of SUT1 was higher in mature stems than the mature leave from all three Saccharum species (Fig. 7b), and thus supported the previous conclusions. Moreover, Saccharum SUT1s displayed much higher expression level in the seedling leave tissues than in both of the seedling stems and all the mature tissues, suggesting SUT1 may play an enhanced role in directing sucrose in source tissue before sucrose accumulation in Sacchaurm species. Similar to the expression of SbSUT1 in sorghum [13], the SUT family expression analysis revealed that SUT1 was the most expressed among SUTs in Saccharum species. Nevertheless, the only direct evidence for the SUT1 function was from the Saccharum close related maize sut1 mutant, which exhibited a phenotype of shorter stature and carbohydrate accumulation in their source leaves [63]. SUT1 in Saccharum may have similar function as ZmSUT1 since the two orthologs shared high sequence similarity.
Comparative analyses of SUT expression among the three Saccharum species revealed that SUT1 had lower expression level in all tissue types from high sugar species (S.officinarum) than the lower sugar species (S.spontaneum and S.robustum), which was consistent to sorghum that SUT1 had lower gene expression in high sucrose Rio. than in the grain type genotype RTx623 [13]. Similar to SUT1, besides the leaf tissues, SUT2 was more abundant in S.spontaneum and S.robustum than in S.officinarum (Fig. 7b). These results supported the notion that sinks demand in the mature plant might be stronger than in the lower sucrose content plants of Andropogoneae tribe.
We compared the expression level of SUTs of all Saccharum species in both mature and seedling plant. In mature plants, SUT1 had higher expression level in sink than source tissues; in contrast, SUT1 had a lower expression level in sink than source tissues. SUT4 showed a higher expression level in seedling than the mature plants. SUT1 and SUT4 accounted for above 70 % of transcripts in this gene family (Fig. 8). These results indicated that sucrose transport were active before the sucrose accumulation and both SUT1 and SUT4 were involved in the plant development in Saccharum.
Fig. 8.
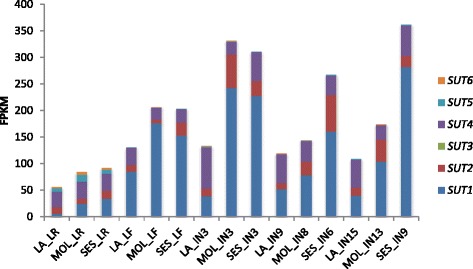
Total accumulative expression level of the SUTs in tissues and in stage of three Saccharum species. IN, internode; LR, leaf roll. Internnodes 3, 9, 15, internodes 3, 8, 13 and internodes 3, 6, 9 were from Saccharum officinarum LA-Purple, Saccharum robustum Molokai6081 and Saccharum spontaneum SES208 respectively
In the Saccharum species, SUT4 showed similar expression level in both seedling and mature stage tissues. These results were different from its close orthologs including OsSUT2 [64], PtaSUT4 [8], and SbSUT2 [13]. Thus, SUT4 might contribute to the characteristics of sucrose accumulation in Saccharum species. The orthologous of SUT4 phylogenetic group was proved to be localized in the tonoplast [64–66]. In model plant Oryza sativa [11] and Arabidopsis [67], SUT4 played a role for transporting sucrose from mesophyll vacuoles to their cytoplasm. This information may not be sufficient for discovering the gene function of SUT4, but is an indication for further functional study of SUT4 in sugarcane.
Both SUT5 and SUT6 in SUT5 group (Fig. 5) were revealed to be recent duplication and through rapid evolution accompanied by the multiple amino acid differences in their allelic haplotypes (Table 4), suggesting that these two genes may have similar gene expression profiles. Gene expression analysis showed that these two genes have much lower gene expression level among the SUTs families and had similar gene expression profiles in the mature tissues (Fig. 7b), supporting that the two genes were derived from a recent duplication. Nevertheless, in the seedling plants, SUT6 was absent while SUT5 had higher expression in stems than leaves (Fig. 7b), hence, SUT5 may contribute to phloem loading before the sucrose accumulation in Saccharum. In sorghum, great variation of gene expression level for SbSUT5 and SbSUT6 were observed among the tissues from of vegetative stages and anthesis [13]. Of them, SbSUT5 showed higher expression in spikelet tissue and inflorescence, and thus was suggested to play a role for inflorescence development; similarly, SUT6 in Saccharum species was more abundant in leaves than the other tissues [13]. Based on above genomic analysis, SUT5 and SUT6 have gone through rapid evolution after the split of Sorghum and Saccharum, suggesting that these two genes have functional divergence between these two species. These two gene expression profiles in Saccharum and Sorghum were different from their closest orthologous OsSUT5, which exhibited broad expression level across source and sink tissues as well as in filling rice grains [11]. Phylogenetic and comparative analysis revealed that there was a single SUT in group SUT5 from rice. These differences can be explained by the single gene OsSUT5 in rice response for function of two duplication genes in the Andropogoneae tribe.
Soluble sugar such as sucrose usually increases in plant under drought stress. to identify which SUT gene responsible to drought stress, we examined the SUTs expression level under drought stress in the seedling plant leaves of three Saccharum species. Under drought stress, in the four detectable SUTs in the seeding, SUT1 and SUT2 were down regulated, in contrast, SUT4 and SUT5 were up regulated, indicating that the SUT4 and SUT5 are important in response to drought stress and may involved in transporting sugar into cell for osmotic adjustment. SUT families in Saccharum presented a great gene expression diversity in response to drought stress. SUT4 was the predominant expression member in the SUTs families in Saccharum, therefore, the up-regulation of SUT4 expression resulted in the higher total SUTs transcript level. A possible explanation for this phenomenon could be that the source tissue reduced the sucrose product level under drought stress thus down regulated the SUT expression level. An expression profile for Saccharum plants under water stress with different time points could be further used for verifying this notion. Similar experiment was performed for the five SUT members in rice, which revealed that OsSUT2 was only member display up-regulated during drought and salinity treatments [68]. Therefore, it is most likely that plant SUT gene members possess diversity pattern in response to stress tolerance.
Conclusion
In this study, we presented the first report of a gene family consists of six SUTs in Saccharum. We provided the comprehensive evaluation of the evolutionary genesis, gene allelic haplotypes, phylogenetic relationships, gene structure, and gene expression pattern for the SUT gene family in Saccharum species. Our results revealed that SsSUT5 and SsSUT6 were recent duplication genes companied by rapid evolution, while, SsSUT2 and SsSUT4 were the ancient members in the families. Gene size extensions caused by sequence insertions in introns were observed in the SUT families. Despite the high polyploidy level, the examined SUTs exhibited conserved gene structures and amino acid sequences among the allelic haplotypes. Both SUT1 and SUT4 had predominant expression in Saccharum SUT families. SUT1, which displayed lower expression in the high sucrose species S.officinarum, might be involved in saccharide unloading in the sink tissue of mature plant, phloem loading in early developmental stage of Saccharum. SUT2 likely contributed to both phloem loading and sink development. SUT4 was more important at early developmental stage than in mature plants. Both SUT5 and SUT6 had lower expression level than other gene members, and had higher expression in source leaves than in other tissues, thus, supposed to play roles in phloem loading. In the seedling plant leaves with drought stress treatment, four genes SUT1, SUT2, SUT4 and SUT5 were detectable, among which, SUT1 and SUT4 were down regulated, while, SUT2 and SUT5 were up regulated. To further reveal these genes’ roles under stress, experiments such as, characterizing the spatio-temporal expression dissection, enzyme activity assay, and gene editing technology like CRISPR-Cas9 system, would be necessary. The results offered useful foundation and framework for future research for understanding the physiological roles for each SUT gene and molecular mechanisms of sucrose metabolism in sugarcane.
Availability of supporting data
The 14 sequences of SsSUTs(allele haplotypes) were deposited into Genbank (accession numbers: KT284760-KT284773).
Phylogenetic data (alignments and phylogenetic trees) have been deposited to TreeBase and are accessible via the URL: http://purl.org/phylo/treebase/phylows/study/TB2:S18751.
Acknowledgements
This project was supported by grants from the 863 program (2013AA100604), NSFC (31201260) and Fujian Provincial Department of Education (No. JA12082). We are grateful for the invaluable help and comments of Dr. Jianping Wang.
Abbreviations
- BACs
bacterial artificial chromosomes
- eEF-1a
Eukaryotic elongation factor 1a
- EST
Expressed Sequence Tag
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase gene
- RPKM
Reads Per Kilobasesper Million reads
- SUTs
Sucrose transporters
Additional files
The probe primers for SUT BAC hybridization in S. spontaneum . (DOC 32 kb)
BLAST results for SsSUTs EST in NCBI database. (DOC 32 kb)
Alignment of the amino acid sequences of SsSUT haplotypes. (DOC 114 kb)
The qRT-PCR primers for SUTs in this study. (DOC 32 kb)
qRT-PCR verification of SUT gene expressions in partial issues of three Saccharum species. (DOC 824 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
QZ and JZ conceived the study and designed the experiments. QZ,WH, FZ, LW, QY, RM and JZ carried out the experiments and analyzed the data. QZ and JZ wrote the manuscript. All authors read and approved the final paper.
Contributor Information
Qing Zhang, Email: 297160480@qq.com.
Weichang Hu, Email: 654919671@qq.com.
Fan Zhu, Email: fanzhu2@illinois.edu.
Liming Wang, Email: 849473519@qq.com.
Qingyi Yu, Email: qyu@ag.tamu.edu.
Ray Ming, Email: rming@life.illinois.edu.
Jisen Zhang, Email: zjisen@126.com.
References
- 1.Slewinski TL, Braun DM. Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Sci. 2010;178(4):341–9. doi: 10.1016/j.plantsci.2010.01.010. [DOI] [Google Scholar]
- 2.Chang AB, Lin R, Studley WK, Tran CV, Saier MH. Phylogeny as a guide to structure and function of membrane transport proteins (Review) Mol Membr Biol. 2004;21(3):171–81. doi: 10.1080/09687680410001720830. [DOI] [PubMed] [Google Scholar]
- 3.Saier MH., Jr Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35(4):699–710. doi: 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 4.Riesmeier JW, Willmitzer L, Frommer WB. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11(13):4705–13. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kuhn C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006;45(2):180–92. doi: 10.1111/j.1365-313X.2005.02572.x. [DOI] [PubMed] [Google Scholar]
- 6.Sivitz AB, Reinders A, Ward JM. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008;147(1):92–100. doi: 10.1104/pp.108.118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava AC, Ganesan S, Ismail IO, Ayre BG. Effective carbon partitioning driven by exotic phloem-specific regulatory elements fused to the Arabidopsis thaliana AtSUC2 sucrose-proton symporter gene. BMC Plant Biol. 2009;9:7. doi: 10.1186/1471-2229-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payyavula RS, Tay KH, Tsai CJ, Harding SA. The sucrose transporter family in Populus: the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J. 2011;65(5):757–70. doi: 10.1111/j.1365-313X.2010.04463.x. [DOI] [PubMed] [Google Scholar]
- 9.Chincinska I, Gier K, Krugel U, Liesche J, He H, Grimm B, et al. Photoperiodic regulation of the sucrose transporter StSUT4 affects the expression of circadian-regulated genes and ethylene production. Front Plant Sci. 2013;4:26. doi: 10.3389/fpls.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arabidopsis Genome I. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408(6814):796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 11.Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT. The sucrose transporter gene family in rice. Plant Cell Physiol. 2003;44(3):223–32. doi: 10.1093/pcp/pcg030. [DOI] [PubMed] [Google Scholar]
- 12.Aoki N, Whitfeld P, Hoeren F, Scofield G, Newell K, Patrick J, et al. Three sucrose transporter genes are expressed in the developing grain of hexaploid wheat. Plant Mol Biol. 2002;50(3):453–62. doi: 10.1023/A:1019846832163. [DOI] [PubMed] [Google Scholar]
- 13.Milne RJ, Byrt CS, Patrick JW, Grof CPL. Are sucrose transporter expression profiles linked with patterns of biomass partitioning in Sorghum phenotypes? Front Plant Sci. 2013;4:223. doi: 10.3389/fpls.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirose T, Imaizumi N, Scofield GN, Furbank RT, Ohsugi R. cDNA cloning and tissue specific expression of a gene for sucrose transporter from rice (Oryza sativa L.) Plant Cell Physiol. 1997;38(12):1389–96. doi: 10.1093/oxfordjournals.pcp.a029134. [DOI] [PubMed] [Google Scholar]
- 15.Scofield GN, Aoki N, Hirose T, Takano M, Jenkins CL, Furbank RT. The role of the sucrose transporter, OsSUT1, in germination and early seedling growth and development of rice plants. J Exp Bot. 2007;58(3):483–95. doi: 10.1093/jxb/erl217. [DOI] [PubMed] [Google Scholar]
- 16.Chen JY, Liu SL, Siao W, Wang SJ. Hormone and sugar effects on rice sucrose transporter OsSUT1 expression in germinating embryos. Acta Physiologiae Plantarum. 2010;32(4):749–56. doi: 10.1007/s11738-009-0459-0. [DOI] [Google Scholar]
- 17.Siao W, Chen JY, Hsiao HH, Chung P, Wang SJ. Characterization of OsSUT2 Expression and Regulation in Germinating Embryos of Rice Seeds. Rice. 2011;4(2):39–49. doi: 10.1007/s12284-011-9063-1. [DOI] [Google Scholar]
- 18.Chen HJ, Wang SJ. Molecular regulation of sink-source transition in rice leaf sheaths during the heading period. Acta Physiologiae Plantarum. 2008;30(5):639–49. doi: 10.1007/s11738-008-0160-8. [DOI] [Google Scholar]
- 19.Truernit E, Sauer N. The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of beta-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta. 1995;196(3):564–70. doi: 10.1007/BF00203657. [DOI] [PubMed] [Google Scholar]
- 20.Martens HJ, Roberts AG, Oparka KJ, Schulz A. Quantification of plasmodesmatal endoplasmic reticulum coupling between sieve elements and companion cells using fluorescence redistribution after photobleaching. Plant Physiol. 2006;142(2):471–80. doi: 10.1104/pp.106.085803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava AC, Ganesan S, Ismail IO, Ayre BG. Functional characterization of the Arabidopsis AtSUC2 Sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol. 2008;148(1):200–11. doi: 10.1104/pp.108.124776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci U S A. 2000;97(25):13979–84. doi: 10.1073/pnas.250473797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weise A, Barker L, Kuhn C, Lalonde S, Buschmann H, Frommer WB, et al. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell. 2000;12(8):1345–55. doi: 10.1105/tpc.12.8.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker L, Kuhn C, Weise A, Schulz A, Gebhardt C, Hirner B, et al. SUT2, a putative sucrose sensor in sieve elements. Plant Cell. 2000;12(7):1153–64. doi: 10.1105/tpc.12.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki N, Hirose T, Furbank RT. Sucrose transport in higher plants: from source to sink. In: Photosynthesis. Springer; 2012. 34:703–729.
- 26.Cuadrado A, Acevedo R, Moreno Diaz de la Espina S, Jouve N, de la Torre C. Genome remodelling in three modern S. officinarumxS. spontaneum sugarcane cultivars. J Exp Bot. 2004;55(398):847–54. doi: 10.1093/jxb/erh093. [DOI] [PubMed] [Google Scholar]
- 27.D'Hont A, Grivet L, Feldmann P, Rao S, Berding N, Glaszmann JC. Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Mol Gen Genet. 1996;250(4):405–13. doi: 10.1007/BF02174028. [DOI] [PubMed] [Google Scholar]
- 28.Piperidis G, Christopher MJ, Carroll BJ, Berding N, D'Hont A. Molecular contribution to selection of intergeneric hybrids between sugarcane and the wild species Erianthus arundinaceus. Genome. 2000;43(6):1033–7. doi: 10.1139/gen-43-6-1033. [DOI] [PubMed] [Google Scholar]
- 29.Moore P. Temporal and spatial regulation of sucrose accumulation in the sugarcane stem. Funct Plant Biol. 1995;22(4):661–79. [Google Scholar]
- 30.Casu RE, Grof CP, Rae AL, McIntyre CL, Dimmock CM, Manners JM. Identification of a novel sugar transporter homologue strongly expressed in maturing stem vascular tissues of sugarcane by expressed sequence tag and microarray analysis. Plant Mol Biol. 2003;52(2):371–86. doi: 10.1023/A:1023957214644. [DOI] [PubMed] [Google Scholar]
- 31.ElSayed AI, Ramadan MF, Komor E. Expression of sucrose transporter (ShSUT1) in a Hawaiian sugarcane cultivar infected with Sugarcane yellow leaf virus (SCYLV) Physiol Mol Plant P. 2010;75(1-2):56–63. doi: 10.1016/j.pmpp.2010.08.006. [DOI] [Google Scholar]
- 32.Rae AL, Perroux JM, Grof CPL. Sucrose partitioning between vascular bundles and storage parenchyma in the sugarcane stem: a potential role for the ShSUT1 sucrose transporter. Planta. 2005;220(6):817–25. doi: 10.1007/s00425-004-1399-y. [DOI] [PubMed] [Google Scholar]
- 33.Reinders A, Sivitz AB, Hsi A, Grof CPL, Perroux JM, Ward JM. Sugarcane ShSUT1: analysis of sucrose transport activity and inhibition by sucralose. Plant Cell Environ. 2006;29(10):1871–80. doi: 10.1111/j.1365-3040.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Arro J, Chen Y, Ming R. Haplotype analysis of sucrose synthase gene family in three Saccharum species. BMC Genomics. 2013;14:314. doi: 10.1186/1471-2164-14-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore PH. Anatomy and morphology. In: Heinz DJ, editor. Sugarcane Improvement through Breeding. Amsterdam: Elsevier; 1987. pp. 85–142. [Google Scholar]
- 36.Ming R, Moore P, Zee F, Abbey C, Ma H, Paterson A. Construction and characterization of a papaya BAC library as a foundation for molecular dissection of a tree-fruit genome. Theor Appl Genet. 2001;102(6-7):892–9. doi: 10.1007/s001220000448. [DOI] [Google Scholar]
- 37.Yu Q, Guyot R, de Kochko A, Byers A, Navajas-Perez R, Langston BJ, et al. Micro-collinearity and genome evolution in the vicinity of an ethylene receptor gene of cultivated diploid and allotetraploid coffee species (Coffea) Plant J. 2011;67(2):305–17. doi: 10.1111/j.1365-313X.2011.04590.x. [DOI] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological). 1995;57:289–300.
- 39.Ling H, Wu Q, Guo J, Xu L, Que Y. Comprehensive selection of reference genes for gene expression normalization in sugarcane by real time quantitative rt-PCR. PloS one. 2014;9(5) doi: 10.1371/journal.pone.0097469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu JM, Bush DR. His-65 in the proton-sucrose symporter is an essential amino acid whose modification with site-directed mutagenesis increases transport activity. Proc Natl Acad Sci U S A. 1998;95(15):9025–30. doi: 10.1073/pnas.95.15.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemoine R. Sucrose transporters in plants: update on function and structure. Biochimica et biophysica acta. 2000;1465(1-2):246–262. doi: 10.1016/S0005-2736(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 42.Pazdernik NJ, Matzke EA, Jessen-Marshall AE, Brooker RJ. Roles of charged residues in the conserved motif, G-X-X-X-D/E-R/K-X-G-[X]-R/K-R/K, of the lactose permease of Escherichia coli. J Membr Biol. 2000;174(1):31–40. doi: 10.1007/s002320001029. [DOI] [PubMed] [Google Scholar]
- 43.Yamada K, Osakabe Y, Mizoi J, Nakashima K, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K. Functional Analysis of an Arabidopsis thaliana Abiotic Stress-inducible Facilitated Diffusion Transporter for Monosaccharides. J Biol Chem. 2010;285(2):1138–1146. doi: 10.1074/jbc.M109.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kühn C, Grof CP. Sucrose transporters of higher plants. Curr Opin Plant Biol. 2010;13(3):287–297. doi: 10.1016/j.pbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Besnard G, Pincon G, D'Hont A, Hoarau JY, Cadet F, Offmann B. Characterisation of the phosphoenolpyruvate carboxylase gene family in sugarcane (Saccharum spp.) Theor Appl Genet. 2003;107(3):470–478. doi: 10.1007/s00122-003-1268-2. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre CL, Goode ML, Cordeiro G, Bundock P, Eliott F, Henry RJ, et al. Characterisation of alleles of the sucrose phosphate synthase gene family in sugarcane and their association with sugar-related traits. Mol Breeding. 2015;35(3):1–14. doi: 10.1007/s11032-015-0286-5. [DOI] [Google Scholar]
- 47.Zhu L, Zhang J, Chen Y, Pan H, Ming R. Identification and genes expression analysis of ATP-dependent phosphofructokinase family members among three Saccharum species. Funct Plant Biol. 2013;40(4):369–378. doi: 10.1071/FP12182. [DOI] [PubMed] [Google Scholar]
- 48.Reinders A, Sivitz AB, Ward JM. Evolution of plant sucrose uptake transporters. Front Plant Sci. 2012;3:22. doi: 10.3389/fpls.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deol KK, Mukherjee S, Gao F, Brule-Babel A, Stasolla C, Ayele BT. Identification and characterization of the three homeologues of a new sucrose transporter in hexaploid wheat (Triticum aestivum L) BMC Plant Biol. 2013;13:181. doi: 10.1186/1471-2229-13-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu GX, Guo CC, Shan HY, Kong HZ. Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci U S A. 2012;109(4):1187–92. doi: 10.1073/pnas.1109047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohno S. Evolution by gene duplication. Berlin: Springer; 1970. [Google Scholar]
- 52.Prince VE, Pickett FB. Splitting pairs: The diverging fates of duplicated genes. Nat Rev Genet. 2002;3(11):827–37. doi: 10.1038/nrg928. [DOI] [PubMed] [Google Scholar]
- 53.Lynch M, Conery JS. The origins of genome complexity. Science. 2003;302(5649):1401–4. doi: 10.1126/science.1089370. [DOI] [PubMed] [Google Scholar]
- 54.Blanc G, Wolfe KH. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell. 2004;16(7):1667–78. doi: 10.1105/tpc.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas BC, Pedersen B, Freeling M. Following tetraploidy in an Arabidopsis ancestor, genes were removed preferentially from one homeolog leaving clusters enriched in dose-sensitive genes. Genome Res. 2006;16(7):934–46. doi: 10.1101/gr.4708406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freeling M. Bias in plant gene content following different sorts of duplication: tandem, whole-genome, segmental, or by transposition. Annu Rev Plant Biol. 2009;60:433–53. doi: 10.1146/annurev.arplant.043008.092122. [DOI] [PubMed] [Google Scholar]
- 57.Throude M, Bolot S, Bosio M, Pont C, Sarda X, Quraishi UM, et al. Structure and expression analysis of rice paleo duplications. Nucleic Acids Res. 2009;37(4):1248–59. doi: 10.1093/nar/gkn1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozkan H, Levy AA, Feldman M. Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops-Triticum) group. Plant Cell. 2001;13(8):1735–47. doi: 10.1105/tpc.13.8.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chantret N, Salse J, Sabot F, Rahman S, Bellec A, Laubin B, et al. Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops) Plant Cell. 2005;17(4):1033–45. doi: 10.1105/tpc.104.029181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tate JA, Ni Z, Scheen AC, Koh J, Gilbert CA, Lefkowitz D, et al. Evolution and expression of homeologous loci in Tragopogon miscellus (Asteraceae), a recent and reciprocally formed allopolyploid. Genetics. 2006;173(3):1599–611. doi: 10.1534/genetics.106.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buggs RJA, Chamala S, Wu W, Gao L, May GD, Schnable PS, et al. Characterization of duplicate gene evolution in the recent natural allopolyploid Tragopogon miscellus by next-generation sequencing and Sequenom iPLEX MassARRAY genotyping. Mol Ecol. 2010;19:132–46. doi: 10.1111/j.1365-294X.2009.04469.x. [DOI] [PubMed] [Google Scholar]
- 62.Rae AL, Grof CPL, Casu RE, Bonnett GD. Sucrose accumulation in the sugarcane stem: pathways and control points for transport and compartmentation. Field Crop Res. 2005;92(2-3):159–68. doi: 10.1016/j.fcr.2005.01.027. [DOI] [Google Scholar]
- 63.Slewinski TL, Meeley R, Braun DM. Sucrose transporter1 functions in phloem loading in maize leaves. J Exp Bot. 2009;60(3):881–92. doi: 10.1093/jxb/ern335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eom JS, Cho JI, Reinders A, Lee SW, Yoo Y, Tuan PQ, et al. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol. 2011;157(1):109–19. doi: 10.1104/pp.111.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, et al. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol. 2006;141(1):196–207. doi: 10.1104/pp.106.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okubo-Kurihara E, Higaki T, Kurihara Y, Kutsuna N, Yamaguchi J, Hasezawa S. Sucrose transporter NtSUT4 from tobacco BY-2 involved in plant cell shape during miniprotoplast culture. J Plant Res. 2011;124(3):395–403. doi: 10.1007/s10265-010-0377-7. [DOI] [PubMed] [Google Scholar]
- 67.Schulz A, Beyhl D, Marten I, Wormit A, Neuhaus E, Poschet G, et al. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 2011;68(1):129–36. doi: 10.1111/j.1365-313X.2011.04672.x. [DOI] [PubMed] [Google Scholar]
- 68.Ibraheem O, Dealtry G, Roux S, Bradley G. The effect of drought and salinity on the expressional levels of sucrose transporters in rice (Oryza sativa Nipponbare) cultivar plants. Plant Omics. 2011;4(2):68–74. [Google Scholar]


