Abstract
Purpose
Rotarix TM was launched in November 2011 in Japan to prevent rotavirus gastroenteritis. Some studies suggest that Rotarix TM may have a temporal association with a risk of intussusception (IS). We assessed a possible association between IS and Rotarix TM vaccination in Japan.
Methods
All IS cases spontaneously reported post‐vaccination (Brighton collaboration levels 1, 2, and 3) were extracted from the GlaxoSmithKline spontaneous report database on the 11th of January 2013. Expected numbers of IS cases were estimated using the number of vaccine doses distributed and the Japanese incidence rate of IS stratified by month of age. The observed versus expected analysis considered the IS cases for each risk period (7 and 30 days post‐vaccination) and for each vaccine dose (two doses).
Results
Before January 2013, approximately 601 000 Rotarix TM doses were distributed in Japan. For a risk period of 7 days post‐dose 1 and post‐dose 2, 10 and five IS cases were observed, whereas 3.4 and 7.6 were expected, providing an observed‐to‐expected ratio of 2.96 (95% confidence interval [CI]: 1.42; 5.45) and 0.66 (95% CI: 0.21; 1.53), respectively. For a risk period of 30 days post‐dose 1 and post‐dose 2, 14 and eight cases were observed, whereas 14.5 and 32.7 were expected, providing an observed‐to‐expected ratio of 0.97 (95% CI: 0.53; 1.62) and 0.24 (95% CI: 0.11; 0.48), respectively.
Conclusion
A statistically significant excess of IS cases was observed within 7 days post‐dose 1, but not post‐dose 2. These results are consistent with previous observations in large post‐marketing safety studies in other world regions. © 2015 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons, Ltd.
Keywords: human rotavirus vaccine, RotarixTM, post‐marketing surveillance, intussusception, Japan, pharmacoepidemiology
Introduction
In 1999, a rhesus‐human reassortant tetravalent rotavirus vaccine, RotaShieldTM (Wyeth Laboratories, Marietta, PA),1 was withdrawn from the US market 9 months after its introduction because it was associated with an attributable risk of intussusception (IS) after the first vaccine dose of about 1 in 10 000 infants aged 2 months or older.2, 3 IS is the most common cause of acute intestinal obstruction among infants and young children; it may become a medical emergency if not treated early and eventually may lead to death.4 It is now recognized that IS can occur as a rare adverse event following rotavirus vaccination.5
In Japan, the background incidence of IS is relatively high and ranges between 143.5 and 191 per 100 000 children‐years in the first year of life.6, 7, 8, 9 This incidence rate is one of the highest in the world, together with other countries, such as Israel (219 per 100 000 children‐years), Vietnam (302), and South Korea (328).10, 11
A live attenuated monovalent rotavirus vaccine (Rotarix TM; GlaxoSmithKline [GSK] Vaccines, Belgium) was developed for the prevention of rotavirus gastroenteritis in infants. The risk of IS associated with Rotarix TM was evaluated in a large phase III clinical trial, recruiting more than 60 000 infants in 11 Latin American countries and Finland.12 Rotarix TM, administered as two oral doses, significantly reduced the rate of severe gastroenteritis, and no increased risk of IS was identified in the 30‐day post‐vaccination period in this study.12 Based on these data, the World Health Organization recommended in 2009 the use of the Rotarix TM vaccine in routine infant immunization programs worldwide.13 However, recent post‐licensure studies suggested that Rotarix TM may have a temporal association with a risk of IS in infants.14, 15, 16, 17, 18, 19, 20
Rotarix TM was launched in Japan in November 2011 with the indication for the prevention of gastroenteritis caused by rotavirus. The vaccine course consists of two doses; the first dose may be administered from the age of 6 weeks. There should be an interval of at least 4 weeks between doses, and the vaccine course should be completed by the age of 24 weeks. To assess a potential association between IS and Rotarix TM vaccination in Japan, the current study compares the number of IS cases spontaneously reported between November 2011 and January 2013 with that of the number of IS cases expected on the basis of the estimated background incidence of IS in infants.
Methods
We performed an observed versus expected (O/E) analysis, which is a standard method for safety signal strengthening. In this approach, the number of observed IS cases post‐vaccination were compared with that of the number of cases expected to occur naturally if there was no association with vaccination.
All IS cases spontaneously reported after vaccination with Rotarix TM between 21st of November 2011 and 11th of January 2013, and meeting the Brighton Collaboration case definitions for IS levels 1 to 3,5 were considered. The number of IS cases were counted for two risk periods (within 7 and 30 days post‐vaccination) and for each of two vaccine doses.
Vaccine exposure
Because no actual data on vaccine exposure was available, the number of distributed Rotarix TM doses was used as a proxy for exposure. Moreover, it was assumed that all infants in the exposed population received two vaccine doses.
Age distribution
Because the actual age distribution of the vaccine‐exposed population was not available, the distribution of age at vaccination spontaneously reported in Rotarix TM cases, available in the GSK spontaneous report database, was used as a proxy for age distribution of the vaccinated population.
Post‐vaccination intussusception cases in Japan
The Pharmaceuticals and Medical Devices Agency (PMDA) of Japan21 is the primary regulatory agency, working together with the Ministry of Health, Labour, and Welfare to provide the legal basis for pharmacovigilance requirements in Japan.22 PMDA requires that a new drug undergoes Early Post‐marketing Phase Vigilance (EPPV) for the first 6 months after the launch of the new product in Japan.21 EPPV is specified as a condition of approval, and its main objective is to collect the adverse drug reactions from all of the medical institutions where the drugs are used and implement the consequent safety measures and minimize the associated public health risk.22 For Rotarix TM, EPPV was initiated on 21 November 2011.23 After EPPV completion, IS cases are submitted by physicians via spontaneous reporting.
All IS cases spontaneously reported post‐vaccination with Rotarix TM in Japan were extracted from the GSK spontaneous report database. The narratives of the reported cases were reviewed by a physician, and the reports were classified using the Brighton Collaboration case definitions for IS (levels 1 to 4).5 Level 1 of diagnostic certainty is defined as a confirmed or definite case of IS on the basis of surgical, radiological, or autopsy criteria. Levels 2 and 3 IS cases are defined using clinical criteria: two major criteria or one major and three minor criteria for level 2 and more than four minor criteria for level 3. Major criteria include the following: evidence of intestinal obstruction, features of intestinal invagination, and evidence of intestinal vascular compromise or venous congestion. Minor criteria include the following: predisposing factors such as age below 1 year and male, abdominal pain, vomiting, lethargy, pallor, hypovolemic shock, and plain abdominal radiograph showing an abnormal but non‐specific bowel gas pattern. Level 4 is defined as a reported event of IS, with insufficient evidence to meet the case definition.5
Expected intussusception cases
The number of cases expected to occur within 7 and 30 days following vaccination (Ne) was derived from the following formula:
, where
Inc = the incidence of IS cases in the first year of life. The incidence value used for Japan was based on the paper of Noguchi et al. (158/100 000 person‐years).8
Pdi = the proportion of IS cases occurring in the i th month of age. Japanese age‐specific distributions were used.8
Ndi = the number of doses of Rotarix TM distributed in the i th month of age, based on country‐level sales data and an estimate of the age of vaccine administration based on the distribution of age at vaccination spontaneously reported in Rotarix TM cases available in the GSK spontaneous report database.
RP = the risk period in days (e.g., 7 or 30 days).
30.4 = the average number of days in a month.
Statistical analysis
We calculated the ratio of O/E IS cases using an exact Poisson method24 to produce 95% confidence intervals (CI). All analyses were conducted with the use of SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Distributed vaccine doses and intussusception cases reported after vaccination
During the study period, 601 131 doses of Rotarix TM were distributed from the company, and a total of 36 IS cases were reported (regardless of onset time or Brighton criteria). All IS cases were reviewed by a physician and classified according to the Brighton criteria of diagnostic certainty. A total of 29/36 (80.6%) cases (all with known onset time) met the Brighton levels 1 to 3 (level 1, 27 cases; level 2, two cases).
Out of the 29 IS cases meeting the Brighton levels 1 to 3, a total of 22 cases occurred within the 30‐day post‐vaccination risk period. The distribution of the reported IS cases according to infants' age at vaccination and at onset of IS is shown in Figure 1. The number of IS cases varied substantially with age, with the highest values reported for infants aged between 18 and 22 weeks at the time of IS onset (Figure 1B).
Figure 1.
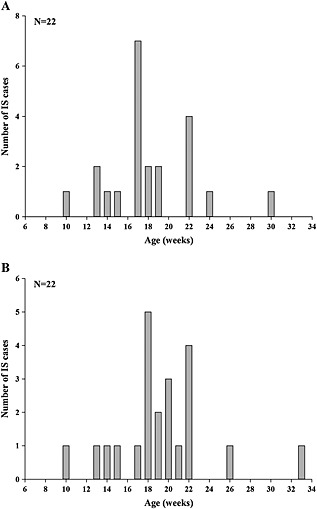
Distribution of the intussusception cases meeting the Brighton levels 1 to 3 and reported to occur in the 30‐day post‐vaccination risk period, according to infants' age (weeks) at vaccination (A) and at onset of intussusception (B)
The 22 IS cases reported to occur within the 30‐day post‐vaccination risk period comprised 13 cases post‐dose 1 (12 cases Brighton level 1 and one case level 2), six cases post‐dose 2 (all Brighton level 1 cases) and three cases with unknown dose information. The majority of those cases (15/22) occurred within the 7‐day post‐vaccination risk period (nine cases post‐dose 1, three cases post‐dose 2, and three cases with unknown dose information; all were Brighton level 1 cases). The cases with unknown dose information were distributed among dose 1 and dose 2 according to the proportion of IS cases reported after dose 1 and 2, resulting in 14 and eight IS cases in the 30‐day risk period post‐dose 1 and 2, respectively, and 10 and five IS cases in the 7‐day risk period post‐dose 1 and 2, respectively. The distribution of the reported IS cases in spontaneous safety reports post‐Rotarix TM vaccination after each dose according to time to onset of IS is shown in Figure 2.
Figure 2.
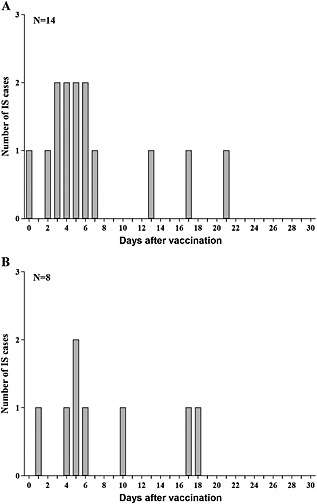
Distribution of the reported intussusception cases according to vaccine dose: post‐dose 1 (A) and post‐dose 2 (B)
Observed versus expected intussusception analysis
The 22 IS cases (14 post‐dose 1 and eight post‐dose 2), which met the Brighton levels 1 to 3 and occurred within the 30‐day post‐vaccination risk period, were included in the O/E analysis (Table 1). The O/E analysis was carried out for 7 days and 30 days post‐vaccination, for both Rotarix TM doses. The O/E ratio was highest in the 7‐day risk period post‐dose 1, with a value of 2.96 (95% CI: 1.42; 5.45). Following the second dose, the calculated O/E ratio was 0.66 (95% CI: 0.21; 1.53). For a risk period of 30 days, the O/E ratios were 0.97 (95% CI: 0.53; 1.62) and 0.24 (95% CI: 0.11; 0.48) post‐dose 1 and post‐dose 2, respectively.
Table 1.
Observed versus expected analysis of IS incidence post‐Rotarix TM vaccination
| Risk period (days) | Dose | Number of distributed Rotarix TM doses | Observed number of IS cases (level 1–3 BCWIG)* | Expected number of IS cases† | O/E ratio [95% Poisson CI] |
|---|---|---|---|---|---|
| 7 | 1 | 300 566‡ | 10 | 3.37 | 2.96 [1.42; 5.45] |
| 7 | 2 | 300 566‡ | 5 | 7.63 | 0.66 [0.21; 1.53] |
| 7 | Any | 601 131 | 15 | 8.81 | 1.70 [0.95; 2.81] |
| 30 | 1 | 300 566‡ | 14 | 14.46 | 0.97 [0.53; 1.62] |
| 30 | 2 | 300 566‡ | 8 | 32.70 | 0.24 [0.11; 0.48] |
| 30 | Any | 601 131 | 22 | 37.75 | 0.58 [0.37; 0.88] |
IS, intussusception; BCWIG, the Brighton Collaboration Intussusception Working Group; O/E, observed versus expected; CI, confidence interval.
Includes cases with attributed dose number;
Calculated based on the incidence rate reported by Noguchi et al. 8
Rounded up for clarity.
Discussion
The present study was designed to compare the number of spontaneously reported IS cases after Rotarix TM vaccination between November 2011 and January 2013 (extracted from the GSK spontaneous report database) with that of the number of IS cases expected on the basis of the estimated background incidence rate of IS. The results showed that in Japan, in the 7 days following the first Rotarix TM dose, the number of IS cases was higher than expected (O/E ratio 2.96 [95% CI: 1.42; 5.45]); no statistically significant increase in the number of IS cases post‐dose 2 was observed.
Our results are compatible with previous observations in several post‐marketing safety studies in other regions of the world.14, 15, 16, 17, 18, 19, 20 In the USA, two recent post‐licensure studies evaluated the risk of IS after vaccination with Rotarix TM.14, 15 In one study,14 the results with respect to the primary analysis (a self‐controlled risk‐interval design that included only vaccinated children) of Rotarix TM were not significant (one IS case in the 21‐day risk window post‐dose 1 and no cases in the control window [22 to 42 days after vaccination]), but the secondary analysis (a cohort design that included exposed and unexposed person‐time) showed a significant attributable risk post‐dose 2 (7.3 [95% CI: 0.8; 22.5] per 100 000 doses), although the study was assumed to be statistically underpowered. In the second US study,15 the risk of IS among children receiving Rotarix TM was compared with historical background rates, using sequential analyses. A total of six cases of IS were identified within 7 days after the administration of either dose of Rotarix TM (207 955 doses), while the expected number of IS cases was 0.72, resulting in a significant relative risk of 8.38. A study in Australia,17 using a self‐controlled case series (SCCS) method reported a relative incidence (frequency of IS occurrence in periods of time after vaccination [‘exposure’] relative to IS occurrence in unexposed time, in case patients only) of IS in the 7‐day period after the first Rotarix TM dose of 6.76 (95% CI: 2.40; 19.01; p <0.001) and a smaller relative incidence post‐dose 2 (2.84 [95% CI: 1.10; 7.34; p = 0.03]). Finally, two studies conducted in Mexico18, 19 and Brazil19 reported an increased risk of IS within 7 days post‐dose 1 of Rotarix TM. One of the studies used the SCCS method and found that the relative incidence of IS within 7 days of vaccination was 6.49 post‐dose 1 (95.5% CI: 4.17; 10.09; p <0.001) and 1.29 post‐dose 2 (95.5% CI: 0.80; 2.11; p = 0.29).18 In the other study,19 an increased risk of IS within 7 days post‐dose 1 was identified among Mexican infants using the case‐series method (incidence ratio, 5.3; 95% CI: 3.0; 9.3). However, no significant risk was identified post‐dose 1 among Brazilian infants (incidence ratio, 1.1; 95% CI: 0.3; 3.3). No elevated relative incidence of IS was observed up to 7 days post‐dose 2 in both countries, but an increase in the rate of IS by a factor of 2 was observed in Mexico during the second and third week after vaccination (incidence ratios: 2.2 [95% CI: 1.1; 4.2] for the second week and 2.2 [95% CI: 1.2; 4.0] during the third week).19 In our current study, the highest O/E ratio was observed within the 7‐day post‐dose 1 period (O/E ratio 2.96; 95% CI: 1.42; 5.45); a cluster of onset of IS was observed in this period. Differences between the results of our study and those of the other post‐marketing studies are difficult to interpret because these studies used different methodologies and the risk estimates values (relative risk, relative incidence, incidence ratio) are not the same as the ratios values obtained from our O/E analysis. However, similar trends in temporal association between the development of IS and receipt of the Rotarix TM vaccine were observed.
In Japan, the peak of natural IS incidence among infants aged less than 1 year was observed between 6 and 11 months of age.6, 7, 8, 9 A comparison with our current data cannot be performed because the age of the vaccine‐exposed population in the current study was below 6 months, considering that according to the manufacturer, the vaccine course should be completed by the age of 24 weeks (5.5 months). In the current study, the number of IS cases post‐Rotarix TM vaccination was highest in infants 18 to 22 weeks old at the time of IS onset. This clustering is related to the infants' age at vaccination and may be because of a reporting bias inherent to spontaneous safety reports and the known risk period.25 Moreover, the aforementioned studies used medical information databases and included all IS cases, regardless of vaccination status of the population,6, 7, 8, 9 while in our O/E analysis, only the IS cases reported to occur within the 30‐day post‐vaccination risk period were considered.
Our study has several limitations. First, the magnitude of IS underreporting inherent to spontaneous reporting is unknown. However, the reporting rate of adverse events might be high because EPPV is mandatory for all new vaccines in Japan.21 To overcome this limitation, we included in the current analysis all IS cases meeting the Brighton levels 1 to 3, which occurred within the 30‐day post‐vaccination risk period. However, only one out of the 22 included IS cases was not level 1. Second, there is an uncertainty on the actual use of the vaccine doses and the age distribution of vaccinated infants. Third, there is an uncertainty on the background incidence rate of IS in Japan because the reported numbers vary between 143.5 and 191 per 100 000 children‐years.6, 7, 8, 9 Finally, the O/E analysis is a method for signal detection and strengthening and is not designed for estimating relative or attributable risks; therefore, coparisons with data from other studies should be made with caution.
In conclusion, in this study, a statistically significant excess of IS cases was observed within 7 days after administration of the first Rotarix TM dose to Japanese infants. No statistically significant excess of IS cases was observed after the second dose. IS was identified as a rare adverse reaction post‐vaccination with Rotarix TM. These results are consistent with the data available on risk estimates from other regions. The high background incidence and reporting rates of IS in Japan might have contributed towards the observation of an excess of IS cases during the short time period after vaccine launch.
Conflict of Interest
Vincent Bauchau, Lionel Van Holle, Olivia Mahaux, Katsiaryna Holl, and Hubert Buyse are employed by the GSK group of companies. Keiji Sugiyama is employed by Japan Vaccine Co., Ltd., Tokyo, Japan. Vincent Bauchau, Lionel Van Holle, Katsiaryna Holl, and Hubert Buyse own shares in GSK.
Key Points.
Rotarix TM was launched in 2011 in Japan to prevent rotavirus gastroenteritis in infants.
Vaccination with Rotarix TM may have a temporal association with a risk of intussusception.
In Japan, a statistically significant excess of intussusception cases was observed within 7 days post‐dose 1 of Rotarix TM, but not post‐dose 2.
The results are consistent with previous observations in other world regions.
Financial Disclosures and Support
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript.
Ethics Statement
The data used to perform the analyses described in the present manuscript consists of adverse events following vaccination received by GSK through spontaneous reporting via worldwide sources including health care professionals, regulatory authorities, members of the public, and literature sources. These data are from a passive safety surveillance system and have been voluntarily reported to GSK. Hence, no ethics approval was required for the present analyses.
Trademarks
Rotarix is a trademark of the GSK group of companies.
RotaShield is a trademark of Wyeth‐Lederle Vaccines.
Author Contributions
All named authors have contributed in the design/acquisition of data or analysis and interpretation of data. They have provided substantial intellectual and scientific input in the development of this manuscript. All authors were involved in critically reviewing the content and revising the manuscript.
Acknowledgements
The authors thank Roeland Van Kerckhoven (Consultant for Keyrus Biopharma) for publication management and Vasile Coman (XPE Pharma & Science, Belgium) for writing support.
Bauchau, V. , Van Holle, L. , Mahaux, O. , Holl, K. , Sugiyama, K. , and Buyse, H. (2015) Post‐marketing monitoring of intussusception after rotavirus vaccination in Japan. Pharmacoepidemiol Drug Saf, 24: 765–770. doi: 10.1002/pds.3800.
Prior postings and presentations: These data were presented at the 45th Annual Meeting of the Japanese Society for Pediatric Infectious Diseases, in October 2013.
References
- 1. Kapikian AZ, Hoshino Y, Chanock RM, Perez‐Schael I. Efficacy of a quadrivalent rhesus rotavirus‐based human rotavirus vaccine aimed at preventing severe rotavirus diarrhea in infants and young children. J Infect Dis 1996; 174(Suppl 1): S65–72. doi:10.1093/infdis/174.Supplement_1.S65. [DOI] [PubMed] [Google Scholar]
- 2. Kramarz P, France EK, Destefano F, et al. Population‐based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J 2001; 20(4): 410–416. doi:10.1097/00006454-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 3. Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344(8): 564–572. doi:10.1056/nejm200102223440804. [DOI] [PubMed] [Google Scholar]
- 4. Bines J, Ivanoff B. Acute Intussusception in Infants and Young Children: Incidence, Clinical Presentation and Management: A Global Perspective. WHO/V&B/02.19. World Health Organization, Department of Vaccines and Biologicals: Geneva, 2002. [Google Scholar]
- 5. Bines JE, Kohl KS, Forster J, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004; 22(5‐6): 569–574. doi:10.1016/j.vaccine.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 6. Miura M, Sato K, Muto H, Gopala K, Holl K. Intussusception in Japanese infants: analysis of health insurance claims database. Open J Pediatr 2013; 03(04): 311–316. doi:10.4236/ojped.2013.34056. [Google Scholar]
- 7. Nakagomi T, Takahashi Y, Arisawa K, Nakagomi O. A high incidence of intussusception in Japan as studied in a sentinel hospital over a 25‐year period (1978–2002). Epidemiol Infect 2006; 134(1): 57–61. doi:10.1017/s0950268805004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noguchi A, Nakagomi T, Kimura S, et al. Incidence of intussusception as studied from a hospital‐based retrospective survey over a 10‐year period (2001–2010) in Akita Prefecture, Japan. Jpn J Infect Dis 2012; 65(4): 301–305. doi:10.7883/yoken.65.301. [PubMed] [Google Scholar]
- 9. Takeuchi M, Osamura T, Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S. Intussusception among Japanese children: an epidemiologic study using an administrative database. BMC Pediatr 2012; 12: 36. doi:10.1186/1471-2431-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bines JE, Patel M, Parashar U. Assessment of postlicensure safety of rotavirus vaccines, with emphasis on intussusception. J Infect Dis 2009; 200(Suppl 1): S282–290. doi:10.1086/605051. [DOI] [PubMed] [Google Scholar]
- 11. Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS One 2013; 8(7): e68482. doi:10.1371/journal.pone.0068482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruiz‐Palacios GM, Perez‐Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354(1): 11–22. doi:10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 13. Meeting of the Immunization Strategic Advisory Group of Experts. April 2009–conclusions and recommendations. Wkly Epidemiol Rec 2009; 84(23): 220–236. [PubMed] [Google Scholar]
- 14. Yih WK, Lieu TA, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med 2014; 370(6): 503–512. doi:10.1056/NEJMoa1303164. [DOI] [PubMed] [Google Scholar]
- 15. Weintraub ES, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med 2014; 370(6): 513–519. doi:10.1056/NEJMoa1311738. [DOI] [PubMed] [Google Scholar]
- 16. Haber P, Patel M, Pan Y, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006–2012. Pediatrics 2013; 131(6): 1042–1049. doi:10.1542/peds.2012-2554. [DOI] [PubMed] [Google Scholar]
- 17. Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clin Infect Dis 2013; 57(10): 1427–1434. doi:10.1093/cid/cit520. [DOI] [PubMed] [Google Scholar]
- 18. Velazquez FR, Colindres RE, Grajales C, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J 2012; 31(7): 736–744. doi:10.1097/INF.0b013e318253add3. [DOI] [PubMed] [Google Scholar]
- 19. Patel MM, Lopez‐Collada VR, Bulhoes MM, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 2011; 364(24): 2283–2292. doi:10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 20. Buttery JP, Danchin MH, Lee KJ, et al. Intussusception following rotavirus vaccine administration: post‐marketing surveillance in the National Immunization Program in Australia. Vaccine 2011; 29(16): 3061–3066. doi:10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- 21. Pharmaceuticals and medical devices agency , Japan. Available at: http://www.pmda.go.jp [24 July 2014].
- 22. Biswas P. Pharmacovigilance in Asia. J Pharmacol Pharmacother 2013; 4(Suppl 1): S7–S19. doi:10.4103/0976-500x.120941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pharmaceuticals and medical devices safety information, No. 290, April 2012. Available at: http://www.pmda.go.jp/english/service/pdf/precautions/PMDSI‐290.pdf [24 July 2014].
- 24. Garwood F. Fiducial limits for the Poisson distribution. Biometrika 1936; 28(3/4): 437–442. doi:10.1093/biomet/28.3-4.437. [Google Scholar]
- 25. Guideline on good pharmacovigilance practices (GVP) product‐ or population‐specific considerations I: vaccines for prophylaxis against infectious diseases. EMA/488220/2012. Available at: http://www.ema.europa.eu [24 July 2014].


