Abstract
OBJECTIVE
Pediatric inflammatory bowel disease (IBD) often presents insidiously and standard blood tests are normal in 20% of patients. We hypothesize that fecal occult blood testing (FOBT) and the perianal examination in addition to blood tests provide important information during the screening process for IBD. The goal of this study was to measure the diagnostic value of adding FOBT and perianal examination to standard screening labs in evaluating children and adolescents for IBD.
METHODS
The medical records of consecutive patients undergoing ileocolonoscopy for IBD were reviewed. Laboratory test results, FOBT, and perianal examination prior to the decision to perform the ileocolonoscopy were recorded. Standard limits of laboratory tests were used. Multivariate logistic regression was performed on a discovery cohort and applied to an independent validation cohort.
RESULTS
The discovery cohort included 335 patients (85 IBD and 250 non-IBD). 61.2% had FOBT and perianal examination performed prior to the decision to perform the ileocolonoscopy. 119 patients had complete blood testing, FOBT, and perianal exam available for full analysis. The sensitivity of the lab testing was 80.5% for IBD and the sensitivity of FOBT with perianal examination was 65.9%. However, the combined sensitivity of lab testing and FOBT with perianal examination was 97.6%. The most predictive model included CRP, platelets and FOBT with perianal examination and was superior to the lab value-only model (p<0.001) which was validated in a separate cohort.
CONCLUSIONS
Perianal examination and FOBT improves sensitivity in screening children for IBD.
Keywords: pediatric, inflammatory bowel disease, fecal occult blood testing, perianal exam, sensitivity and specificity
BACKGROUND
Inflammatory bowel disease (IBD) is a chronic inflammatory condition that involves the gastrointestinal tract and includes both ulcerative colitis (UC) and Crohn’s disease (CD).1 Symptoms often include abdominal pain, weight loss, hematochezia, and diarrhea although some patients may present with more insidious gastrointestinal and extra-intestinal manifestations.2, 3 A delay in diagnosis of IBD, especially CD remains common in children and potential complications from these delays necessitate an effective and efficient evaluation process.4, 5
Given the complications of a delayed diagnosis of IBD in a child, prompt identification of patients likely to have IBD is crucial. Children with IBD frequently present with anemia and elevated inflammatory markers which prompts further diagnostic testing. However, it is well-understood that 10-20% of children with IBD present with a normal laboratory examination.6-8 Although serology directed at microbial antigens (e.g. ASCA, OmpC) offers promise as a diagnostic screen, current antibody screens are no more effective at screening for IBD in children than standard laboratory markers.9 More recently, c-reactive protein (CRP) determination has been shown to correlate with disease activity in pediatric CD and improves the sensitivity of lab-based evaluations for IBD. 10, 11 However, there is high potential for a delay in diagnosis for a child with IBD who has a normal lab evaluation given the overlap of some symptoms seen in IBD and irritable bowel syndrome (IBS). The optimal evaluation to identify these patients is not clear.
The digital rectal exam (DRE) has been a longstanding part of the physical exam. However, DRE is not performed consistently in children with abdominal pain.12-14 Potentially useful information obtained from a DRE (during an evaluation for IBD) includes the presence of occult blood in the stool and perianal disease. However, the possible discomfort of a DRE and the unclear role that FOBT and perianal examination play in an evaluation for pediatric IBD may contribute to some patients not being examined.
We hypothesize that the addition of FOBT and perianal examination to a lab-based testing strategy improves the screening process of children for IBD. The aim of this study was to assess whether FOBT and perianal examination in children suspected to have IBD offered additional diagnostic yield compared to standard laboratory blood testing.
METHODS
The discovery cohort included consecutive patients who were undergoing initial endoscopic evaluation for IBD at MassGeneral Hospital for Children between August 2009 and December 2011. The diagnosis of IBD was based on standard endoscopic, histologic, and radiographic criteria.15 Patients who already had a diagnosis of IBD and patients whose evaluation lasted >3 months prior to ileocolonoscopy were excluded. The medical charts were reviewed for demographic data, documentation of perianal examination and FOBT during the clinic visit (prior to the decision to perform an ileocolonoscopy), and laboratory values obtained prior to the ileocolonoscopy. The perianal examination was considered abnormal if the examiner noted a large fissure (large enough to result in bleeding or perianal pain), skin tag, perianal fistula, or perianal abscess.
Abnormal lab testing was based on standard limits of normal lab values and included CRP > 8.0g/L, erythrocyte sedimentation rate (ESR) >20mm/hr, platelets > 450,000/mL, and albumin <3.5g/dL (as used previously).9 Age-specific cutoffs for normal value for hematocrit were used (0-2yo 31.5, 2-12yo 34.5, >12yo female 35.1, and >12yo male 40.5).9 The validation cohort consisted of an independent group of 50 consecutive patients with newly diagnosed IBD and 50 consecutive patients who had a normal ileocolonoscopy during an evaluation for IBD at MassGeneral Hospital for Children between December 2011 and September 2013. For inclusion in the validation cohort, all subjects must have had FOBT, perianal examination, as well as the laboratory values that were included in the predictive model from the discovery cohort. The validation cohort size was powered to find a difference in AUC of 0.05 for the model including the perianal exam and FOBT compared to the best lab-only model (based on results generated from the discovery cohort). This study was approved by the institutional review board at Massachusetts General Hospital.
The sensitivity and specificity of individual laboratory testing were determined using the predetermined cut-off values. Multivariate, stepwise logistic regression was performed on the discovery cohort using both the dataset that included only the five recorded laboratory values as well as the dataset that included the five recorded laboratory values and FOBT with perianal examination. Subjects in the discovery cohort that did not have all five laboratory values measured within 12 weeks of the endoscopic procedures were excluded from this analysis.
The analysis of the validation cohort involved multivariate logistic regression on the datasets that contained only the predictive laboratory markers and the dataset that included both the predictive laboratory markers as well as FOBT and perianal examination results. Multivariate, stepwise logistic regression and additional statistical analysis were performed using SAS 9.1 (SAS Institute, Cary, NC). ROC curves were derived in XL-STAT. Comparisons were performed via Chi square analysis for categorical variables and by student’s t test for continuous variables. A p value of <0.05 was used as the threshold for significance.
RESULTS
We identified 335 patients including 85 patients with IBD (49 CD, 24 UC, 12 indeterminate colitis) diagnosed by ileocolonoscopy and 250 patients who were evaluated by ileocolonoscopy for symptoms consistent with IBD (such as diarrhea, rectal bleeding, abdominal pain, or weight loss) but were found to not have IBD. The IBD cohort was 56.5% male compared to the non-IBD cohort that was 51.6% (p=0.45). The average ages of the IBD and non-IBD patients were 12.9 and 12.5 years, respectively (p=0.46). The distribution of disease location amongst the CD patients was 6.1% L1 (ileal), 34.7% L2 (colonic), and 59.2% L3 (ileocolonic). The majority of the non-IBD cases had a normal ileocolonoscopic evaluation (80.8%) but others in this group had findings that included non-specific colitis (including enteric infections) 12.4%, solitary rectal ulcer syndrome 1.2%, colonic polyps 3.6%, and H. pylori gastritis 0.8%. The demographics and mean lab values are shown in Table 1.
Table 1.
Demographics and Initial Lab Testing in Total Discovery Cohort
| IBD cohort (n=85) | Control cohort(n=250) | P value | |
|---|---|---|---|
| Age (years) | 12.9 (11.0-16.0) | 12.5 (9.0-16.0) | 0.46 |
| Gender (M%) | 56.5% | 51.6% | 0.45 |
| CRP (mg/L) | 26.8 (1.6-32) | 5.9 (0.35-3.65) | <0.001 |
| ESR (mm/hr) | 27.8 (11.5-40) | 10.8 (5-13) | <0.001 |
| Hematocrit (%) | 36.5 (33.6-40.1) | 38.1 (35.6-40.8) | 0.01 |
| Albumin (g/dL) | 4.0 (3.7-4.4) | 4.5 (4.3-4.8) | <0.001 |
| Platelets (K/uL) | 401.6 (295.5-482.3) | 294.7 (242-334) | <0.001 |
Presented values represent mean and interquartile range.
Prior to the decision to perform an ileocolonoscopy, 61.2% of the discovery cohort had FOBT and perianal examination. FOBT and perianal examination were performed in 67.1% (57/85) of IBD patients and 59.2% (148/250) of non-IBD patients (p=0.25). (All patients had a DRE immediately prior to colonoscope insertion to rule-out an anal stricture.) The discovery cohort was 52.8% male (177/338). The gender of the medical provider and patient differed in 42.4% of patients (142/338). The performance of FOBT and perianal examination during office visits with gender discordance between provider and patient was as frequent as visits without gender discordance (62.7% vs. 69.9%, p=0.20). Patients who reported gross blood in their stool were as likely as those without gross blood to have FOBT and perianal examination (66.0% vs. 58.1%, p=0.17). The sensitivity and specificity of FOBT and perianal examination to predict IBD was 65.9% (54.8-75.8%) and 72.0% (66.0-77.5%). There were significant differences between IBD and non-IBD patients in CRP (26.8mg/L vs. 5.9mg/L, p<0.001), ESR (27.8mm/hr vs. 10.8mm/hr, p<0.001), platelets (401.6 × 103 vs. 294.7 × 103, p<0.001), albumin (4.0g/dL vs. 4.5g/dL, p<0.001) and hematocrit (36.5 vs. 38.1, p=0.01). The sensitivity of the lab testing panel (with at least one abnormal value) was 80.5% with the specificity of 51.3%. The sensitivity and specificity of individual lab testing to predict IBD are found in Table 2.
Table 2.
Sensitivity and Specificity to Predict IBD in the Total Discovery Cohort
| Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|
| FOBT and Perianal Examination | 65.9% (54.8-75.8%) | 72.0% (66.0-77.5%) |
| Hematocrit | 59.0% (47.7-69.7%) | 60.4% (53.8-66.8%) |
| Platelets | 33.8% (23.6-45.2%) | 95.5% (91.9-97.8%) |
| Albumin | 18.4% (10.5-29.0%) | 97.4% (94.0-99.2%) |
| CRP | 50.7% (38.4-63.0%) | 89.2% (82.8-93.8%) |
| ESR | 54.4% (42.8-65.6%) | 90.8% (85.9-94.5%) |
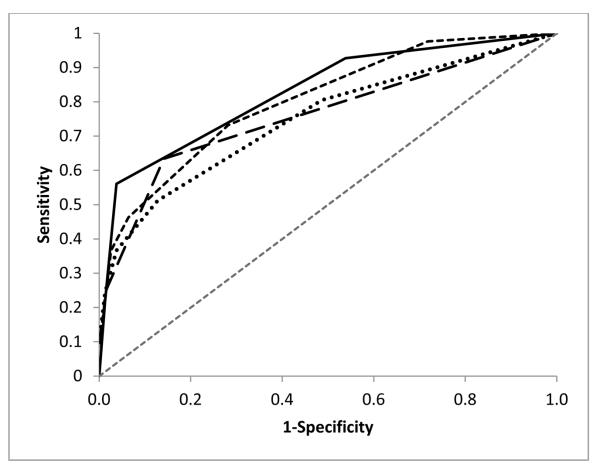
Out of the total discovery cohort, 35.5% of patients (n = 119, 41 IBD and 78 non-IBD) had all five lab values and FOBT with perianal examination documented in the clinic visit prior to the ileocolonoscopy. A normal laboratory panel was found in 19.5% (8/41) IBD patients. Seven of these IBD patients with a normal laboratory panel had abnormalities on FOBT and perianal examination resulting in a sensitivity of 97.6% for the lab panel combined with FOBT and perianal examination. Logistic regression modeling found the most predictive model for IBD included FOBT with perianal examination, CRP, and platelets.(Table 3) This model was superior to the best lab-only model (CRP and platelet count) (p<0.001). The sensitivity and specificity of this model in the discovery cohort was 92.7% and 79.7%. Figure 1 displays the ROC curves for the model with perianal exam, FOBT, CRP, and platelets (AUC=0.828).
Table 3.
Binary Logistic Regression from Discovery Cohort (who had all 6 markers)
| Odds Ratio (95% CI) |
P value | Sensitivity (95% CI) |
Specificity (95% CI) |
Positive Predictive Value (95% CI) |
Negative Predictive Value (95% CI) |
|
|---|---|---|---|---|---|---|
| CRP | 8.85 (2.79- 27.78) |
0.0002 | 51.2% (35.9- 66.5%) |
91.0% (84.7- 97.4) |
75.0% (59.0- 91.0) |
78.0% (69.5- 86.5) |
| Platelet Count |
6.13 (1.26- 29.41) |
0.0247 | 36.6% (21.9- 51.3) |
93.6% (88.2- 99.0) |
75.0% (56.0- 94.0) |
73.7% (65.0- 82.4) |
| FOBT and Perianal Examination |
6.10 (2.08- 17.86) |
0.001 | 82.9% (71.4- 94.4) |
56.4% (45.4- 67.4) |
50.0% (38.1- 61.9) |
86.3% (76.9- 95.7) |
Figure 1.
ROC Curve for Perianal/FOBT/CRP/Platelet model (solid line, AUC=0.828), CRP/Platelet Model (long dashed line, AUC=0.760), CRP/ESR/Hematocrit/Platelet/Albumin Model (dotted line, AUC=0.749), and Perianal/FOBT/CRP/ESR/Hematocrit/Platelet/Albumin Model (short dashed line, AUC=0.803).
The validation cohort was comprised of 50 IBD patients and 50 non-IBD patients. The average age of the IBD cohort was 14.6 years old while the average age of the non-IBD cohort was 14.0 years old (p=0.29). The sensitivity and specificity were 18% and 100% for platelet count, 58.0% and 90% for CRP, and 78.0% and 82.0% for FOBT with perianal examination. Logistic regression found that the model that included FOBT with perianal examination, CRP, and platelet count was better at predicting IBD than the model that included only the CRP and platelet count (p<0.001).
DISCUSSION
Complications may result from the delayed diagnosis of IBD in a child. With the increased reliance on laboratory testing, the frequency of performing a complete physical exam has diminished. In our study, 19.5% of IBD patients had normal lab findings at the time of diagnosis, a finding that is consistent with previous studies.8, 9 Our study demonstrates that while FOBT and perianal examination were not universally performed during an evaluation for IBD in children, the data acquired from these evaluations helps to identify children with IBD that otherwise would be overlooked if the screening focused on blood tests alone.
This study demonstrates that the tendency to perform FOBT and perianal examination when IBD was a diagnostic consideration was relatively low but these examinations do improve screening for IBD. Given our data and the ability to identify other relevant physical examination findings (e.g. rectal polyp, firm stool in the rectal vault), a DRE may add further information. The reasons for deferral of FOBT or perianal examination could not be ascertained given the retrospective design of this study. In some patients, FOBT was not performed due to significant perianal disease that alone raised significant concern for IBD. Other patients had concerning symptoms, abnormal laboratory results, grossly abnormal imaging studies, or significant family history of IBD that prompted further endoscopic evaluation regardless of FOBT and perianal findings. In all of these scenarios, DRE was still performed at the time of the ileocolonoscopy to rule-out anal strictures.
One limitation of this study is that the cohort was comprised of patients referred to a tertiary care center for abdominal complaints. The results may not be applicable to every child and adolescent with abdominal pain. The study was not able to capture the reasons why perianal examination or FOBT was not performed in some patients at the time of the initial evaluation. Some patients were noted to have significant perianal disease (but did not have FOBT). The exclusion of such patients from the analysis likely undervalues the role of the perianal exam. Furthermore, there was a trend for the discovery cohort to be older than the validation cohort, but this did not reach statistical significance (data not shown). Finally, we were unable to assess the role that stool inflammatory markers would have played in the screening for IBD. Fecal calprotectin was recently shown to be a cost-effective method for screening for IBD in children.16 Routine availability to fecal calprotectin measurement was not possible during the majority of the testing period.
Increasing reliance on laboratory testing is part of the evaluation for IBD, but our study provides evidence that perianal examination and FOBT continues to provide important information that enhances diagnostic accuracy.
What is currently known?
Pediatric IBD remains elusive to diagnose
Delays in the diagnosis of pediatric IBD is common
Standard screening labs may fail to identify 10-20% of children with IBD
What is new?
Fecal occult blood testing (FOBT) and perianal examination are not always included in IBD screening in children
FOBT and perianal examination improves IBD screening in children
FOBT and perianal exam should be included in the evaluation for pediatric IBD
Acknowledgements
We thank the Massachusetts General Hospital Clinical Research Program for assistance with biostatistical calculations. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and NCATS, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Relevant Funding Sources:
This work was supported in part by philanthropic donations from Martin Schlaff (HSW) and The B. Hasso Family Foundation (May Serrano, President) (HSW).
Footnotes
Conflicts of Interest
None of the authors have conflicts of interest or relevant funding sources with regard to the work contained within this manuscript.
References
- 1.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauer CG, Kugathasan S. Pediatric inflammatory bowel disease: highlighting pediatric differences in IBD. Gastroenterology clinics of North America. 2009;38(4):611–28. doi: 10.1016/j.gtc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Jose FA, Heyman MB. Extraintestinal manifestations of inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2008;46(2):124–33. doi: 10.1097/MPG.0b013e318093f4b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoepfer AM, Dehlavi MA, Fournier N, et al. Diagnostic delay in Crohn's disease is associated with a complicated disease course and increased operation rate. The American journal of gastroenterology. 2013;108(11):1744–53. doi: 10.1038/ajg.2013.248. [DOI] [PubMed] [Google Scholar]
- 5.Vavricka SR, Spigaglia SM, Rogler G, et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(3):496–505. doi: 10.1002/ibd.21719. [DOI] [PubMed] [Google Scholar]
- 6.Sabery N, Bass D. Use of serologic markers as a screening tool in inflammatory bowel disease compared with elevated erythrocyte sedimentation rate and anemia. Pediatrics. 2007;119(1):e193–9. doi: 10.1542/peds.2006-1361. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera-Abreu JC, Davies P, Matek Z, Murphy MS. Performance of blood tests in diagnosis of inflammatory bowel disease in a specialist clinic. Archives of disease in childhood. 2004;89(1):69–71. [PMC free article] [PubMed] [Google Scholar]
- 8.Mack DR, Langton C, Markowitz J, et al. Laboratory values for children with newly diagnosed inflammatory bowel disease. Pediatrics. 2007;119(6):1113–9. doi: 10.1542/peds.2006-1865. [DOI] [PubMed] [Google Scholar]
- 9.Benor S, Russell GH, Silver M, Israel EJ, Yuan Q, Winter HS. Shortcomings of the inflammatory bowel disease Serology 7 panel. Pediatrics. 2010;125(6):1230–6. doi: 10.1542/peds.2009-1936. [DOI] [PubMed] [Google Scholar]
- 10.Tsampalieros A, Griffiths AM, Barrowman N, Mack DR. Use of C-reactive protein in children with newly diagnosed inflammatory bowel disease. The Journal of pediatrics. 2011;159(2):340–2. doi: 10.1016/j.jpeds.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Tilakaratne S, Lemberg DA, Leach ST, Day AS. C-reactive protein and disease activity in children with Crohn's disease. Digestive diseases and sciences. 2010;55(1):131–6. doi: 10.1007/s10620-009-1017-8. [DOI] [PubMed] [Google Scholar]
- 12.Nensi A, Chande N. A survey of digital rectal examination training in Canadian medical schools. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2012;26(7):441–4. doi: 10.1155/2012/681357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholer SJ, Pituch K, Orr DP, Dittus RS. Use of the rectal examination on children with acute abdominal pain. Clinical pediatrics. 1998;37(5):311–6. doi: 10.1177/000992289803700506. [DOI] [PubMed] [Google Scholar]
- 14.Safder S, Rewalt M, Elitsur Y. Digital rectal examination and the primary care physicians: a lost art? Clinical pediatrics. 2006;45(5):411–4. doi: 10.1177/0009922806289615. [DOI] [PubMed] [Google Scholar]
- 15.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflammatory bowel diseases. 2011;17(6):1314–21. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Clark N, Park KT. Effectiveness and cost-effectiveness of measuring fecal calprotectin in diagnosis of inflammatory bowel disease in adults and children. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(2):253–62. e2. doi: 10.1016/j.cgh.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]