Abstract
Objective
To determine the effectiveness and safety of an expanded perioperative venous thromboembolism (VTE) prophylaxis strategy in women undergoing complex gynecologic surgery.
Methods
We performed a cohort study of 527 patients undergoing major surgery at a single institution over a thirty-month interval during which the gynecologic oncology service implemented an expanded approach to VTE prophylaxis. We compared rates of VTE pre- and post-intervention as well as bleeding and infectious complications.
Results
Prior to the intervention, there were 23 VTE events in 345 patients (rate of 6.67%): 8 deep vein thrombosis (DVTs) and 15 pulmonary emboli (PEs). Post intervention, there were 5 VTE events in 182 patients (2.7%): 3 DVTs and 2 PEs (RR=0.4 p=0.056). Time-to-event analysis showed a significantly higher incidence of VTE events in the pre-intervention time frame compared to the post-intervention period (p = 0.049). There were no significant differences in bleeding or infection complications between groups.
Conclusions
Implementation of a perioperative VTE prophylaxis protocol was safe, feasible and resulted in a clinically significant reduction in symptomatic VTE. Preoperative single-dose unfractionated heparin for all patients, combined with two weeks of thromboprophylaxis in gynecologic cancer patients, may decrease VTE events without increasing bleeding or infection.
Keywords: extended prophylaxis, gynecologic surgery, venous thromboembolism, perioperative outcomes
Introduction
Venous thromboembolism (VTE), consisting of deep venous thrombosis (DVT) or pulmonary embolism (PE), remains common and potentially fatal. VTE affects up to 2 million people in the United States, with an annual incidence of 200,000-400,000. It is the proximate cause of death in up to 100,000 each year[1-3]. Gynecologic surgical patients, especially those with malignancy, have an elevated risk: without prophylaxis, rates of VTE are as high as 35%. Even with prophylaxis, clinically significant PEs are found in 5-18% of women undergoing complex pelvic surgery[4-6]. Preoperative lower extremity screening is not useful, in part because many gynecologic oncology patients with postoperative PEs have no evidence of DVT pre- or post-operatively.[7].
Both immediate and extended perioperative pharmacologic prophylaxis with low molecular weight heparin decrease rates of VTE and of hospital readmission in medical and surgical patients with malignancy [8, 9]. The American College of Chest Physicians (ACCP) and the American College of Obstetricians & Gynecologists (ACOG) recommend unfractionated heparin, low molecular weight heparin, or unfractionated or low molecular weight heparin combined with pneumatic sequential compression devices (SCDs) for patients at moderate or high risk who undergo gynecologic surgery, with extended prophylaxis for 2-4 weeks after discharge [10].
Despite ample guidelines, adherence continues to be suboptimal, ranging in published series from 39-59%.[11-13]. While inpatient postoperative VTE prophylaxis has gained acceptance, use of preoperative or extended prophylaxis has been inconsistent and/or infrequent except in certain orthopedic [14] and cancer populations. [15]. Potential concerns with perioperative anticoagulation include perceived risks for wound infection or hematoma, surgical blood loss, and concomitant increased hospital lengths of stay [16].
In 2010, in response to higher-than-expected rates of VTE (as defined by hospital-specific American College of Surgeons National Surgery Quality Improvement Program (NSQIP) data), the Hospital of the University of Pennsylvania launched an initiative to adopt the use of a preoperative dose of thromboprophylaxis consistently. In patients undergoing pancreatic surgery at this institution, VTE incidence decreased from 17.6% to 2.76% following implementation of this protocol [15]. Here, we report how expanded thromboprophylaxis affected VTE incidence among our patients undergoing surgery on the gynecologic oncology service.
Methods
Institutional review board approval was obtained to perform a prospective cohort study of consecutive patients undergoing major abdominal surgery by the gynecologic oncology service over a thirty-month interval. A protocol was developed in 2010 at the Hospital of the University of Pennsylvania to encourage the routine use of preoperative thromboprophylaxis for high-risk patients. This protocol included a mandatory checklist to be completed by the attending physician prior to surgery that included the questions “1) Heparin use prior to induction in operating room? (yes/no), 2) Dosage of subcutaneous unfractionated heparin injection? (5000 Units vs. 7500 Units based on weight), 3) Intermittent sequential compression devices (SCDs) to be applied in operating room? (yes/no).” The nursing preoperative checklist for safety was expanded to include a mandatory review of the form along with administration of prophylaxis as ordered. Current ACCP guidelines were distributed and posted in patient care areas to assist faculty with the determination of the appropriate regimen.
Prior to this intervention, all patients on the gynecologic oncology service (regardless of cancer status) received dual inpatient prophylaxis consisting of perioperative SCDs and subcutaneous heparin three times daily beginning 6 hours after surgery; they received neither the preoperative dose nor the extended thromboprophylaxis after hospital discharge. In February of 2010, the Division of Gynecologic Oncology initiated the above VTE prophylaxis regimen of a dose of pre-operative subcutaneous unfractionated heparin given to all patients undergoing major abdominal surgery on the operating room table prior to the time of anesthesia induction. Patients undergoing exploratory laparotomy received an epidural prior to induction for postoperative pain relief; for these patients, the heparin dose was given 15 minutes later. This pre-operative dose was combined with dual inpatient prophylaxis (consisting of SCDs and subcutaneous heparin). Patients wore SCDs while in bed and initially received subcutaneous unfractionated heparin three times daily. If hemodynamically stable, cancer patients were transitioned to daily dosing of low molecular weight heparin (LMWH) on the evening of postoperative day 1. Patients with malignancy were discharged with up to 14 days of prophylactic dosing of low-molecular weight heparin (Figure 1). Insurance coverage was arranged and patients' acceptance of co-pay for extended prophylaxis was documented by social work services prior to discharge; providers assessed compliance with the regimen at each patient's two-week postoperative visit.
Figure 1. Schematic of intervention strategy.
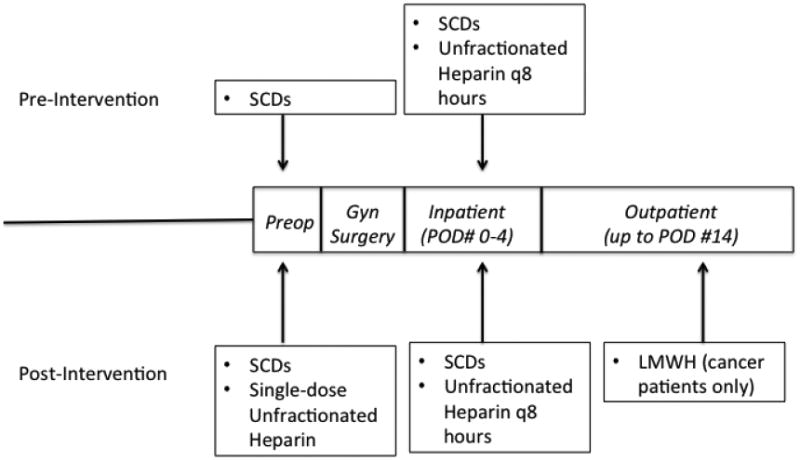
SCDs = sequential compression devices, Preop = Preoperative dose given in operating room prior to anesthesia induction and surgery; POD = postoperative day; LMWH = low molecular weight heparin.
“Complex gynecologic surgery” was defined as all major surgery performed by the Division of Gynecologic Oncology requiring an inpatient stay during the designated time frame. Systematic chart abstraction was performed for all patients who underwent surgery by our gynecologic oncology service from January 2008 though July 2010. Charts were reviewed of patients undergoing surgery within a 24-month period prior to the new VTE prophylaxis regimen change as well as 6 months following the change. All charts were reviewed for follow up for at least one year following surgery. Primary outcomes were: 1) incidence of symptomatic, clinically significant VTE as documented at postoperative clinic visits or readmission before and after the regimen change and 2) post-operative bleeding or infections. The post-operative time period was defined as 90 days.
To aid in clarification of the complexity of surgery, we assigned each individual patient a Caprini Score (CS) using the Caprini Risk Assessment Model (RAM), which has recently been validated for gynecologic oncology surgery[12]. Comorbidities and perioperative risk factors accounted for within this scoring system include age, malignancy, surgery > 45 minutes, family history of VTE, prior personal history of VTE, thrombophilia, chronic pulmonary disease, body mass index (BMI), and history or recent stroke or hip, pelvis or leg fracture. Scores were then calculated into a Caprini risk category as follows: low (0-1 point, with VTE incidence 2%), moderate (2 points, VTE incidence 10-20%), higher (3-4 points, incidence 20-40%), highest risk (≥5 points, incidence 40-80%).
Continuous variables were compared using the student t test or Wilcoxon rank sum tests as appropriate. Categorical variables were compared using the Chi square or Fisher's exact tests. Kaplan-Meier plots were utilized to perform a time-to-event analysis, along with tests for equality by the method of Tarone-Ware. A Cox proportional hazards model was generated to quantify risk before and after implementation. A multivariate analysis was performed to assess risk factors associated with VTE events.
Results
Over a thirty-month interval (24 months prior to the implementation, and 6 months following the change), 273 cancer patients and 254 patients with benign pathology were identified. Patient and pathologic characteristics were not significantly different before and after the intervention (Table 1). Specifically, Caprini risk scores did not differ significantly between groups, with over 93% of each cohort falling into either the high-risk or highest-risk categories.
Table 1.
Patient characteristics.
| Characteristics | Pre-Intervention n=345 | Post-Intervention n=182 | p |
|---|---|---|---|
| Age (mean ±SD) | 55.1 ±13.1 | 54.2 ±12.5 | 0.451 |
| BMI (kg/m2) (mean ±SD) | 29.6 ±8.1 | 29.1 ±8.1 | 0.488 |
| Race (n, %) | 0.301 | ||
| Black | 64 (19) | 39 (21.4) | |
| White | 248 (72) | 130 (71.4) | |
| Asian | 6 (1.8) | 6 (3.4) | |
| Other/Unknown | 25 (7.2) | 7 (3.8) | |
| Surgical Procedure (n, %) | |||
| Laparoscopy | 74 (21.4) | 47 (25.8) | 0.96 |
| Laparotomy | 271 (78.6) | 135 (74.2) | |
| Lymphadenectomy | 112 (32.5) | 67 (36.8) | 0.316 |
| Cytoreduction | 49 (14.2) | 32 (17.6) | 0.31 |
| Caprini Score Category | 0.594 | ||
| Low risk | 0 | 0 | |
| Moderate risk | 19 (5.5) | 11 (6) | |
| High risk | 128 (37.1) | 75 (41.2) | |
| Highest risk | 198 (57.4) | 96 (52.7) | |
| Histology (n, %) | 0.886 | ||
| Benign | 166 (48.1) | 88 (48.4) | |
| Serous | 54 (15.7) | 33 (18.1) | |
| Endometrioid | 54 (15.7) | 33 (18.1) | |
| Squamous | 18 (5.2) | 8 (4.4) | |
| Clear Cell | 14 (4.1) | 5 (2.7) | |
| Mucinous | 9 (2.6) | 3 (1.6) | |
| Sarcoma | 9 (2.6) | 2 (1.1) | |
| MMMT | 8 (2.3) | 3 (1.6) | |
| Other | 13 (3.8) | 7 (3.8) | |
| Tumor Origin (n, %) | 0.981 | ||
| Ovary | 67 (37.4) | 37 (39.4) | |
| Endometrium | 77 (43) | 41 (43.6) | |
| Uterine stroma | 7 (4) | 2 (2.1) | |
| Cervical | 17 (9.5) | 9 (9.6) | |
| Other | 11 (6.1) | 5 (5.3) | |
| Grade | 0.664 | ||
| I-II | 83 (46.4) | 41 (43.6) | |
| III | 96 (53.6) | 53 (56.4) | |
| Stage | 0.735 | ||
| I-II | 99 (55.3) | 54 (57.4) | |
| III-IV | 80 (44.7) | 40 (42.6) |
Tumor origin, grade & stage percentages are reported for patients with malignancy only
Table 2 highlights the significant difference in risk factors for VTE between patients with benign versus malignant disease. The majority of our patients with benign disease (76%) fell into the high-risk category, while the majority of patients with malignancy (96.3%) and a smaller portion of patients with benign pathology (12%) fell into the highest risk category. Regardless of presence or absence of malignancy, there were no significant differences in bleeding or infection complications between groups (Table 3).
Table 2. Comparison of Caprini scores between benign and malignant cases.
| Benign n = 254 | Malignant n = 273 | p value | |
|---|---|---|---|
| Mean Caprini score ± SD (range) | 3.53 ± 1.00 (2-8) | 6.14 ± 1.19 (4-11) | <0.001 |
| Caprini Risk Score Category | <0.001 | ||
| Low risk | 0 | 0 | |
| Moderate risk | 30 (11.8) | 0 | |
| High risk | 193 (76) | 10 (3.7) | |
| Highest risk | 31 (12) | 263 (96.3) |
Low risk = Caprini score (CS) 0-1, Moderate risk = CS 2, High risk = CS 3-4, Highest risk = CS ≥5
Table 3. Comparison of perioperative outcomes pre- and post-implementation of expanded prophylaxis.
| Surgical Outcome | Pre-Intervention n=345 Mean ±SD or n (%) | Post-Intervention n=182 Mean ±SD or n (%) | p |
|---|---|---|---|
| Length of surgery (min) | 143 ± 83 | 148 ± 109 | 0.59 |
| Surgical EBL (mL) | 295 ± 463 | 297 ± 415 | 0.95 |
| Hgb drop (g/dL) | 1.48 ± 8.9 | 1.56 ± 8.9 | 0.92 |
| PRBC transfusion (units) | 0.32 ± 1.4 | 0.35 ± 1.3 | 0.79 |
| Days in Hospital | 4.0 ± 4.1 | 3.9 ± 4.5 | 0.94 |
| Hematoma | 3(0.9) | 3 (1.6) | 0.42 |
| Wound infection | 28 (8.1) | 15 (8.2) | 0.96 |
| Lymphocele | 1 (0.3) | 0 | 0.47 |
| HIT | 1 (0.3) | 0 | 0.47 |
EBL = estimated blood loss; HIT = heparin-induced thrombocytopenia
A post-operative VTE was defined as a VTE occurring within 90 days of surgery. These 527 patients had a total of 28 VTEs (median 25 events/year). VTE events were counted as individual events; no patients were documented as having both a PE and a DVT. Mean follow up time for the pre-intervention group was 145.3 months ± 5.3, while the mean follow up time for the post-intervention group was 27.2 months ± 0.23. Over 95% of each cohort was followed for the full 90 days of interest. Prior to the intervention, there were 23 VTE events in 345 patients (6.67%): 8 DVTs and 15 PEs. Post intervention, there were 5 VTE events in 182 patients (2.7%): 3 DVTs and 2 PEs (p=0.056; relative risk [RR]=0.4; risk reduction of 60%) (Table 4). Prior to the intervention, 19/23 VTE events occurred in cancer patients compared to 5/5 in the post intervention group.
Table 4.
Comparison of VTE event rates before and after implementation of expanded prophylaxis, along with clinical and pathologic characteristics of patients experiencing VTE.
| Pre Intervention n=345 n (%) | Post Intervention n=182 n (%) | p | OR (95% CI) | RR R (%) | |
|---|---|---|---|---|---|
| VTE | 23 (6.7) | 5 (2.7) | 0.056 | 0.40 (0.15-1.1) | 60 |
| DVT | 8 (2.3) | 3 (1.6) | 0.76 | 0.71 (0.19-2.7) | 30 |
| PE | 15 (4.3) | 2 (1.1) | 0.07 | 0.24 (0.06-1.1) | 74 |
| Surgical Procedure | 0.234 | ||||
| Laparoscopy | 3 (0.9) | 0 | |||
| Laparotomy | 20 (5.8) | 5 (2.7) | |||
| CS Category | 0.801 | ||||
| Low/Moderate | 0/19 | 0/11 | |||
| High/Highest | 23 (7) | 5 (2.9) | |||
| Histology | 0.762 | ||||
| Benign | 4 (2.4) | 0 | |||
| Serous | 10 (18.5) | 2 (6) | |||
| Endometrioid | 1 (1.9) | 0 | |||
| Squamous | 1 (5.6) | 0 | |||
| Clear Cell | 3 (21.4) | 2 (40) | |||
| Mucinous | 0 | 0 | |||
| Sarcoma | 0 | 0 | |||
| MMMT | 3 (37.5) | 1 (33.3) | |||
| Other | 1 (7.7) | 0 | |||
| Grade | 0.491 | ||||
| I-II | 2 (2.4) | 0 | |||
| III | 17 (17.7) | 5 (9.4) | |||
| Stage | 0.902 | ||||
| I-II | 5 (5) | 1 (1.85) | |||
| III-IV | 14 (17.5) | 4 (10) |
OR= Odds ratio; RRR= Relative risk reduction; CS = Caprini Score
A total of 28 events occurred in 28 patients. No patients were documented as having both PE and DVT. Percentages shown for pathologic characteristics indicate rates of VTE in each category pre- and post-intervention.
When using a 90-day post-surgical follow up, Kaplan-Meier time-to-event analysis showed a higher incidence of VTE events in the pre-intervention group compared to the post-intervention group (p = 0.049; Figure 2). An interaction term for malignancy was added, with no change seen in the overall result.
Figure 2. Time-to-event analysis, comparing incidence of VTE pre- and post-intervention.
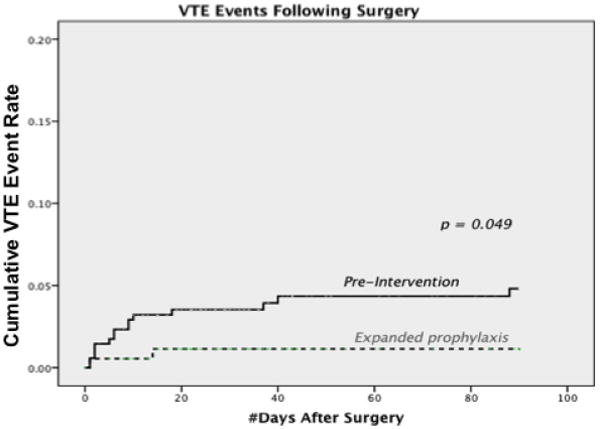
Breakdown of the clinical and pathologic characteristics for patients experiencing VTE events are further provided in Table 4. Specifically for minimally invasive surgery (MIS) procedures, there were 3 VTE events (0.9%) pre-intervention and 0 events post-intervention (p = 0.269). For laparotomy cases, there were 20 events (5.8%) pre-intervention and 5 events (2.7%) post-intervention (p = 0.147). No VTE events occurred in patients in either low or moderate risk CS categories. Similar to the overall group, the VTE event rate was 7% in high and highest risk CS categories pre-intervention, and 2.9% post intervention. 17.7% of patients with grade III disease experienced a VTE event rate prior to the intervention, versus 9.4% post-intervention. With regard to histology, the highest incidence of VTE was seen in patients with serous, clear cell and mixed malignant mullerian tumors (MMMT); event rates for clear cell (21% to 40%) and MMMT (37.5% to 33%) remained steady post-intervention, while the VTE rate for serous fell from 18.5% to 6%.
When controlling for intervention status and stage, a multivariable analysis for VTE showed the odds ratios (and 95% CI) for VTE to be increased with Grade III vs I/II cancer (7.6; 1.6-36); age per year (1.04; 1.0-1.08); and BMI per kg/m2 (1.06; 1.01-1.11)
Discussion
Our data support the use of expanded thromboprophylaxis for gynecologic surgery, and concur with a growing body of literature advocating for this [7, 12, 17, 18]. With a straightforward uniform practice change for women undergoing complex gynecologic surgery, we demonstrate safety and effectiveness of a single preoperative dose of subcutaneous heparin (given at the time of anesthesia induction) combined with inpatient dual prophylaxis and extended prophylaxis for cancer patients. We found a clinically significant decrease in VTE rate from 6.7% to 2.7%. There was no significant difference in bleeding or infection complications.
Strengths of our study include a long follow up period, allowing for a time-to-event analysis, helping to account for the difference in our n pre- and post-intervention and showing a decrease in symptomatic VTE events after implementation of enhanced perioperative prophylaxis. We report detailed information regarding the clinical and pathologic characteristics for those patients experiencing VTE that despite lack of power for this study could be used in future meta-analyses. Furthermore, as 48% of the patients in our population had benign disease, our findings support the safety of pre-operative thromboprophylaxis in gynecologic patients with benign disease undergoing open pelvic surgery. While “complex gynecologic surgery” is a subjective term, we have provided Caprini scores to aid the readers in comparing our population to their own. The majority of our benign patients (76%) fell into the high-risk category, while the majority of malignant patients (96.3%) and a smaller portion of the benign patients (12%) fell into the highest risk category. From our data, we would suggest that enhanced prophylaxis, i.e., a preoperative dose of pharmacologic prophylaxis followed by dual prophylaxis with SCDs and pharmacologic prophylaxis while hospitalized, be given to all gynecologic patients who fall into the high-risk or highest risk Caprini categories, regardless of the performing surgeon's subspecialty.
Optimal timing for preoperative dosing of pharmacologic prophylaxis remains unclear. Meta-analyses of preoperative heparin dosing have not been powered to make conclusions regarding initial timing of VTE prophylaxis [7, 19]. Common practices have included initiation 12 hours pre-operatively, 1-2 hours preoperatively or 6-12 hours post-operatively. Clarke-Pearson et al performed the largest randomized three-arm study in gynecologic patients, comparing subcutaneous heparin given 2 or 8 hours prior to surgery versus no prophylaxis. The arms with preoperative regimens had decreased rates of VTE compared to no prophylaxis and had no increase in bleeding [20]. More recently, in a retrospective study of 239 gynecologic patients undergoing laparotomy, Whitworth et al found a risk reduction in clinical DVTs from 8% to 1.9% with the addition of enoxaparin 40 mg given subcutaneously 2 hours prior to surgery[17].
We found that administering unfractionated heparin at the time of anesthesia induction, or 15 minutes thereafter in the setting of epidural placement, allowed for convenience and ensured documentation and awareness of administration by all surgical personnel. In our experience, heparin administration did not interfere with preoperative epidural placement. We do acknowledge a recent study by Courtney-Brooks et al [21] where the implementation of more consistent epidural placement improved pain scores but resulted in a higher rate of VTE at their institution. It is possible that concern for complications with a newer epidural policy may have decreased compliance with previously established VTE prophylaxis. While we did not specifically collect epidural placement data, this practice has been standard for several years on our service for patients undergoing planned exploratory laparotomy. As there were no practice changes in frequency of epidural placement at any time point during the study, the lack of documentation of epidural placement should not confound our analysis.
Once hospitalization is complete, optimal duration of extended VTE prophylaxis for patients undergoing gynecologic cancer surgery remains unclear (21 22). Prior studies have demonstrated safety and efficacy of extended thromboprophylaxis. Both the PREVENT [14] and MEDENOX trials[9] demonstrated lower VTE rates with minimal to no bleeding side effects after 14 days of LMWH in nonsurgical medically ill patients. The ENOXACAN II trial of 332 patients undergoing open abdominal or pelvic cancer surgery found that incidence of VTE decreases from 12% to 4.8% with 3 weeks of LMWH prophylaxis compared to inpatient prophylaxis alone [8]. A Cochrane Collaboration meta-analysis of patients undergoing open abdominal surgery for malignancy, which included women with gynecological cancers, demonstrated a VTE incidence of 14.3% of those treated with inpatient prophylaxis alone vs. 6.1% of those treated for 28 days with low molecular weight heparin [22]. Our selected duration of 14 days was within current guidelines at the time [23], but 28 days of extended thromboprophylaxis (as recommended in the 2012 ACCP guidelines) may be a more effective course for a gynecologic oncology surgery population [1]. We chose a 90-day surgical follow-up for our time-to-event analysis as recent VTE data for gynecologic cancers suggest that 75% are occurring greater than 7 days from initial surgery; up to 40% of VTEs occur 21-90 days from surgery [24, 25]. Development of risk prediction models incorporating tumor type, stage, grade and histology of gynecologic cancers may help decrease uncertainty of duration for extended prophylaxis for these women. Our study was not powered to look at these differences, but we do note a trend in decreased VTE events in patients with Stage III-IV disease and high-grade histology, specifically serous.
Our study provides motivation for development and implementation of VTE risk stratification models in a gynecologic surgery population. Risk stratification scores, such as the Caprini RAM [26], are becoming increasingly utilized in national surgical improvement efforts. Incorporating VTE stratification into computer-alert programs increases use of thromboprophylaxis and decreases VTE [27] and has been shown to decrease incidence of VTE[28]. While our initial effort at University of Pennsylvania was done with paper charting on the day of surgery, the system has now evolved to incorporate these orders into the preoperative EMR documentation, thereby preventing unnecessary delays on the day of surgery and allowing for careful and timely consideration of preoperative VTE risks. As the Caprini score has now been validated in a gynecologic surgery population [12], further studies should focus on implementation strategies to encourage guideline adherence.
We acknowledge several limitations of this non-randomized study. We focused on clinically significant VTE events only, which may have underestimated the true event rate. Our study was originally designed to establish safety of our enhanced protocol with regard to bleeding and infection risks, and was unfortunately not powered on VTE events within the 6-month time frame post-intervention. To improve power, utilization of lower extremity venous Doppler screening in our postoperative population could have been employed. Furthermore, while we identified eligible patients in a prospective manner, ascertainment of VTE events was done by chart review. Especially for patients with benign disease who may not have had reason to stay within our hospital system, we cannot exclude the possibility that patients presented elsewhere with VTE events or other postoperative complications. Compliance with in-hospital dual prophylaxis strategies was confirmed based on computerized hospital records, but post-discharge compliance was primarily assumed based on documentation from social work services and the first postoperative visit, leaving room for error. Despite these weaknesses, we do feel our study population and follow-up data are representative of a “real world” gynecologic oncology patient population. Finally, while there was an uneven distribution of patients pre- and post-intervention, with a larger number of patients studied before versus after the intervention, our time-to-event analysis helps overcome this, showing a significant decrease in VTE event rate within 90 days of surgery following the implementation.
Only 23% of our cohort underwent minimally invasive surgery (MIS). While there were no significant complications noted in this subpopulation due to implementation of expanded thromboprophylaxis, the benefits of this strategy in MIS may be modest. Recent studies have questioned the need for any prophylaxis—either inpatient or extended regimens—given the lower risks of VTE that early ambulation and decreased hospitalization afford to these women compared to those undergoing laparotomy[29, 30]. Further stratification of VTE risk with MIS is needed.
Finally, our study was not designed to evaluate costs associated with our implementation, but some patients required consultation with our inpatient social workers. While cost has been a perceived barrier to extended prophylaxis, recent studies lend support to the cost-effectiveness of this strategy [31, 32], especially when considering costs of cancer-related VTE [33, 34]. The MAGELLAN trial evaluating thromboprophylaxis in acutely ill medical patients showed the novel oral factor Xa inhibitor rivaroxaban to be noninferior to enoxaparin at day 10, but superior to placebo after day 35 [35]. Future studies of new oral agents could help address perceived cost barriers, as well as examine patient compliance and risk of bleeding in a postoperative setting.
Perioperative prophylaxis strategies remain difficult to study, given the overall rarity of VTE events when all patients include post-operative thromboprophylaxis. VTE outcome measures are being incorporated into many quality improvement programs and public reporting initiatives, including the Centers for Medicare & Medicaid Services 2015 Value-based Purchasing program [36-38]. The Agency for Healthcare Research and Quality has designated a risk-adjusted postoperative VTE rate measure, Patient Safety Indicator (PSI-12). In this context, continued efforts to risk stratify and prophylax gynecologic surgery populations are warranted. In conclusion, this study demonstrates that expanded thromboprophylaxis (preoperative subcutaneous heparin given at the time of anesthesia induction and 14 days of low-molecular-weight heparin after discharge for patients with malignancy), is effective and safe in a complex gynecologic surgery population.
Highlights.
We examine VTE rates before and after a uniform change in practice.
Expanded prophylaxis resulted in a decreased VTE rate (6.7% to 2.7%).
There was no significant difference in bleeding or infection complications.
Acknowledgments
This publication was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and U54 HL112303 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Presented at the 19th Annual SGO Winter Meeting, Breckenridge, CO, February 2014.
Conflict of Interest Statement: Dr. Gage reports consultation for Boehringer Ingelheim in 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gould MK, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–77S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman MG, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 Suppl):S495–501. doi: 10.1016/j.amepre.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Pannucci CJ, et al. A validated risk model to predict 90-day VTE events in postsurgical patients. Chest. 2014;145(3):567–73. doi: 10.1378/chest.13-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol. 2007;105(3):813–9. doi: 10.1016/j.ygyno.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez AO, et al. Venous thromboembolism in ovarian cancer. Gynecol Oncol. 2007;105(3):784–90. doi: 10.1016/j.ygyno.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Tateo S, et al. Ovarian cancer and venous thromboembolic risk. Gynecol Oncol. 2005;99(1):119–25. doi: 10.1016/j.ygyno.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Clarke-Pearson DL, Abaid LN. Prevention of venous thromboembolic events after gynecologic surgery. Obstet Gynecol. 2012;119(1):155–67. doi: 10.1097/AOG.0b013e31823d389e. [DOI] [PubMed] [Google Scholar]
- 8.Bergqvist D, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346(13):975–80. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 9.Samama MM, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Practice Bulletins--Gynecology, A.C.o.O. and Gynecologists. ACOG Practice Bulletin No. 84: Prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol. 2007;110(2 Pt 1):429–40. doi: 10.1097/01.AOG.0000263919.23437.15. [DOI] [PubMed] [Google Scholar]
- 11.Schleyer AM, et al. Adherence to guideline-directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2011;26(3):174–80. doi: 10.1177/1062860610382289. [DOI] [PubMed] [Google Scholar]
- 12.Stroud W, et al. Validation of a venous thromboembolism risk assessment model in gynecologic oncology. Gynecol Oncol. 2014;134(1):160–3. doi: 10.1016/j.ygyno.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 13.Cohen AT, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371(9610):387–94. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 14.Prandoni P, et al. Prolonged thromboprophylaxis with oral anticoagulants after total hip arthroplasty: a prospective controlled randomized study. Arch Intern Med. 2002;162(17):1966–71. doi: 10.1001/archinte.162.17.1966. [DOI] [PubMed] [Google Scholar]
- 15.Reinke CE, et al. Timing of preoperative pharmacoprophylaxis for pancreatic surgery patients: a venous thromboembolism reduction initiative. Ann Surg Oncol. 2012;19(1):19–25. doi: 10.1245/s10434-011-1858-1. [DOI] [PubMed] [Google Scholar]
- 16.Jensen CD, et al. Return to theatre following total hip and knee replacement, before and after the introduction of rivaroxaban: a retrospective cohort study. J Bone Joint Surg Br. 2011;93(1):91–5. doi: 10.1302/0301-620X.93B1.24987. [DOI] [PubMed] [Google Scholar]
- 17.Whitworth JM, et al. Double prophylaxis for deep venous thrombosis in patients with gynecologic oncology who are undergoing laparotomy: does preoperative anticoagulation matter? Int J Gynecol Cancer. 2011;21(6):1131–4. doi: 10.1097/IGC.0b013e31821dc9f0. [DOI] [PubMed] [Google Scholar]
- 18.Einstein MH, et al. A protocol of dual prophylaxis for venous thromboembolism prevention in gynecologic cancer patients. Obstet Gynecol. 2008;112(5):1091–7. doi: 10.1097/AOG.0b013e31818b1486. [DOI] [PubMed] [Google Scholar]
- 19.Rahn DD, et al. Venous thromboembolism prophylaxis in gynecologic surgery: a systematic review. Obstet Gynecol. 2011;118(5):1111–25. doi: 10.1097/AOG.0b013e318232a394. [DOI] [PubMed] [Google Scholar]
- 20.Clarke-Pearson DL, et al. A controlled trial of two low-dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol. 1990;75(4):684–9. [PubMed] [Google Scholar]
- 21.Courtney-Brooks M, et al. Continuous epidural infusion anesthesia and analgesia in gynecologic oncology patients: less pain, more gain? Gynecol Oncol. 2015;136(1):77–81. doi: 10.1016/j.ygyno.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009;(1):CD004318. doi: 10.1002/14651858.CD004318.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Geerts WH, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 24.Peedicayil A, et al. Incidence and timing of venous thromboembolism after surgery for gynecological cancer. Gynecol Oncol. 2011;121(1):64–9. doi: 10.1016/j.ygyno.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 25.Agnelli G, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243(1):89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motykie GD, et al. Risk factor assessment in the management of patients with suspected deep venous thrombosis. Int Angiol. 2000;19(1):47–51. [PubMed] [Google Scholar]
- 27.Kucher N, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–77. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 28.Cassidy MR, Rosenkranz P, McAneny D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization program. J Am Coll Surg. 2014;218(6):1095–104. doi: 10.1016/j.jamcollsurg.2013.12.061. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, et al. Risk of postoperative venous thromboembolism after minimally invasive surgery for endometrial and cervical cancer is low: a multi-institutional study. Gynecol Oncol. 2013;130(1):207–12. doi: 10.1016/j.ygyno.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Bouchard-Fortier G, et al. Is venous thromboprophylaxis necessary in patients undergoing minimally invasive surgery for a gynecologic malignancy? Gynecol Oncol. 2014;134(2):228–32. doi: 10.1016/j.ygyno.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Teoh D, et al. Cost comparison of strategies for the management of venous thromboembolic event risk following laparotomy for ovarian cancer. Gynecol Oncol. 2011;122(3):467–72. doi: 10.1016/j.ygyno.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Cain K, et al. Patient cost associated with filling a prescription for extended-duration venous thromboembolism (VTE) prophylaxis following surgery for gynecologic cancer. Gynecol Oncol. 2012;127(1):18–21. doi: 10.1016/j.ygyno.2012.07.096. [DOI] [PubMed] [Google Scholar]
- 33.Elting LS, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164(15):1653–61. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 34.Kourlaba G, et al. The humanistic and economic burden of venous thromboembolism in cancer patients: a systematic review. Blood Coagul Fibrinolysis. 2014 doi: 10.1097/MBC.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 35.Cohen AT, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513–23. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Medicare & Medicaid Services. Hospital value-based purchasing. [Accessed May 1st, 2014]; Available from: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/hospital-value-based-purchasing/
- 37.Agency for Healthcare Research and Quality. Postoperative pulmonary embolism or deep vein thrombosis rate. [Accessed May 1st, 2014]; Available from: http://www.qualityindicators.ahrq.gov/Downloads/Modules/PSI/V44/TechSpecs/PSI12PostoperativePEorDVTRate.pdf.
- 38.Bilimoria KY, et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310(14):1482–9. doi: 10.1001/jama.2013.280048. [DOI] [PubMed] [Google Scholar]


