Abstract
Background
A diverse body of evidence suggests that lycopene might inhibit prostate cancer development. We conducted a 6-month repeat biopsy randomized trial among men with HGPIN. Here we report results for serum lycopene, PSA and IGF proteins, histopathological review, and tissue markers for proliferation (MCM-2) and cell cycle inhibition (p27).
Methods
Participants consumed placebo or tomato extract capsules containing 30 mg/day lycopene. Pre- and post-treatment biopsies were immunostained and digitally scored. Serum lycopene was determined by LC-MS-MS. In secondary analyses, pathologists blindly reviewed each biopsy to score histological features.
Results
58 men completed the trial. Serum lycopene increased 0.55 μmol/L with treatment and declined 0.29 μmol/L with placebo. We observed no meaningful differences in PSA, IGF-1 or IGFBP3 concentrations between groups, nor any differences in expression of MCM-2 or p27 in epithelial nuclei. Prevalences of cancer, HGPIN, atrophy or inflammation post-treatment were similar; however, more extensive atrophy and less extensive HGPIN was more common in the lycopene group.
Conclusions
Despite large differences in serum lycopene following intervention, no treatment effects were apparent on either the serum or benign tissue endpoints. Larger studies are warranted to determine whether changes observed in extent of HGPIN and focal atrophy can be replicated.
Keywords: prostate cancer, lycopene, HGPIN, clinical trial
BACKGROUND
A diverse and sizable, although not entirely consistent, body of evidence from observational epidemiology, animal models and cell culture experiments suggests that tomato foods or other sources of lycopene could inhibit the development and/or progression of prostate cancer. A comprehensive review by the World Cancer Research Fund in 2007 concluded that foods containing lycopene probably protect against prostate cancer, and estimated, based on a meta-analysis of data from prospective studies, that there is a 4% decrease in prostate cancer risk for every 10 μg/L increase in serum or plasma lycopene.(1) However, some more recent epidemiological studies have not observed a protective association, which might be attributable to shifts towards detection of more indolent cancers during the PSA screening era.(2-4) Meanwhile, clinical trial data remain relatively sparse, especially in relation to the effects of lycopene or tomato foods on histologically benign prostate tissue that could harbor field effects due to the presence of neoplasia in the gland. Lycopene, an acyclic carotenoid that is not a vitamin A precursor, displays potent antioxidant activity in vitro and is found in relatively high concentrations in the prostate.(5) It is the most abundant carotenoid in serum surveys of the U.S. population, largely due to consumption of tomato foods such as pizza, salsa and ketchup. Preclinical studies have indicated that lycopene, apart from its antioxidant properties, could exert anti-carcinogenic effects through mechanisms involving suppression of androgen or IGF-1 signaling pathways, with resultant effects on cell proliferation and growth. (6-10)
We conducted a Phase II repeat biopsy trial in men with high grade prostatic intraepithelial neoplasia (HGPIN) who were randomly assigned to consume either a placebo or capsules of Lyco-Mato®, a tomato oleoresin delivering 30 mg per day of lycopene for a period of approximately six months. We obtained blood samples and paraffin blocks containing benign prostate tissue from needle biopsies performed before and after treatment, enabling each participant to serve as his own control. In this report we present the results for treatment-associated changes in several endpoints, including immunohistochemical analysis of epithelial cell proliferation and cell cycle inhibition, as well as serum PSA, IGF-1 and IGFBP3. Histopathology outcomes were evaluated as secondary endpoints due to the relatively small size of the study.
METHODS
Trial design, selection of participants, and specimen collection
This was a Phase II randomized, double-blind, placebo-controlled trial. Patients were enrolled at Northwestern Memorial Hospital and the Jesse Brown Veterans Administration Medical Center in Chicago. The study protocol was reviewed and approved by the Institutional Review Boards at Northwestern University and the University of Illinois at Chicago. Eligible participants were men with a confirmed diagnosis of isolated HGPIN (no prostate cancer or ASAP), no use of lycopene, other antioxidant supplements or agents affecting hormone metabolism within one month of randomization, no prior non-skin cancer or else remission for at least 5 years, dietary fat intake between 23-48% of total calories, and AUA urinary function symptom score less than 26. Potential participants were pre-screened by telephone using the Block Dietary Fat screening questionnaire (NutritionQuest, Berkeley, CA) to ascertain whether fat intake would be adequate to allow optimal absorption of carotenoids. The attending urologists planned to perform a repeat biopsy in approximately six months. After providing informed consent, trial participants completed a demographic/medical questionnaire, a 110-item Block Food Frequency Questionnaire (NutritionQuest, Berkeley, CA), and a supplemental diet questionnaire to obtain more detail regarding carotenoid intake. Potential participants with lycopene intakes greater than 15 mg per day received brief dietary counseling to reduce intake prior to starting the trial; all participants agreed to limit their intake of lycopene-containing foods while on study.
Participants were randomly assigned to a treatment group and a non-fasting venous blood sample was obtained. A single data manager and the hospital pharmacists were the only individuals who were aware of each participant’s assigned treatment; the participants themselves and all other study staff were blinded. Blood was processed into serum and plasma aliquots of 0.5 ml and cryovials were stored in nitrogen vapor tanks at −140°C. Paraffin blocks from the pre- and post-treatment biopsies were retrieved from the relevant pathology archives. All biopsies were performed under ultrasound guidance with an 18-gauge needle; 8-12 needle cores were obtained during each biopsy session. The initial protocol called for collection of expressed prostatic fluid prior to the post-treatment biopsy; however, this procedure was discontinued due to poor acceptability from the urologists and patient participants.
Intervention characteristics
Participants in the lycopene group consumed two daily capsules of Lyc-O-Mato®, a tomato oleoresin containing 15 mg of lycopene per soft gel capsule (Lycored, Ltd, Beer Sheva, Israel). The men were instructed to take one capsule with breakfast and one with dinner, and were informed that absorption is improved when capsules are taken with dietary fat. In addition to lycopene, each capsule contains 1.4 mg phytoene, 1.1 mg phytofluene, 0.7 mg β-carotene and 4 mg α-tocopherol. Placebo soft gel capsules contained medium-chain triglycerides and red food coloring. Study staff contacted participants by telephone at 1, 3 and 5 months during the treatment period to evaluate compliance and respond to questions. Samples of active and placebo capsules were assayed twice during the trial to ensure that the lycopene concentrations remained stable.
Laboratory methods
For all serum assays, pre- and post-treatment samples from the same subject were included in the same batch. In every batch, at least two samples, labeled anonymously, contained aliquots from a pool of quality control serum. Serum lycopene concentrations were measured in duplicate by LC-MS-MS as described previously.(11) Intra-assay and inter-assay CVs were 7.8% and 12.6%, respectively. PSA was measured using an ultrasensitive automated immunoenzymometric assay (Tosoh Bioscience, King of Prussia, PA) with Tandem® Hybritech monoclonal antibodies. Intra-assay and inter-assay CVs were 2.8% and 5.2%. Serum IGF-1 and IGFBP3 were measured by enzyme immunoassay using commercial kits from Diagnostic Systems Laboratories (Webster, TX). Intra-assay and inter-assay CVs for IGF-1 were 8.1% and 11.2%, and 4.6% and 9.3% for IGFBP3. Serum samples were either unavailable or unusable for 1 and 2 subjects in the lycopene and placebo groups, respectively, for serum lycopene and PSA assays. An additional subject in the placebo group had an unusable specimen for serum IGF-1 and IGFBP3.
Immunohistochemistry and digital image analysis
Four micron-thick sections were cut from paraffin blocks and mounted on sialinized glass slides. Slides not stained immediately were stored at 4°C in vacuum-sealed plastic bags containing oxygen-absorbing tablets. Following deparaffinization and antigen retrieval using Universal Decloaker solution (Biocare Medical, Concord, CA) in a steamer at 95° C, slides were treated with a peroxidase blocking solution and Background Sniper (Biocare), a casein-based universal blocking reagent. For MCM-2, we used a rabbit monoclonal antibody (clone D7G11) at a 1:2000 dilution from Cell Signaling Technology (Danvers, MA). For p27, we used a rabbit monoclonal antibody (clone Y236) from Epitomics (Burlingame, CA) at a dilution of 1:1200. Slides were incubated with Mach 3® anti-rabbit secondary probes (Biocare) and Mach 3® HRP detection polymer followed by 5 minutes exposure to DAB chromagen and one minute exposure to 50% hematoxylin. Additional sections were stained with hematoxylin and eosin for standard histopathological analysis. Two experienced pathologists, blinded to treatment assignment, reviewed each H&E slide and classified them by consensus for the presence and extent of prostate cancer, HGPIN, focal atrophy (including proliferative inflammatory atrophy, PIA), and inflammation. Focal atrophy was classified using consensus criteria published by DeMarzo, et al.(12) Inflammation was classified on a 4-point ordinal scale.(13)
All slides were scanned at 400x on an Aperio Scanscope CS® whole slide digital microscopy system (Leica Biosystems, Buffalo Grove, IL). Images were stored on a dedicated server running Spectrum® software for image management and ImageScope® for slide viewing. A machine learning program (Genie®, Aperio) was used to separately map epithelial and stromal areas on the biopsy slides. The Nuclear Image Analysis Program® (Aperio) was used to quantify the percentage of epithelial nuclei staining positive at each of four pre-determined ordinal staining intensity levels. Results were expressed as the percent of positive-staining epithelial cells and as the H-score, an index ranging from 0-3 based on weights equal to the proportion of nuclei at each intensity level. Experience in our lab supports a high level of agreement between manual scoring by pathologists and the automated approach to nuclear marker scoring used in this study.
Data analysis
With 30 participants randomized per group, the trial was powered (at power = 80%) to detect approximately a 30% difference at significance level of 0.05 for the primary endpoints, expression of MCM-2 and p27. We first examined all key variables to detect outliers and assess whether variables were normally distributed. Natural log transformations were used to support parametric analysis for PSA and IGFBP3; anti-logged geometric means are presented in the Results section. For continuous variables, we used paired T tests to compare treatment groups according to intention-to-treat principles; for trial outcomes, comparisons were based on the mean difference between the pre- and post-treatment values. Linear regression models were used to determine whether confounding existed due to treatment group differences in baseline age, BMI, PSA or gland volume. None of these covariates altered the estimated treatment effects and thus unadjusted results are presented. We performed two analyses to account for non-compliance: first, we excluded participants who failed to consume at least 75% of their assigned capsules, and second, we ignored treatment group and calculated the Spearman and Pearson correlation coefficients for the change in serum lycopene versus the key outcome variables. SAS-PC version 9 (SAS, Cary, NC) was used for all analyses.
RESULTS
Selected characteristics of the participants in each randomized group are shown in Table 1. A total of 58 patients completed the trial. Placebo group participants were slightly older, but all other baseline characteristics were similar. Dietary intake of lycopene and pro-vitamin A carotenoids was moderate in both groups and presumably declined further before starting the trial since all participants were counseled to reduce intake at that point. Compliance with assigned treatment was excellent. Average gland volume rose slightly to an equal degree in both groups.
Table 1.
Selected characteristics of participants in the HGPIN tomato extract trial, by treatment assignment
| Lycopene n |
Placebo n |
P Value | |
|---|---|---|---|
| Pre- and post-treatment biopsy | 26 | 32 | - |
| Pre- and post-treatment blood obtained | 25 | 31 | - |
| Race | |||
| White | 19 | 23 | 0.55 |
| African-American | 6 | 9 | |
| Other | 1 | 0 | |
| Family history of PCa (1st degree) | 1 | 1 | - |
| Suspicious DRE (before baseline biopsy) | 4 | 5 | 0.98 |
|
| |||
| mean (sd) | mean (sd) | ||
|
| |||
| Age, baseline, years | 62.9 (8.3) | 67.1 (8.1) | 0.06 |
| Clinical PSA before enrollment, ng/ml | 4.89 (2.51) | 4.72 (2.66) | 0.81 |
| Research PSA at baseline, ng/ml | 5.50 (3.73) | 6.18 (4.57) | 0.55 |
| Time on treatment, days | 175.6 (31.8) | 200.2 (70.2) | 0.10 |
| Pre-treatment gland volume, cc+ | 45.3 (20.3) | 53.9 (30.4) | 0.26 |
| Post-treatment gland volume | 46.7 (20.6) | 51.9 (29.5) | 0.54 |
| Serum lycopene, baseline, μmol/L | 0.94 (0.50) | 0.95 (0.50) | 0.96 |
| Lycopene intake, baseline, mg/day | 3.98 (3.29) | 3.42 (3.36) | 0.54 |
| Tomato food intake, baseline, servings/week | 7.95 (7.23) | 6.44 (4.62) | 0.34 |
| Pro-vitamin A intake, baseline, mg/day | 4.36 (3.44) | 5.81 (10.0) | 0.49 |
| Total dietary fat intake, baseline, g/day | 67.6 (59.4) | 60.9 (33.8) | 0.61 |
| Capsules consumed* | n (%) | n (%) | |
| > 75% | 24 (92) | 28 (88) | 0.68 |
| 50-75% | 2 (8) | 4 (12) | |
| < 50% | 0 | 0 | |
By TRUS, unless unavailable, then by DRE
By pill count, unless unavailable, then by self-report.
Table 2 shows the results for histological diagnoses that were determined by study pathologists. We emphasize that these were not primary endpoints for this Phase II trial. Prostate cancer was detected in four men from each group. As expected, the prevalence of HGPIN upon repeat biopsy declined in both groups. The prevalence of HGPIN after treatment was slightly lower in the lycopene group (42% vs. 56%), but was well within bounds expected due to chance. There was a small, non-significant decrease in the proportion of participants in the lycopene group with HGPIN in multiple cores, and a 24% greater decrease (62% versus 38%) among men assigned to lycopene in the mean number of biopsy cores with HGPIN. No differences between treatment groups were observed for overall prevalence of PIA, all focal atrophy or inflammation, although in the lycopene group there was an increase in the number of men with extensive focal atrophy (≥ 5 cores, P = 0.05).
Table 2.
Pre- and post-treatment histopathology results: randomized, placebo-controlled trial of lycopene supplement in men with HGPIN
| Lycopene (n = 26) | Placebo (n = 32) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pre-Rx | Post-Rx | P * | Pre-Rx | Post-Rx | P | |
| Prostate cancer, n (%) | 0 | 4 (15) | 0.11 | 0 | 4 (13) | 0.11 |
| HGPIN, n (%) | ||||||
| Present, any no. cores | 26 (100) | 11 (42) | < 0.01 | 32 (100) | 18 (56) | < 0.01 |
| 1 core positive | 16 (62) | 7 (27) | 0.03 | 21 (66) | 11 (34) | 0.02 |
| ≥ 2 cores positive | 10 (38) | 4 (16) | 0.12 | 11 (34) | 7 (22) | 0.40 |
| Mean no. HGPIN cores | 1.73 | 0.65 | <0.001 | 1.41 | 0.88 | 0.005 |
|
Proliferative inflammatory atrophy, n (%) |
6 (23) | 5 (19) | 0.99 | 10 (31) | 11 (34) | 0.99 |
| Focal atrophy, n (%) | ||||||
| Present, any no. cores | 16 (62) | 17 (65) | 0.99 | 22 (69) | 20 (63) | 0.79 |
| 1-2 cores | 7 (27) | 6 (23) | 0.99 | 10 (31) | 8 (25) | 0.78 |
| 3-4 cores | 8 (31) | 4 (15) | 0.32 | 6 (19) | 4 (13) | 0.73 |
| ≥ 5 cores | 1 (4) | 7 (27) | 0.05 | 6 (19) | 8 (25) | 0.77 |
| Mean no. cores | 1.73 | 2.81 | 0.05 | 2.66 | 2.34 | 0.58 |
| Inflammation, n (%) | ||||||
| Present, any no. cores | 20 (77) | 16 (62) | 0.37 | 18 (56) | 21 (66) | 0.61 |
| 1-2 cores | 13 (50) | 8 (31) | 0.26 | 13 (41) | 13 (41) | 0.99 |
| 3-4 cores | 6 (23) | 6 (23) | 0.99 | 3 (9) | 6 (19) | 0.47 |
| ≥ 5 cores | 1 (4) | 2 (8) | 0.99 | 2 (6) | 2 (6) | 0.99 |
| Mean no. cores | 1.65 | 1.73 | 0.88 | 1.25 | 1.66 | 0.30 |
P values are Fisher exact (2-tailed) for comparison of proportions and paired T test (2-tailed) for comparison of means.
The waterfall plots in Figure 1 show that serum lycopene concentrations increased in 21 of 25 men assigned to tomato oleoresin with a mean increase of 77%, whereas serum lycopene decreased in 20 of 30 men in the placebo group by an average of 19% (P < 0.001). At the exit biopsy, serum lycopene levels on average were 2.16 times higher in the lycopene compared to the placebo group. Results for the changes in serum PSA are shown in Figure 2. In the lycopene group, the median change in PSA was a 0.08 ng/ml increase, while in the placebo group the median change was a 0.18 ng/ml decrease (non-parametric P = 0.46). When men with prostate cancer detected at exit biopsy were excluded from each group, the changes in PSA were still essentially equivalent between groups (P = 0.95).
Figure 1.
Change in serum lycopene concentrations in the Lycopene (n = 25) and Placebo (n = 30) groups from baseline to 6-month follow-up.
Figure 2.
Change in serum PSA concentrations in the Lycopene (n = 25) and Placebo (n = 30) groups from baseline to 6-month follow-up.
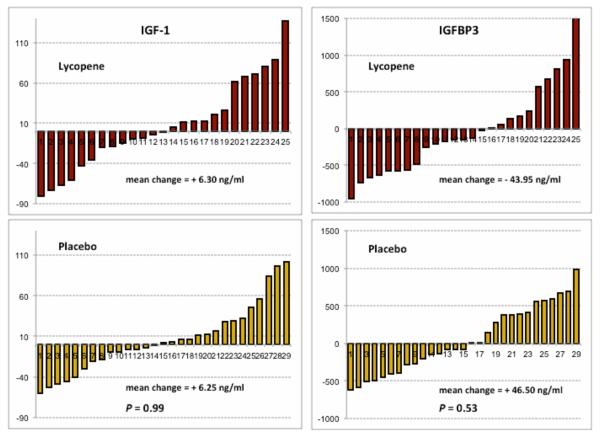
The pre- to post-treatment changes in serum IGF-1 and IGFBP3 are shown in Figure 3. The changes in IGF-1 were virtually identical between groups (P = 0.99). There were also no differences in the change in serum IGFBP3 (P = 0.53) and the ratio IGF-1:BP3 (P = 0.68).
Figure 3.
Changes in serum IGF-1 and IGFBP3 concentrations in the Lycopene (n = 25) and Placebo (n = 29) groups from baseline to 6-month follow-up.
Table 3 compares the treatment groups on changes in expression of MCM-2 and p27 in the benign prostate tissue. There were no differences between groups in either the change in percent of positive nuclei or the H-score index that combines percent positive and expression intensity. As expected, both biomarkers showed predominantly nuclear expression. Also as expected, MCM-2 expression was greater in the basal as compared to the luminal epithelial cells, while the opposite was true for p27.
Table 3.
Changes in expression of proliferation (MCM-2) and cell cycle inhibition (p27) biomarkers in benign prostate tissue following random assignment to lycopene-rich tomato extract or placebo capsules
| Lycopene |
Placebo |
|||||
|---|---|---|---|---|---|---|
| Biomarker | Pre-Rx | Post-Rx | Mean Δ (95% CI) |
Pre-Rx | Post-Rx | Mean Δ (95% CI) |
| MCM-2, % positive epithelial cells |
7.58 | 8.24 | 0.66 (−1.08-2.40) |
8.45 | 8.74 | 0.29 (−1.51 -2.08) |
| MCM-2, H-Score (% positive × intensity) |
0.12 | 0.13 | 0.01 (−0.02-0.04) |
0.13 | 0.14 | 0.01 (−0.02-0.04) |
| p27, % positive epithelial nuclei |
52.96 | 52.01 | −0.95 (−8.16-6.27) |
56.34 | 54.71 | −1.63 (−9.75-6.50) |
| p27, H-Score(% positive × intensity) |
1.00 | 0.98 | −0.02 (−0.21-0.17) |
1.10 | 1.03 | −0.07 (−0.26-0.12) |
While the analyses above were all conducted according to the intention-to-treat principle, additional analyses that accounted for non-compliance in either randomized group also failed to show treatment effects on the tissue biomarkers or serum levels of PSA, IGF-1 or IGFBP3 (data not shown). First, non-compliers were excluded from the intergroup comparison based on pill count or change in serum lycopene. Second, correlations between the change in serum lycopene and change in each endpoint were calculated, including all subjects in the trial.
DISCUSSION
The results presented here from this placebo-controlled randomized trial among men with HGPIN indicate that consumption of a lycopene-rich tomato extract for approximately six months had no discernible effect on proliferation or cell cycle inhibition in benign prostatic epithelium, despite a substantial increase in serum lycopene. Moreover, we observed no effect of the intervention on circulating levels of PSA, IGF-1 or IGFBP3, the principal inhibitory binding protein for IGF-1. An identical lycopene intervention in an earlier trial produced a significant increase in the lycopene concentration in prostate tissue after only 21 days of exposure.(11) A large number of field effects - molecular or other subtle abnormalities detectable in benign regions associated with a tumor - have now been documented in the prostate and even have validated clinical applications, and thus it is relevant to ask whether a potential chemopreventive agent, aimed at primary prevention, can have beneficial effects on these disturbed fields.(14, 15) In earlier work, we reported data showing that proliferation rates in benign epithelium were elevated in tissue that was spatially associated with cancer lesions, suggesting that this is a field effect characteristic of high-risk histologically normal prostate.(16) In contrast to the present findings, studies of benign prostatic epithelial cells in culture have observed that lycopene reduced tritiated thymidine incorporation and inhibited cell cycle progression.(17) Moreover, members of our group recently reported proteomic analyses of primary benign prostatic epithelial cells indicating that lycopene induced anti-proliferative and pro-apoptotic responses.(18)
Although the trial was not powered for histopathological endpoints, an exploratory analysis revealed no remarkable differences in the prevalence of cancer or HGPIN upon repeat biopsy except for a relative decrease in the number of biopsy cores positive for HGPIN in the lycopene group. Participants assigned to lycopene also had a relative increase following treatment in the number of cores showing any type of focal atrophy, as well as the number of men with extensive atrophy. To our knowledge, this is the first trial to obtain data on the effects of a lycopene supplement on proliferation markers or histology in the benign prostate. Mohanty, et al reported a decrease in the detection of cancer among 20 men with HGPIN diagnosed following transurethral prostatectomy who received only 8 mg/day of lycopene as tomato oleoresin, compared to a similar group that received no intervention.(19) However, the trial was neither blinded nor placebo-controlled, and the criteria for performing follow-up biopsies was unclear. Recently, Mariani, et al. reported results of a trial in which 32 men with HGPIN received 20-25 mg/day lycopene as tomato paste and were re-biopsied at 6 months.(20) The prevalence of cancer at repeat biopsy was similar to historical data (9/32), but prostatic lycopene concentrations were significantly lower in the men who had prostate cancer detected compared to those who did not.
Several other clinical trials involving lycopene supplementation in men without confirmed cancer have used serum PSA as an endpoint, with mixed results. Schwarz, et al. compared 15 mg/day of lycopene versus placebo with 6-month follow-up among 37 men with histologically confirmed BPH.(21) The lycopene group had a small (11.3%) but statistically significant decrease in serum PSA. A reduction in serum PSA was also seen in the aforementioned trial in India, after one year of exposure to only 8 mg/day of lycopene.(19) However, Bunker et al, conducted a trial among Caribbean men with HGPIN using the same lycopene intervention as the present study and found no change in PSA after four months.(22)
We can speculate that discrepancies in the results for PSA among these trials could be due to differences in the dosage, formulation or duration of exposure; however, it is obvious that the logic of using PSA as an intermediate endpoint should be dependent on the context of the trial. In healthy men or men with clinically apparent BPH, a decrease in PSA could be attributed to an generalized anti-androgen effect on the prostate, whereas a decrease in PSA among men with PCa on active surveillance would be attributed to a reduction in tumor volume or change in tumor architecture that diminishes leakage of PSA into the blood. Since isolated HGPIN is not associated with increases in serum PSA, it must be assumed that the majority of the men in the present trial had PSA elevations due to concurrent BPH or prostatitis, and therefore this trial should be compared to others within that context. Our failure to detect an effect on PSA after relatively brief exposure may not be surprising, because it seems unlikely that lycopene would have the same potency as a pharmaceutical agent such as a 5α-reductase inhibitor in terms of causing a generalized anti-androgen effect in the prostate. On the other hand, lycopene was observed via proteomic analysis to downregulate the androgen receptor signaling pathway in primary prostatic epithelial cells and thus could reduce expression of PSA, a classical androgen response protein.(18)
The null results in the present trial for effects on circulating IGF-1 and IGFBP3 are not in concordance with some previously published intervention studies. In a placebo-controlled crossover trial of a tomato drink with healthy subjects, Riso, et al reported that changes in serum lycopene were inversely associated with serum IGF-1 levels.(23) Also, in a trial among patients with untreated colon cancer, brief treatment with the same 30 mg/day of the tomato lycopene extract used in our trial reduced plasma IGF-1 by 25%.(24) However, other human trials found no effects on circulating IGF-1 or IGFBP3, or on prostate tissue RNA expression for IGF-1.(21, 25-27) It could be noteworthy that in an observational study reflecting long-term diet patterns and IGF-1 levels, Mucci, et al observed a strong inverse relationship between serum IGF-1 and consumption of cooked tomato foods among men in Greece.(28) Additional studies on the effects of lycopene on IGF signaling in prostate tissue, which are pending, could be more informative.
Major strengths of this study include the randomized double-blind, placebo-controlled design and the ability to compare biopsy tissue samples from the same subject before and after treatment. This repeat sampling of the same organ increases statistical power by reducing variance through having each participant act as his own control. The presence of HGPIN on an initial biopsy presents one of the few opportunities to conduct trials in which repeat biopsy can reliably be conducted at a predictable interval. Compliance with the assigned treatment was generally excellent and serum lycopene levels were substantially elevated in the intervention group and reduced in the control group, as expected. Finally, the use of whole slide digital image analysis, assisted by machine learning to score only the epithelial compartment, permitted objective and more fine-grained scoring of nuclear biomarker expression than could be obtained with conventional scoring by eye. In previous work, we and others demonstrated that minichromosome maintenance proteins such as MCM-2 are important biomarkers for proliferation in high-risk benign prostate epithelium, due to their expression in a higher proportion of cells compared to Ki-67, a shift in expression from basal to luminal cells as neoplasia progresses, and indications of their association with field cancerization.(16, 29) The choice of p27 as an intermediate endpoint biomarker was also significant, because this cell cycle inhibitor has been implicated in the early stages of prostate carcinogenesis and androgen-mediated regulation of benign epithelial proliferation.(30, 31) There is evidence of its up-regulation during the prostate cell response to antioxidants,(32, 33) and to lycopene in particular.(34-36)
The chief limitation of this trial lies in its relatively small size and restricted statistical power. Although the study was adequately powered for detection of approximately a 25% change in expression of the tissue markers – the primary endpoints in the analysis – power was considerably lower for histological changes. In particular, no conclusions can be drawn regarding progression from HGPIN to prostate cancer due to the small number of cancers diagnosed on the follow-up biopsies. Presence of HGPIN as an endpoint was also problematic in terms of statistical power because its detection following treatment in men with HGPIN at baseline is decreased simply due to spatial sampling error during the follow-up biopsy. Larger trials are needed to determine if the suggestive effects of treatment observed in this trial on secondary endpoints such as the extent of HGPIN and focal atrophy can be replicated. It is possible that higher doses of lycopene might be required, although the present dose was approximately 8-9 times higher than the average daily intake among men in the U.S.(37) It is also possible that exposure must be more prolonged or must occur during an earlier stage of life. While the tomato oleoresin capsules used in this trial contain essentially all lipid soluble components from the tomato, there are water-soluble compounds in tomato, such as ascorbic acid and quercetin, that could have relevant biological activity. Therefore, different effects could be seen in studies employing whole tomato foods, especially in cooked form. Moreover, it is reasonable to conjecture that lycopene would only affect the endpoints used in this trial when it is part of a complete dietary pattern, such as that represented by the typical Mediterranean diet.(38)
Ongoing and future studies are examining additional intermediate endpoints in prostate tissue. Meanwhile, the present results do not support the idea of conducting Phase III prostate cancer chemoprevention trials with lycopene supplementation at this time. Lastly, some observers, in considering why some recent epidemiological studies during the PSA era have failed to detect an association between lycopene and prostate cancer risk, have argued that the most important effect of lycopene might be to prevent the transition from indolent cancer to progressive, clinically significant disease.(3) Therefore, the optimal context for future trials might lie in the active surveillance setting.
CONCLUSION
The results of this randomized trial indicate no effect of a lycopene-rich tomato extract on serum PSA, IGF-1, or IGFBP3 concentrations, nor any effect on proliferation or cell cycle inhibition in benign tissue of men with HGPIN. Small increases in extensive focal atrophy and decreases in extensive HGPIN in the intervention group were seen in secondary analyses. Future trials might benefit from longer duration or the use of alternate biomarker endpoints.
Acknowledgments
The authors wish to express appreciation to Michael Schlicht, Peter Nguyen, Galina Khramtsova, Rachel Poon, Zohar Nir, Linlin Dong, Sheano Gold, Lindsay Gallagher, Roohalah Sharifi and Kevin McVary for their contributions to this project. The study was funded by the National Institutes of Health/National Cancer Institute through RO1 CA90759 (PI: P. Gann). The funding agency had no role in the design or conduct of the study, or the preparation of the manuscript.
List of Abbreviations
- HGPIN
high-grade prostatic intraepithelial neoplasia
- ASAP
atypical small acinar proliferation
- PSA
prostate specific antigen
- IGF-1
insulin-like growth factor 1
- IGFBP3
insulin-like growth factor binding protein 3
- MCM-2
minichromosome maintenance protein 2
- p27
p27(Kip1) or Cyclin-dependent kinase inhibitor 1B (CDKN1B)
- CV
coefficient of variation
- BPH
benign prostatic hyperplasia
Footnotes
Trial Registration: ClinicalTrials.gov Identifier NCT00416325
Previous Publication/Concurrent Submission Statement
The authors declare that the material in this manuscript has not been published elsewhere and is not concurrently under review elsewhere.
REFERENCES
- 1.WCRF . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. World Cancer Research Fund / American Institute for Cancer Research; Washington, DC: 2007. [Google Scholar]
- 2.Kristal AR, Till C, Platz EA, Song X, King IB, et al. Serum lycopene concentration and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer epidemiology, biomarkers & prevention. 2011;20:638–46. doi: 10.1158/1055-9965.EPI-10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannucci E. Commentary: Serum lycopene and prostate cancer progression: a re-consideration of findings from the prostate cancer prevention trial. Cancer causes & control. 2011;22:1055–9. doi: 10.1007/s10552-011-9776-x. [DOI] [PubMed] [Google Scholar]
- 4.Wei MY, Giovannucci EL. Lycopene, Tomato Products, and Prostate Cancer Incidence: A Review and Reassessment in the PSA Screening Era. Journal of oncology. 2012;2012:271063. doi: 10.1155/2012/271063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sies H, Stahl W. Lycopene: antioxidant and biological effects and its bioavailability in the human. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1998;218:121–4. doi: 10.3181/00379727-218-44285a. [DOI] [PubMed] [Google Scholar]
- 6.Herzog A, Siler U, Spitzer V, Seifert N, Denelavas A, et al. Lycopene reduced gene expression of steroid targets and inflammatory markers in normal rat prostate. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:272–4. doi: 10.1096/fj.04-1905fje. [DOI] [PubMed] [Google Scholar]
- 7.Siler U, Herzog A, Spitzer V, Seifert N, Denelavas A, et al. Lycopene effects on rat normal prostate and prostate tumor tissue. The Journal of nutrition. 2005;135:2050S–2S. doi: 10.1093/jn/135.8.2050S. [DOI] [PubMed] [Google Scholar]
- 8.Tang L, Jin T, Zeng X, Wang JS. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. The Journal of nutrition. 2005;135:287–90. doi: 10.1093/jn/135.2.287. [DOI] [PubMed] [Google Scholar]
- 9.Nahum A, Zeller L, Danilenko M, Prall OW, Watts CK, et al. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr. 2006;45:275–82. doi: 10.1007/s00394-006-0595-x. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Allen JD, Arnold JT, Blackman MR. Lycopene inhibits IGF-I signal transduction and growth in normal prostate epithelial cells by decreasing DHT-modulated IGF-I production in co-cultured reactive stromal cells. Carcinogenesis. 2008;29:816–23. doi: 10.1093/carcin/bgn011. [DOI] [PubMed] [Google Scholar]
- 11.van Breemen RB, Sharifi R, Viana M, Pajkovic N, Zhu D, et al. Antioxidant effects of lycopene in African American men with prostate cancer or benign prostate hyperplasia: a randomized, controlled trial. Cancer prevention research. 2011;4:711–8. doi: 10.1158/1940-6207.CAPR-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Marzo AM, Platz EA, Epstein JI, Ali T, Billis A, et al. A working group classification of focal prostate atrophy lesions. The American journal of surgical pathology. 2006;30:1281–91. doi: 10.1097/01.pas.0000213289.50660.be. [DOI] [PubMed] [Google Scholar]
- 13.Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, et al. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379–84. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nonn L, Ananthanarayanan V, Gann PH. Evidence for field cancerization of the prostate. Prostate. 2009;69:1470–9. doi: 10.1002/pros.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partin AW, Van Neste L, Klein EA, Marks LS, Gee JR, et al. Clinical validation of an epigenetic assay to predict negative histopathological results in repeat prostate biopsies. J Urol. 2014;192:1081–7. doi: 10.1016/j.juro.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ananthanarayanan V, Deaton RJ, Yang XJ, Pins MR, Gann PH. Alteration of proliferation and apoptotic markers in normal and premalignant tissue associated with prostate cancer. BMC cancer. 2006;6:73. doi: 10.1186/1471-2407-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obermuller-Jevic UC, Olano-Martin E, Corbacho AM, Eiserich JP, van der Vliet A, et al. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro. The Journal of nutrition. 2003;133:3356–60. doi: 10.1093/jn/133.11.3356. [DOI] [PubMed] [Google Scholar]
- 18.Qiu X, Yuan Y, Vaishnav A, Tessel MA, Nonn L, et al. Effects of lycopene on protein expression in human primary prostatic epithelial cells. Cancer prevention research. 2013;6:419–27. doi: 10.1158/1940-6207.CAPR-12-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohanty NK, Saxena S, Singh UP, Goyal NK, Arora RP. Lycopene as a chemopreventive agent in the treatment of high-grade prostate intraepithelial neoplasia. Urologic oncology. 2005;23:383–5. doi: 10.1016/j.urolonc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Mariani S, Lionetto L, Cavallari M, Tubaro A, Rasio D, et al. Low prostate concentration of lycopene is associated with development of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. International journal of molecular sciences. 2014;15:1433–40. doi: 10.3390/ijms15011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz S, Obermuller-Jevic UC, Hellmis E, Koch W, Jacobi G, et al. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. The Journal of nutrition. 2008;138:49–53. doi: 10.1093/jn/138.1.49. [DOI] [PubMed] [Google Scholar]
- 22.Bunker CH, McDonald AC, Evans RW, de la Rosa N, Boumosleh JM, et al. A randomized trial of lycopene supplementation in Tobago men with high prostate cancer risk. Nutr Cancer. 2007;57:130–7. doi: 10.1080/01635580701274046. [DOI] [PubMed] [Google Scholar]
- 23.Riso P, Brusamolino A, Martinetti A, Porrini M. Effect of a tomato drink intervention on insulin-like growth factor (IGF)-1 serum levels in healthy subjects. Nutr Cancer. 2006;55:157–62. doi: 10.1207/s15327914nc5502_6. [DOI] [PubMed] [Google Scholar]
- 24.Walfisch S, Walfisch Y, Kirilov E, Linde N, Mnitentag H, et al. Tomato lycopene extract supplementation decreases insulin-like growth factor-I levels in colon cancer patients. European journal of cancer prevention. 2007;16:298–303. doi: 10.1097/01.cej.0000236251.09232.7b. [DOI] [PubMed] [Google Scholar]
- 25.Graydon R, Gilchrist SE, Young IS, Obermuller-Jevic U, Hasselwander O, et al. Effect of lycopene supplementation on insulin-like growth factor-1 and insulin-like growth factor binding protein-3: a double-blind, placebo-controlled trial. Eur J Clin Nutr. 2007;61:1196–200. doi: 10.1038/sj.ejcn.1602632. [DOI] [PubMed] [Google Scholar]
- 26.Vrieling A, Voskuil DW, Bonfrer JM, Korse CM, van Doorn J, et al. Lycopene supplementation elevates circulating insulin-like growth factor binding protein-1 and -2 concentrations in persons at greater risk of colorectal cancer. The American journal of clinical nutrition. 2007;86:1456–62. doi: 10.1093/ajcn/86.5.1456. [DOI] [PubMed] [Google Scholar]
- 27.Chan JM, Weinberg V, Magbanua MJ, Sosa E, Simko J, et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer causes & control : CCC. 2011;22:141–50. doi: 10.1007/s10552-010-9684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mucci LA, Tamimi R, Lagiou P, Trichopoulou A, Benetou V, et al. Are dietary influences on the risk of prostate cancer mediated through the insulin-like growth factor system? BJU Int. 2001;87:814–20. doi: 10.1046/j.1464-410x.2001.02191.x. [DOI] [PubMed] [Google Scholar]
- 29.Padmanabhan V, Callas P, Philips G, Trainer TD, Beatty BG. DNA replication regulation protein Mcm7 as a marker of proliferation in prostate cancer. J Clin Pathol. 2004;57:1057–62. doi: 10.1136/jcp.2004.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdamar S, Yang G, Harper JW, Lu X, Kattan MW, et al. Levels of expression of p27KIP1 protein in human prostate and prostate cancer: an immunohistochemical analysis. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1999;12:751–5. [PubMed] [Google Scholar]
- 31.Waltregny D, Leav I, Signoretti S, Soung P, Lin D, et al. Androgen-driven prostate epithelial cell proliferation and differentiation in vivo involve the regulation of p27. Mol Endocrinol. 2001;15:765–82. doi: 10.1210/mend.15.5.0640. [DOI] [PubMed] [Google Scholar]
- 32.Sinha R, El-Bayoumy K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Current cancer drug targets. 2004;4:13–28. doi: 10.2174/1568009043481614. [DOI] [PubMed] [Google Scholar]
- 33.Venkateswaran V, Fleshner NE, Klotz LH. Modulation of cell proliferation and cell cycle regulators by vitamin E in human prostate carcinoma cell lines. The Journal of urology. 2002;168:1578–82. doi: 10.1016/S0022-5347(05)64524-7. [DOI] [PubMed] [Google Scholar]
- 34.Nahum A, Hirsch K, Danilenko M, Watts CK, Prall OW, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001;20:3428–36. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 35.Venkateswaran V, Klotz LH, Ramani M, Sugar LM, Jacob LE, et al. A combination of micronutrients is beneficial in reducing the incidence of prostate cancer and increasing survival in the Lady transgenic model. Cancer prevention research. 2009;2:473–83. doi: 10.1158/1940-6207.CAPR-08-0124. [DOI] [PubMed] [Google Scholar]
- 36.Palozza P, Colangelo M, Simone R, Catalano A, Boninsegna A, et al. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31:1813–21. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- 37.Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, et al. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. Journal of the Academy of Nutrition and Dietetics. 2012;112:222–9. doi: 10.1016/j.jada.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 38.Itsiopoulos C, Hodge A, Kaimakamis M. Can the Mediterranean diet prevent prostate cancer? Molecular nutrition & food research. 2009;53:227–39. doi: 10.1002/mnfr.200800207. [DOI] [PubMed] [Google Scholar]