Abstract
Optimal sleep is integral to health but is commonly not obtained. Despite its wide ranging public health impact, sleep health is under-appreciated by the general public and is only rarely considered by policy makers, employers, schools, and others whose policies and structures can adversely affect sleep. Inadequate sleep duration and quality are prevalent in minority and low-income populations and may play a fundamental role in racial and socioeconomic status (SES) inequities for a wide range of health conditions including cardiovascular disease (CVD).The goal of this review is to examine the relationship between sleep and CVD health disparities. To this end, we describe the overall public health importance of sleep and the role of sleep duration as well as the two most common disorders (sleep apnea and insomnia) as risk factors for a number of chronic diseases. We then focus on the potential link between sleep and CVD disparities. A multilevel model developed for the analysis of population health and health disparities as a part of the National Cancer Institute’s Centers on Population Health and Health Disparities served as our conceptual framework. It is based on the notion that individual behaviors, like sleep, are influenced by complex and dynamic interrelations among the individual and his or her physical and social environments across the lifespan. Using this model, we describe modifiable factors that contribute to insufficient sleep and circadian misalignment, propose potential interventions in various sectors (e.g. neighborhoods, schools, workplaces) that address social structures that contribute to disparities, and conclude by recommending critical areas for future sleep research. We ultimately suggest that integrating sleep into public health research will identify novel approaches for closing the gap in health disparities, such as CVD.
Keywords: Sleep, Cardiovascular Disease, Health Disparities
Introduction
The Institute of Medicine identified inadequate sleep and sleep disorders as a public health issue in a 2006 report (132). This report estimated that while 50 to 70 million Americans have a chronic sleep disorder, there is a low awareness of sleep health both in the general public and in professional communities. It also highlighted the high prevalence of sleep apnea and short sleep duration in blacks as well as the potential for these problems to contribute to chronic health conditions. The biological, social or environmental bases of these sleep disorders were not addressed. This review aims to further examine the relationship between sleep and chronic disease health disparities, with an emphasis on cardiovascular disease (CVD). Health disparities or inequities are defined as differences in health between groups (e.g. race/ethnicity; socioeconomic status) that are not only unnecessary and avoidable, but are also unfair and unjust (21). Racial/ethnic and socioeconomic health disparities are embedded in larger historical, geographic, sociocultural, economic, as well as political contexts (165). Inequality in the built and social environments underlies key health disparities and prevalent risk factors (e.g. physical inactivity, obesity) for CVD (57). Although inadequate sleep duration and quality may substantially contribute to racial and socioeconomic status (SES) inequities for a wide range of health conditions, sleep health is understudied by researchers and underappreciated by the general public, policy makers, and other stakeholders.
We begin with an overview of the physiology of sleep and the mechanisms by which sleep may increase risk of chronic diseases for which there are persistent racial/ethnic and socioeconomic disparities. We emphasize CVD as a particularly underappreciated potential consequence of suboptimal sleep and focus on sleep duration as well as the two most common sleep disorders, obstructive sleep apnea (OSA) and insomnia. We then describe racial/ethnic and socioeconomic disparities in sleep and sleep disorders, focusing on Black-White sleep disparities, as data for other race/ethnicities are sparse. We subsequently present a conceptual framework for how the environmental context likely affects racial/ethnic and socioeconomic sleep-related health disparities. We conclude by suggesting critical areas of future research that will help to unpack the complex interplay between sleep, health, and health disparities, and will provide targets for novel sleep-focused interventions that could reduce persistent disparities in CVD. We suggest that understanding the complex interplay among sleep, social determinants of health, and CVD health is critical if we are to design, implement and evaluate clinical and public health initiatives for improving overall population health as well as the health of racial/ethnic minorities and low-SES populations.
The Public Health Importance of Sleep
Overview of Sleep Physiology
Sleep is an essential neurophysiologic state that is an integral part of overall health and a source of physiological and psychological resilience (157). Universal across species, sleep is considered critical for rest and restoration of brain and body functions. Sleep also is important for learning and memory consolidation. Major physiological functions influenced by sleep include protein synthesis, release of hormones, and modulation of the autonomic nervous system.
There are distinct stages of sleep, characterized by distinct patterns of brain electroencephalographic activity. Typically, sleep begins in a light stage (termed stage N1), and over approximately 90 minute repetitive cycles, progresses into deeper periods of non-rapid eye movement (non-REM) (stages N2 and N3) and REM sleep. Stage N3 (also termed “slow wave sleep”) is when the brain is least likely to arouse to external stimuli and when growth hormone and other hormones important for metabolism are released. Autonomic nervous system activity- critical in the regulation of cardiovascular functions- vary with sleep stage, with parasympathetic nervous system tone highest in N3 sleep and sympathetic activation highest in REM sleep. The regular transition of sleep stages (without excessive arousals or fragmentation) is needed for sleep to be “restorative.”
Curtailment of overall sleep duration, disruption of normal cycling of sleep stages, selective curtailment of deeper sleep, increased sleep fragmentation, and sleep-associated breathing abnormalities each can lead to acute and chronic health problems and contribute to neuroendocrine abnormalities that affect, for instance, lipid and glucose metabolism as well as vascular health. Among physiological pathways influenced by sleep are the hypothalamic-pituitary-adrenal axis, the autonomic nervous system, pro-inflammatory hormone release, glucose homeostasis and vascular control (24; 118).
Over the lifespan, sleep duration decreases from average values of approximately 16 hours a day in infancy to 7 or 8 hours in adulthood. At any given age, there is likely some inter-individual variability in the duration of sleep associated with optimal health and functioning. Most studies suggest that short sleep duration consisting of < 11 hours per night in infancy, less than 7 hours per night in adolescence and less than 6 hours per night in adulthood are associated with adverse health outcomes.
Sleep Disorders and Public Health
The two most common sleep disorders are OSA and insomnia, which each affect approximately 15% of the population (122; 124). OSA is the occurrence of repetitive periods of obstructed breathing during sleep (apneas and hypopneas), associated with drops in oxygen levels, arousals, and mechanical stresses on the heart and lungs. It is associated with symptoms of sleep disruption, snoring, and daytime sleepiness. Insomnia is a disorder characterized by chronic difficulties in initiating or maintaining sleep, or frequent early morning awakenings. In addition, sleep may be impaired due to irregular sleep patterns, particularly when sleep occurs outside of the normal sleep-wake circadian cycles, as occurs in shift workers, which is referred to as “circadian misalignment” (45; 134).
Sleep deficiency is defined as insufficient quantity or inadequate quality of sleep obtained relative to that needed for optimal health, performance, and well-being (25; 31). Although it is unclear if sleep duration has decreased over time (13), it is estimated that 50–70 million Americans suffer from a chronic sleep disorder. For instance, more than 12 million Americans have OSA (37). Regarding performance, suboptimal sleep contributes to poor performance in everyday activities, including academic underachievement and behavioral problems in children and adolescents in addition to work-related productivity among adults (78). Inadequate sleep is also associated with an increased risk of motor vehicle crashes, occupational injuries, and a loss in work-related productivity (35; 64; 104; 134). Almost 20% of all serious car crash injuries in the general population are associated with driver sleepiness.
Recent recognition of the population impact of sleep deficiency has informed Healthy People 2020 sleep health objectives (78), which include an: 1) increase in the proportion of adolescents obtaining adequate sleep (baseline: 31%); 2) increase in the proportion of adults obtaining adequate sleep (baseline: 63%); 3) decrease in the number of motor vehicle incidents attributed to drowsy driving (baseline: 2.7/100 million miles); and 4) increase the proportion of adults with apnea symptoms seeking medical attention (baseline: 10%).
Sleep Disorders and Chronic Disease Risk
Billions of dollars a year are spent on direct medical costs related to sleep disorders (162). Suboptimal sleep is associated with mood disorders and physical health outcomes, including increased incidence, progression and severity of CVD, diabetes, obesity, cancer, and mortality (11; 25; 53; 54; 119). This section provides a brief review of the evidence linking sleep with chronic disease outcomes, and is followed by a more detailed section examining CVD.
Weight gain and obesity
Findings in 31 cross-sectional and 5 prospective cohort studies of children suggested that short sleep duration was strongly and consistently associated with concurrent and future obesity (125). A systematic review of 30 studies found that self-reported short sleepers were 55% more likely to be obese than those reporting ≥7 h of sleep per night (31).
Hypertension
Epidemiological studies have shown statistically significant independent associations between habitual sleep duration (especially during middle age), OSA, insomnia, restless leg syndrome, as well as periodic limb movement and an increased risk or prevalence of hypertension (27). Approximately 50% of patients with OSA are hypertensive, and more than 30% of patients with hypertension have OSA (167). OSA is also present in up to 90% of patients with resistant hypertension (167), which is more commonly observed in blacks.
Blood pressure normally decreases by approximately 10% during sleep compared to wakefulness, a pattern termed “dipping.” “Non-dipping” of blood pressure, associated with an increased risk of cardiovascular disease and mortality (44), is particularly common in African Americans, and can occur secondary to OSA (161), secondary to intermittent hypoxia, and due to arousals from sleep (19). Activation of the hypothalmic-pituitary-adrenal axis and the sympathetic nervous system as seen in insomnia may also increase hypertension risk (16).
Diabetes
Both quantity and quality of sleep have been demonstrated to significantly predict the risk of type 2 diabetes (30). Snoring and OSA also have been associated with abnormal glucose control and risk of diabetes, with some evidence that OSA treatment improves this risk (153). Insomnia has been associated with a relative risk range from 1.84 to 2.95 (159).
Cancer
Sleep duration is dependent on circadian rhythm that controls a variety of key cellular functions, and circadian disruption is implicated in cancer risk. Both short and long sleep durations have been associated with an increase in the risk of colorectal cancer (CRC) in postmenopausal women and short sleep has been associated with breast cancer (160). OSA also has been reported to be associated with cancer incidence (28) and mortality (120), attributed to hypoxemia influencing angiogenesis, apoptosis, and tumor metastases.
Mood disorders
Sleep and circadian rhythm disturbances are common in many mood and psychiatric disorders (11). There is evidence that these associations are bi-directional, and some may represent abnormalities in common neuropsychiatric pathways. Sleep disturbances often precede the onset of anxiety and depression and are risk factors for relapse of depression and for suicidality (26).
Behavior and Cognition
Insufficient sleep and OSA have been linked to externalizing behaviors (negative behaviors that are directed toward the external environment, such as impulsivity, fighting and refusal to follow rules/laws), and emotion regulation, internalizing behaviors (negative behaviors that are directed towards oneself, such as social withdrawal), and executive functioning (7; 36). Untreated OSA has been linked to poor school performance (58), and appropriate recognition and surgical treatment of OSA in children with ADHD may prevent the need for long-term stimulant treatment (90). Adults with OSA have also been shown to have higher rates of divorce (66). Sleep disturbances thus adversely affect behaviors and physiologic processes critical for social and cognitive development as well as for academic and occupational performance (56; 58; 152).
Mortality
Given the number of chronic health conditions associated with suboptimal sleep, it is not surprising that it is also associated with increased mortality (53). Among 16 studies, the pooled relative risk (RR) for all-cause mortality for short sleep duration was 1.10 [95% confidence interval (CI): 1.06, 1.15] (53). Similarly, among the 17 studies reporting on long sleep duration and mortality, the pooled RRs comparing the long sleepers with medium-length sleepers were 1.23 (95% CI: 1.17, 1.30) for all-cause mortality, 1.38 (95% CI: 1.13, 1.69) for cardiovascular-related mortality, and 1.21 (95% CI: 1.11, 1.32) for cancer-related mortality (53).
The Role of Sleep in CVD Disparities
We first provide a brief review of CVD disparities prior to describing the relationship between sleep and CVD, followed by an overview of the evidence for disparities in sleep and sleep disorders, and then finally followed by the introduction of a conceptual framework to illustrate the relationship between sleep and CVD disparities from a multilevel perspective.
Cardiovascular Disease Disparities
Despite advances in CVD prevention, racial/ethnic as well as low SES groups have a disproportionately high prevalence of CVD (42; 95; 112; 140). Table 1 shows the age-adjusted death rates and potential life years lost for all causes, heart disease and cerebrovascular disease by race in addition to heart disease prevalence by SES between and within racial/ethnic populations. CVD disparities are attributable to an increased prevalence of CVD risk factors, often with early onset and poor control. Difficult to control hypertension is considered a “lynchpin” that contributes to excessive rates of heart failure and stroke among racial/ethnic minorities (especially blacks) (18; 52; 111). Obesity, abnormal glucose metabolism, and frank Type 2 diabetes also are more common in most racial/ethnic minority populations (20; 41; 87; 121).
Table 1.
Age-adjusted Death Rates and Potential Life Years Lost for All causes, Heart Disease, and Cerebrovascular Disease by Race as well as Heart Disease Prevalence by SES between and within Racial/Ethnic Populations
| Age-adjusted Death Rate (per 100,000) in 2010 (1) | |||||||||
| Black | White | ||||||||
| All causes | 898.2 | 741.8 | |||||||
| Heart disease | 224.9 | 176.9 | |||||||
| Cerebrovascular disease | 53.0 | 37.7 | |||||||
| Years of Potential Life Lost before Age 75 Years (per 100,000) in 2010 (1) | |||||||||
| Black | White | ||||||||
| All causes | 9,832.5 | 6,342.8 | |||||||
| Heart disease | 1,691.1 | 900.9 | |||||||
| Cerebrovascular disease | 358.1 | 142.7 | |||||||
| Relative Risk by Education and Income within Racial/Ethnic Population (6) | |||||||||
|
Relative Risk compared to 12 Years of Education |
Relative Risk compared to $20,000–$29,999 |
||||||||
| ≤ 8 years | 9–11 | 13+ | <$10,000 | $10,000–19,000 | ≥ $30,000 | Foreign | |||
| Heart disease prevalence | |||||||||
| Longitudinal Study of Aging | |||||||||
| White (N=7,003) | 1.17* | 1.20* | 0.83 | 1.26* | 0.98 | 0.92 | |||
| Black (N=888) | 1.40 | 0.81 | 1.91 | 1.01 | 1.28 | 1.34 | |||
| Hispanic (N=315) | 1.15 | 0.81 | 0.90 | 1.48 | 0.91 | 1.17 | 1.03 | ||
| AHEAD 70+ | |||||||||
| White (N=5,896) | 1.33* | 1.23* | 1.00 | 1.31* | 1.19* | 1.03 | |||
| Black (N=1,023) | 2.26* | 1.90* | 0.85 | 0.78 | 0.94 | 1.09 | |||
| Hispanic (N=406) | 1.09 | 1.36 | 1.08 | 2.69 | 1.63 | 2.22 | 1.35 | ||
| HRS 51–61 | |||||||||
| White (N=5,936) | 1.21 | 1.39* | 0.89 | 1.49* | 1.12 | 0.81* | |||
| Black (N=1,483) | 0.82 | 0.78 | 0.75 | 2.21* | 1.04 | 0.80 | |||
| Hispanic (N=772) | 1.39 | 1.31 | 1.03 | 2.41 | 1.77 | 1.18 | 1.16 | ||
| Relative Risk by Race/Ethnicity, Education, and Income (6) | |||||||||
| Relative to Non-Hispanic Whites |
Relative to 12 Years of Education |
Relative to $20,000–$29,999 | |||||||
| Blacks |
U.S.-born Hispanics |
Foreign- born Hispanics |
≤ 8 Yrs | 9–11 | 13+ | <$10,000 |
$10,000– $19,999 |
≥$30,000 | |
| Heart disease prevalence | |||||||||
| Longitudinal Study of Aging 70+ | |||||||||
| N=8,333 (1) | 0.81* | 0.88 | 0.91 | ||||||
| N=8,206 (2) | 0.74* | 0.82 | 0.82 | 1.24 | 1.18 | 0.85 | |||
| N=8,206 (3) | 0.71* | 0.81 | 0.86 | 1.19* | 1.15 | 0.86 | 1.24* | 1.00 | 0.93 |
| AHEAD 70+ | |||||||||
| N=7,342 (1) | 0.76* | 0.75 | 0.58* | ||||||
| N=7,342 (2) | 0.67* | 0.63* | 0.49* | 1.43* | 1.29* | 0.98 | |||
| N=7,342 (3) | 0.66* | 0.62* | 0.48* | 1.39* | 1.27* | 1.00 | 1.26* | 1.17 | 1.04 |
| HRS 51–61 | |||||||||
| N=9,456 (1) | 1.16 | 0.67 | 0.71 | ||||||
| N=9,456 (2) | 1.06 | 0.59* | 0.60* | 1.32 | 1.35* | 0.85* | |||
| N=9,456 (3) | 0.95 | 0.55* | 0.56* | 1.13 | 1.24* | 0.89 | 1.72* | 1.23 | 0.83* |
Equation 1=Relative risk adjusted for age and sex without control for SES
Equation 2=Relative risk adjusted for age, sex, and 4 categories of education
Equation 3= Relative risk adjusted for age, sex, 4 categories of education, and income
Significant at 0.05 level or below.
Data sources: Health 2013, United States. http://www.cdc.gov/nchs/data/hus/hus13.pdf. Accessed October 2014
Anderson NB, RA; Cohen B. 2004. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: The National Academies Press
Sleep and Cardiovascular Disease Risk
Some health outcomes (e.g. obesity, hypertension, and diabetes) most notably associated with CVD are also affected by suboptimal sleep. Sleep abnormalities are linked to abnormalities in blood pressure, lipid and glucose metabolism, and weight, and thus may significantly contribute to excess CVD. Intermediate pathways by which sleep influences CVD include effects on diurnal patterns of blood pressure and heart rate, insulin sensitivity, autonomic nervous system activity, and salt and fluid homeostasis (10; 67; 97). The intermittent hypoxemia and swings in intrathoracic pressure that occur with OSA also negatively impact cardiovascular health through adverse effects on endothelial function, myocardial contractility, oxidative stress and systemic inflammation (19). Sleep also may indirectly influence CVD risk via effects on behaviors such as diet and physical activity (117). For instance, sleep deprivation alters appetite regulating hormones such as ghrelin and leptin, increasing hunger, leading to increased caloric intake (145; 170). Imaging studies indicate that sleep deprivation affects brain centers associated with reward behaviors, thus contributing to increased energy intake (145; 170). Sleep restriction may lead to fatigue and result in lower physical activity levels (170). Long sleep (> 9 hours sleep per night) has also been linked to adverse physiological functions, and the potential mechanisms underlying the association between long sleep duration and CVD as well as other disease risks are not well understood (61; 88). Depression, unemployment, physical inactivity, poor health status and chronic health conditions have been considered important contributors to suboptimal, long sleep and its association with disease risk.
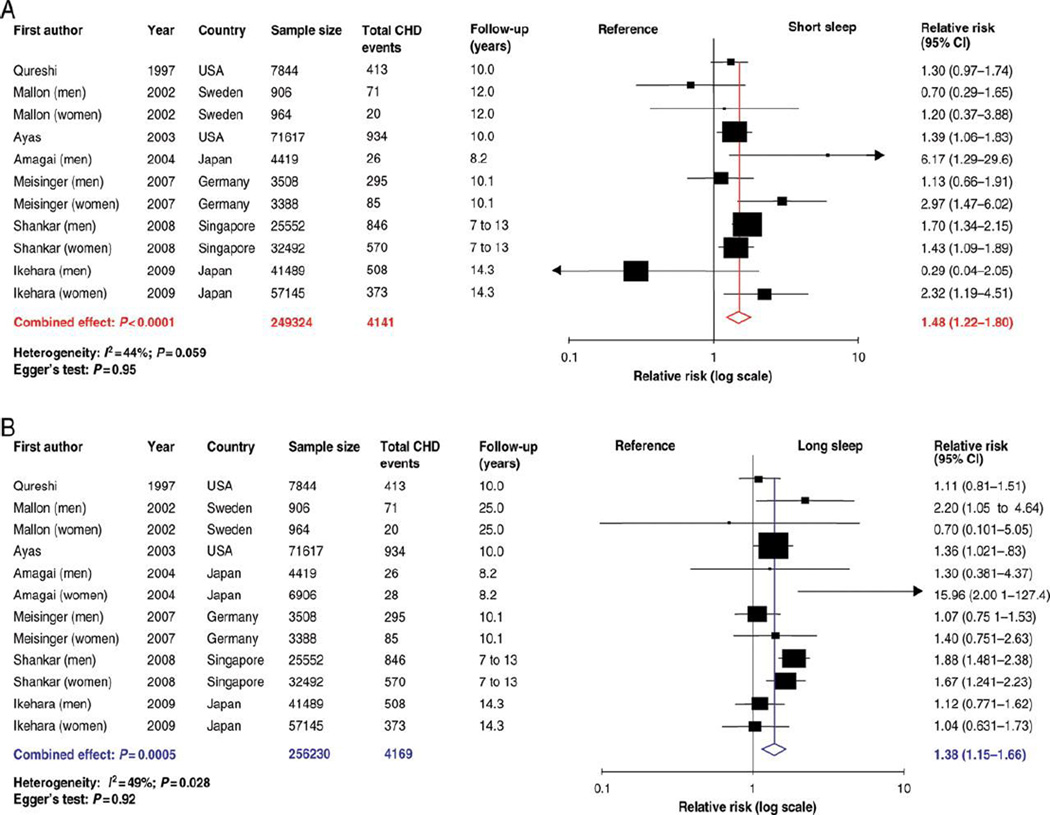
A meta-analysis of 15 studies found that short sleep duration was associated with a greater risk of developing or dying of coronary heart disease (CHD) (RR 1.48, 95% CI 1.22–1.80, P < 0.0001) and stroke (1.15, 1.00–1.31, P = 0.047) (see Figure 1) (29). Long sleep duration was also associated with a greater risk of CHD (1.38, 1.15–1.66, P = 0.0005), stroke (1.65, 1.45–1.87, P < 0.0001), and total CVD (1.41, 1.19–1.68, P < 0.0001) (29). Epidemiological studies have also established OSA as a risk factor for incident hypertension (106), heart failure, CHD, and stroke (131; 151). Moderate-severe OSA doubles the risk for stroke, and increases risk for CHD by 30%. Observational studies suggest that treatment of OSA reduces CVD and CVD-related mortality (107).
Figure 1.
Forest plots of the risk of developing or dying of coronary heart disease associated with (A) short duration of sleep compared with the reference group and (B) long duration of sleep compared with the reference group. (29)
Socioeconomic Disparities in Sleep and Sleep Disorders
Emerging data indicate that individuals from disadvantaged neighborhoods and of low SES experience high rates of extreme sleep durations, low sleep quality, as well as OSA and insomnia (55; 72; 105; 113). Research investigating the basis for sleep disparities is in its early stages, but there is evidence suggesting that greater exposure to stressors (e.g. neighborhood, occupational, psychosocial), and environmental exposures to tobacco, allergens and pollutants may adversely influence sleep quality and duration and also exacerbate OSA (73; 77; 84). Acculturation, which involves the acquisition of the cultural elements (e.g. food choice, language, music) of the dominant society, may also negatively impact sleep quality and increase risk for sleep disorders. Alcohol consumption, depression, shift work, unemployment, physical inactivity, and chronic health conditions also adversely influence sleep (65; 137; 144). Furthermore, despite the higher prevalence of sleep disturbance in low SES groups, they are often under-recognized and under-treated.
Racial/Ethnic Disparities in Sleep and Sleep Disorders
Sleep Duration
Compared to whites, blacks are nearly twice as likely to report short sleep at approximately 31% (93; 149). A meta-analysis of 14 studies found larger effect sizes in studies using objective compared with self-reported measures of sleep duration (135). Blacks are also over 60% more likely to report long sleep (14%) (68). Less research has examined sleep health in Hispanics, but existing evidence suggests that they, too, may be at increased risk for both short and long sleep duration compared with non-Hispanic whites (68; 93; 102), controlling for confounders.
Blacks may also be at risk for more severe consequences of extreme sleep than whites. Analysis of NHIS data revealed that, among individuals reporting short or long sleep duration, blacks were at greater risk of diabetes than were whites (171), even when controlling for age, sex, and income. More work is needed to investigate whether similar effects are present among other racial/ethnic minority groups, and for a range of CVD outcomes. However, extreme sleep durations appear to be disproportionately common in low-income and racial/ethnic minority groups and are associated with intermediate CVD mechanisms as well as subclinical and clinical CVD. The limited longitudinal data available suggests that extreme sleep duration may mediate a portion of the increased CVD burden observed in blacks and Hispanics.
Obstructive Sleep Apnea
White, black and Hispanic Americans (19%-20%) are about twice as likely as Asian Americans (10%) to have been diagnosed with a sleep disorder, including OSA (2). Prevalence of OSA is more than twice as high in blacks (14%) than in whites (6%) or Asians (4%) (34), and 4–6 times higher in black children (6 to 8%) than in white children (17). A cross-sectional study of 280 patients with OSA found that relative to whites: (1) blacks were significantly more obese and had higher rates of hypertension at the time of sleep apnea diagnosis; (2) black females were diagnosed at a significantly younger age than were white females; and (3) black males had a significantly lower oxygen saturation level than did white males (110). Thus, blacks may experience earlier and more severe presentations of OSA than whites, and OSA may contribute to this population’s lifelong increased risk of CVD.
Insomnia
Racial differences in insomnia are not well-understood (68). Insomnia is diagnosed more often in whites (10%) than in Asians (4%) and blacks (3%). In survey (33) and diary (133) studies, whites report more trouble falling asleep and staying asleep than blacks and Hispanics (33). In contrast, cross-sectional (15), survey (126), and prospective studies (141) have found that non-whites suffer from more chronic insomnia, controlling for potential confounders and baseline insomnia symptoms. The mixed findings may be due, in part, to methodological issues, such as differing definitions of insomnia or reliance on self-reports. Of the studies reviewed here, those with the strongest methods (e.g. use of validated self-report measures, prospective designs) found evidence of racial differences in insomnia.
A Conceptual Framework for the Role of Sleep in Cardiovascular Disease Disparities
As previously described, sleep impacts a number of physiological factors that influence CVD outcomes (4). As also reviewed above, socially-determined factors and exposures (e.g. occupational stressors, discrimination, treatment access and adherence) can influence the same mechanisms (65; 85; 142). Thus, we next examine in more detail socially-mediated pathways by which sleep deficiency may be driving CVD disparities.
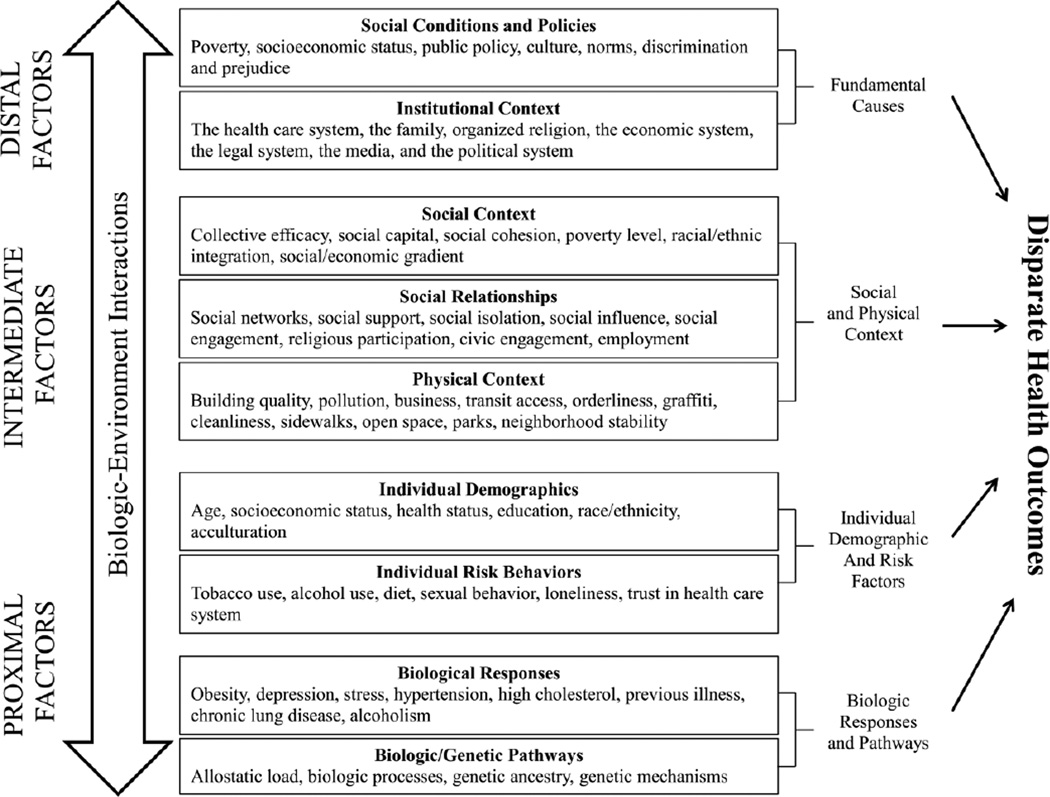
We draw upon a model developed as a part of the National Cancer Institute’s Centers on Population Health and Health Disparities (see Figure 2) (163). The model is based on the notion that individual behaviors, like sleep, are influenced by complex and dynamic interrelations between the individual and his or her physical, social, and institutional environments across the lifespan. This model provides a basis for understanding the complex array of factors likely influencing sleep and its roles in health disparities. Biological pathways and responses are a key part of this model, as reviewed above. Here we focus on individual, physical, social, and institutional factors.
Figure 2.
The Socioecological Model and Determinants of Sleep and Cardiovascular Health
Distal determinants, as described in the figure, are considered fundamental causes of population health and health disparities because their influence contributes to variation in health and disease. An example of a distal determinant is the establishment of city ordinances that control the working hours of construction sites to protect residents from noise that could disrupt sleep. Intermediate determinants, such as neighborhoods, represent the physical and social contexts where distal determinants are realized. Availability and accessibility of certain tangible and social resources (e.g. social cohesion, lighting) can influence sleep environment characteristics. It is believed that intermediate determinants link the environment to individuals, and influence biological responses and more proximal determinants (e.g. stress/anxiety, sleep homeostasis, circadian rhythm misalignment).
Proximal Factors-- Individual Risk Behaviors
Risk Behaviors
Lifestyle factors such as nutrition, physical activity, alcohol consumption and smoking are established CVD risk factors and can influence sleep quality. Sleep behaviors such as regularity of bedtimes and limiting use of substances (alcohol, tobacco) and certain activities (TV watching in bed), considered “sleep hygiene,” also influence sleep quality and duration (8; 60; 133).
Recent trends in technology use and affordability have resulted in a 24-hour society where individuals are perpetually available and capable of communicating and engaging in work in ways that may displace sleep differentially across groups (39; 62). Screen exposure is particularly disruptive to sleep due to the alerting effects of light (especially blue) on the sleep centers in the brain. According to a 2011 national poll, 90% of Americans reported using a technological device in the hour before bed, and young adults' sleep patterns were significantly later than other age groups in terms of time going to bed on both weekdays and weekend nights (59). Unlike passive technological devices (e.g., TV), the more interactive technological devices (i.e., computers, cell phones, video games) used in the hour before bed, the more likely difficulties falling asleep and having unrefreshing sleep were reported. Although blacks have lower internet usage than whites (87% of whites and 80% of blacks are internet users), blacks and whites have similar rates of use of social media, especially on mobile platforms; 73% of black internet users—and 96% of those ages 18–29—use a social networking site of some kind (51). The closing of the digital divide in technology may inadvertently contribute to health-disparities.
Proximal Factors Individual Demographics
Acculturation
Recent evidence suggests that the harmful health effects of acculturation, which involves the acquisition of the cultural elements (e.g. food choice, language, music) of the mainstream society, extends to sleep duration among Hispanics (38; 79; 98). A nationally representative study found that Mexican-Americans were 44% more likely to report short sleep duration than were Mexican immigrants, controlling for confounders (73). This effect was modestly attenuated after considering the effects of smoking and self-reported stress. A study of over 300 women of Mexican descent found that acculturation (language preference and socialization in the U.S. < age 18), predicted self-reported sleep disturbance (75). These studies provide initial evidence highlighting acculturation as a mechanism that predisposes Mexican-Americans to higher levels of sleep deficiency than whites.
Other demographic factors
Demographic factors are important to consider given their associations with sleep disorders. In particular, OSA increases in prevalence and severity with advancing age and is more severe in men than women, likely due to the influence of sex hormones on airway patency and ventilation (5; 130). OSA has also been identified to be a risk factor for divorce (66), and its risk is markedly increased after menopause in women (14). Furthermore, insomnia has been shown to be more common in women than men (169).
Intermediate Factors -- Physical Context: Built Environments
Neighborhood and housing disadvantage
Highlighting the interplay across the levels of influence of population health, insufficient sleep and OSA are associated with individual level factors, such as sleep hygiene and obesity, but also with neighborhood disadvantage and low SES (72; 77). Racial/ethnic minority group members are more likely than whites to live in disadvantaged neighborhoods (148; 165), and the adverse effects of stressors and exposures in these neighborhoods may contribute to CVD disparities via an influence on sleep. Residents living in poorer neighborhoods are more likely to be exposed to factors that may contribute to sleep deficiency, such as inopportune light exposure, noise, allergens, and irritants (e.g. environmental tobacco; (96; 108) air pollution (48; 49). Some of these factors (such as particulate air pollution) may also contribute to abnormalities in the autonomic nervous system and increase CV morbidity. Neighborhood disorder may also increase the prevalence of both poor self-reported sleep quality (71; 77) and sleep disordered breathing (168).
Intermediate Factors-- Social Relationships
Sleep patterns and sleep disorders vary by social factors (e.g. discriminatory practices) in the US, and tend to be tied to sociodemographic (e.g. neighborhood) factors that likely start early in life and contribute to disparities in educational attainment, economic opportunity/productivity, and health.
Family Influences
The sleep of caregivers influences the sleep of children and vice versa. Sleep behaviors also reflect the influences of inter-related social, cultural, and environmental factors operating within households. For example, child sleep may be impacted by factors such as family routines, parenting styles (e.g. sleep routines and curfews; TVs in bedrooms), family illness/accident, depressed parental mood, parent work schedules, as well as exposure to intimate partner violence and other traumatic life events. In fact, adverse childhood experiences have been associated with self-reported sleep disturbances in adulthood (33).
A highly important but understudied area is the role that stress may play in sleep and CVD disparities. Stress is linked to both sleep disturbances and CVD; thus, it is critical to carefully consider the inter-relationships among stress, sleep and CVD as fundamental contributors to health disparities. This is supported by the: a) mediating role of stress in linking socioeconomic disadvantage and CVD risk (3; 43); b) physiological links among stress, arousal and disrupted sleep (89; 101); c) associations between negative emotion and life-long discrimination with impaired sleep (150); and d) increase in CVD risk factors in individuals with impaired sleep (9; 88). Prior mediation analyses have estimated that impaired sleep may explain 10–25% of the variance in health outcomes associated with low SES (70). However, mediation analyses were from cross-sectional data and these studies did not objectively measure CVD risk, precluding definitive assessment. The availability of social support to overcome stressors that impact sleep is also important. Although not well studied, sleep is also likely to be influenced by psycho-social interactions within households, such as chaotic family routines, mother-child stress, the level of autonomy allowed and practiced by each family member, and poor sleep patterns of family members (69; 70; 147) --which may influence stress responses leading to increased arousal, a known mediator of disturbed sleep (123; 146).
Intermediate Factors -- Social Context
Racial discrimination
Racial discrimination may be an important determinant of racial disparities in health. For instance, a study of racially-salient chronic stress - "racism-related vigilance" - and sleep difficulty found that blacks reported greater levels of racism-related vigilance, and greater levels of sleep difficulty compared to whites (76). Institutional and interpersonal racial discrimination may lead to chronic psychosocial stress among racial/ethnic minorities (109; 166). Both objective and perceived racial discrimination are psychosocial stressors disproportionately experienced by racial/ethnic minorities, and they may be implicated in sleep disparities (32; 63; 76; 99; 142; 155; 164). Reports of perceived discrimination were positively associated with increased risk of sleep disturbance and daytime fatigue in a survey of over 7,000 black and white adults (63), and there were similar findings in a smaller study of Hispanics (150). Perceived discrimination has also been shown to be associated with reduced time in deeper (stage N3) sleep (154; 158). Hispanics showed a smaller, but non-significant association, while whites showed no association between racial vigilance and sleep difficulty (76).
Another study found that multiple levels of racism, including interpersonal experiences of racial discrimination and the internalization of negative racial bias, operate jointly to accelerate vascular aging, which involves endothelial cells, smooth muscle cells and cardiomyocytes, as measured by telomere length among black men (32; 136). Household income-to-poverty threshold ratio and the interaction between racial discrimination and implicit racial bias was significantly associated with leukocyte telomere length (32). More work is needed to unpack the effects of discrimination on sleep, and the effect of microaggressions or everyday subtle indignities should also be studied. Nonetheless, existing evidence suggests that the stress of discrimination influences racial/ethnic minority group members’ sleep quality and duration, and thus, may have important downstream effects on CVD outcomes.
Distal Factors -- Institutional Context
Occupational Patterns
There is considerable epidemiological evidence that shift work is associated with a range of problems that includes lost productivity, poor concentration, absenteeism, accidents, errors, injuries, fatalities as well as elevated blood pressure, obesity, cancer, and diabetes (46; 74; 86; 89; 92; 100; 115; 116; 139). Shift work is more common in blacks than whites (101; 128), and is likely an important contributor to racial differences in short sleep duration (22).
Short sleep duration varies by industry and occupation among US workers (103), and work has been shown to affect sleep through long/extended work hours, rotating or night shift work, and job-related stress (94; 127; 156). US blacks may be at particularly high risk for the adverse influences of sleep deficiency on morbidity and mortality (79; 91). Blacks, compared to whites, are more likely to report job-related stress, to work in low control/high demand positions (especially those with low decision-making power), to work more than one low-wage job, to live in poverty despite employment, and to experience discrimination (both objective and perceived) (47; 149; 154; 155). It is also likely that workplace exposures to airborne irritants may contribute to OSA disparities.
Using nationally representative data, racial/ethnic differences in short sleep duration by industry of employment and occupation were observed (80). Blacks, regardless of occupational status, had a higher prevalence of short sleep than their white counterparts, and the disparity was widest among professionals. Additionally, the prevalence of short sleep increased with added professional responsibility among blacks, while it decreased with increasing professional roles among whites. The high prevalence of short sleep duration among professional blacks may be partly attributable to limited or less well-connected professional/social networks that can provide financial and emotional support, more taxing interpersonal relationships related to, for example, John Henryism (a coping strategy where prolonged exposure to stressors like discrimination lead to high effort levels that have detrimental health consequences) and discrimination (e.g. microaggressions) in the workplace (82). Discrimination may play an important role in producing psychosocial stress in addition to job strain or limited control over job demands, as illustrated by the well-known Karasek and Theorell demand-control model that has established an association between high-demand/low-control jobs and heart disease (85; 91). The role of insufficient sleep in the context of upward social mobility deserves further study.
Treatment access and adherence
Sources of disparities in health care occur at the provider (e.g. bias, clinical uncertainty, beliefs/stereotypes about the behavior of health of minority patients) and health care systems-levels (e.g. lack of interpretation and translation services, time pressures on physicians, geographic availability, instability in financing and delivery of health care services) (143). Potentially playing an important role in CVD disparities, racial/ethnic minorities with sleep disorders may receive later diagnoses and treatment due in part to reduced access to screening, diagnosis and therapy interventions (17). Federally-qualified health centers (FQHCs) serve as safety nets for over 19.5 million Americans, mostly those from disadvantaged backgrounds. Although care-paths for managing sleep disorders are evolving, sleep diagnostic testing and OSA management usually require involvement of sleep medicine specialists, who often do not practice in these settings. Thus, limited access to sleep-focused specialty services in FQHCs by underserved populations could exacerbate health disparities. Barriers to referral for specialty care by insurance status and location also may lead to persistent disparities. The limited training of primary care providers in sleep medicine contributes to the high proportion of patients with OSA and insomnia who are not diagnosed or treated adequately (83). Disparities in care and outcomes are likely to worsen without interventions to improve access.
There is also evidence suggesting that independent of access to treatment, adherence to sleep disorder treatments is lower among blacks than whites (23; 138), which may unnecessarily increase incidence and severity of CVD outcomes. In a clinical trial of OSA management pathways that provided standardized care to all participants, blacks were observed to have lower treatment adherence than were whites (12). Differences in socioeconomic resources, social support, and perceived benefits/risks of treatment may underlie racial differences in treatment adherence (50). Although there are minimal data identifying best targets for sleep interventions in the primary care setting, several promising areas include patient sleep literacy, doctor-patient communication, care processes, organization practices and health care quality by incentivizing good sleep care. Lessons learned from other healthcare disparities reduction efforts may provide a useful guide.
Distal Factors-- Social Conditions and Policies
There have been limited public policy interventions designed to directly improve the population’s sleep health. A growing number of school districts have implemented later school start times as a strategy for prolonging sleep duration in children, with some evidence that later start times lead to decreased adolescent motor vehicle crashes and improved school attendance (40). However, wider adoption has been limited by financial constraints of school districts unable to budget for altered scheduling and bus routes. Several early initiatives also are underway to improve public and corporate awareness of sleep health and drowsy driving. Industries employing shift workers have begun to develop policies to mitigate the effects of lowered vigilance among workers and medical residents are now limited in their work schedules to minimize sleep deprivation. A recent program initiated by Harvard Medical School (ReCharge America) aims to provide companies and key policy makers with knowledge, technical assistance, communications support, and tools to support healthy sleep in the workplace (129). There are opportunities for numerous federal and local government agencies that oversee public health, safety and transportation to more actively develop policies to improve the sleep of employees, including those whose job responsibilities impact on public safety (e.g. transportation workers) and to implement policies to further mitigate drowsy driving. The Centers for Disease Control partners with various stakeholders to conduct surveys on sleep, and national efforts are now underway to develop consensus documents on sleep needs across the lifespan. The U.S. Department of Housing and Urban Development could play a role, as those living in poorer neighborhoods are more likely to be exposed to factors that may contribute to sleep deficiency, such as inopportune light exposure, noise, allergens, and irritants (e.g. environmental tobacco) (96; 108). Noise ordinances and a focus on community linkages to resources that alleviate stressors that impact sleep should also be considered as a useful policy strategy.
Future Research Directions for Sleep Disparities
In this section, we describe the current data needs in relation to sleep research overall, followed by CVD and CVD disparities, noting that there are significant methodological challenges in the extant research. Most prior sleep research relies on self report, but often surveys have not been validated in the specific groups of interest. Although “patient reported outcomes” are important, inclusion of objective measures of sleep, as obtained by actigraphy or polysomnography (the gold standard), may minimize misclassification of behaviors that may be difficult to report due to their occurrence during sleep (e.g. snoring in individuals without a bed partner; sleep latency), as well as provide quantitative data on degree and type of sleep disruption. Actigraphy, obtained through a small wrist-worn device with recording of movement over multiple days, provides reliable and valid estimates of sleep-wake periods, yielding objective measures of average and night-to-night variability in sleep duration and of sleep efficiency (a function of wake time during the sleep period) (114). Polysomnography monitors multiple physiological variables during an overnight study to identify specific sleep stages and to characterize patterns of breathing, blood oxygen levels, heart rate and leg movements (81).
The existing data support the need to evaluate sleep disorders as a target for both primary and secondary CVD reduction. To advance this research agenda, there is a need for research that examines the possible bidirectional relationships between stress and sleep and their associations with CVD outcomes. Longitudinal or prospective studies will be critical in establishing temporal ordering of sleep duration and sleep problems, stress, and CVD outcomes for different racial/ethnic groups. Therefore, investigators should conduct more research among diverse populations as racial/ethnic minority and low-income groups have been under-represented in sleep research. Furthermore, causal modeling may help discern the interactive roles of stress and sleep on CVD risk. There is also a need to better measure stress across racial/ethnic groups, and to distinguish effects of sudden, daily, and chronic stress, in addition to stresses occurring during developmental periods that may be particularly relevant for their impact on sleep.
Studies are needed to identify key demographic, personality, cultural, environmental, and genetic moderators of the effects of race on sleep, and sleep on CVD, and to better understand whether sleep disturbances differentially contribute to CVD risk in individuals of different ethnic/racial or socioeconomic backgrounds. For example, are shift workers at higher risk for CVD if they also live in a poor, urban neighborhood?
Multi-level research could contribute to further understanding the influences in individual, household, and neighborhood factors on sleep and the sleep-CVD relationship. Studies of environmental exposures could be enhanced by considering biological effects as measured through epigenetic studies. Given that disturbed sleep and elevated CVD risk emerge early in life in racial/ethnic minorities, it is crucial that these associations be evaluated in both children and adults.
In addition to focusing on risk factors, future research should also identify social, cultural, and physical factors associated with resilience or that are protective for sleep and CVD despite adverse environments. An improved understanding of the influences of acculturation on sleep may also help identify the role of a “Westernized” lifestyle on sleep and CVD in addition to potentially identifying how stress associated with acculturation influences sleep and CVD. The emerging data linking sleep with chronic health problems, including CVD and diabetes, provide a strong basis for the public health community to integrate sleep into investigations of behavioral risk factors, and to consider inclusion of sleep health targets in intervention research. Achieving a sustainable, population-level impact on sleep disparities will likely require coordinated efforts that link policy, systems and environmental changes in diverse settings such as schools, workplaces, community centers, and residential settings (e.g. housing developments) as well as the immediate home environment. Although challenging, successful initiatives could be highly impactful given the multifaceted roles that sleep plays in health and well being.
Acknowledgments
Drs. Redline and Jackson were supported by TREC (1U54CA155626-01). The funding sources were not involved in the data collection, data analysis, manuscript writing and publication. We would also like to thank the panelists for the Harvard Catalyst (UL1 TR001102) Symposium on Sleep Health Disparities for their thoughtful discussions, which helped us formulate the content for this review.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS:
Chandra L. Jackson, PhD, MS; Susan Redline, MD, MPH; Karen M. Emmons, PhD
Study concept and design: Jackson, Redline, Emmons.
Acquisition and interpretation of data: Jackson, Redline, Emmons.
Drafting of the manuscript: Jackson, Redline, Emmons.
Critical revision of the manuscript for important intellectual content: Jackson, Redline, Emmons.
Administrative, technical, and material support: Redline, Emmons.
Obtaining funding and study supervision: Redline, Emmons.
Final approval: Jackson, Redline, Emmons.
REFERENCES
- 1. [Accessed October 2014];Health 2013, United States. http://www.cdc.gov/nchs/data/hus/hus13.pdf.
- 2.Adenekan B, Pandey A, McKenzie S, Zizi F, Casimir GJ, Jean-Louis G. Sleep in America: role of racial/ethnic differences. Sleep medicine reviews. 2013;17:255–262. doi: 10.1016/j.smrv.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MA, Glynn RJ, Buring J, Ridker PM. Impact of traditional and novel risk factors on the relationship between socioeconomic status and incident cardiovascular events. Circulation. 2006;114:2619–2626. doi: 10.1161/CIRCULATIONAHA.106.660043. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: a review of the literature. Progress in cardiovascular nursing. 2004;19:56–59. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep medicine. 2009;10(Suppl 1):S7–S11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Anderson NBRA, Cohen B. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 7.Archbold KH, Giordani B, Ruzicka DL, Chervin RD. Cognitive executive dysfunction in children with mild sleep-disordered breathing. Biological research for nursing. 2004;5:168–176. doi: 10.1177/1099800403260261. [DOI] [PubMed] [Google Scholar]
- 8.Baker FC, Wolfson AR, Lee KA. Association of sociodemographic, lifestyle, and health factors with sleep quality and daytime sleepiness in women: findings from the 2007 National Sleep Foundation "Sleep in America Poll". J Womens Health (Larchmt) 2009;18:841–849. doi: 10.1089/jwh.2008.0986. [DOI] [PubMed] [Google Scholar]
- 9.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr The Cook-Medley hostility scale: item content and ability to predict survival. Psychosomatic medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin JA, Lewis KE. Sleep-disordered breathing and cardiovascular disease. Postgraduate medical journal. 2008;84:15–22. doi: 10.1136/pgmj.2007.062836. [DOI] [PubMed] [Google Scholar]
- 11.Bersani FS, Iannitelli A, Pacitti F, Bersani G. Sleep and biorythm disturbances in schizophrenia, mood and anxiety disorders: a review. Rivista di psichiatria. 2012;47:365–375. doi: 10.1708/1175.13027. [DOI] [PubMed] [Google Scholar]
- 12.Billings ME, Rosen CL, Wang R, Auckley D, Benca R, et al. Is the relationship between race and continuous positive airway pressure adherence mediated by sleep duration? Sleep. 2013;36:221–227. doi: 10.5665/sleep.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: a systematic review. Sleep medicine reviews. 2012;16:223–230. doi: 10.1016/j.smrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, et al. Prevalence of sleep-disordered breathing in women: effects of gender. American journal of respiratory and critical care medicine. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 15.Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in central Pennsylvania. Journal of psychosomatic research. 2002;53:589–592. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet MH. Evidence for the pathophysiology of insomnia. Sleep. 2009;32:441–442. doi: 10.1093/sleep/32.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boss EF, Smith DF, Ishman SL. Racial/ethnic and socioeconomic disparities in the diagnosis and treatment of sleep-disordered breathing in children. International journal of pediatric otorhinolaryngology. 2011;75:299–307. doi: 10.1016/j.ijporl.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Bosworth HB, Dudley T, Olsen MK, Voils CI, Powers B, et al. Racial differences in blood pressure control: potential explanatory factors. The American journal of medicine. 2006;119:70, e9–e15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 20.Brancati FL, Whelton PK, Kuller LH, Klag MJ. Diabetes mellitus, race, and socioeconomic status. A population-based study. Ann.Epidemiol. 1996;6:67–73. doi: 10.1016/1047-2797(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 21.Braveman P. Health disparities and health equity: concepts and measurement. Annual review of public health. 2006;27:167–194. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- 22.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. American journal of public health. 2010;100(Suppl 1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budhiraja R, Parthasarathy S, Drake CL, Roth T, Sharief I, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–324. [PubMed] [Google Scholar]
- 24.Buxton OM, Cain SW, O'Connor SP, Porter JH, Duffy JF, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Science translational medicine. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010;138:434–443. doi: 10.1378/chest.09-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, Duran-Cantolla J, Pena Mde L, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. American journal of respiratory and critical care medicine. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 29.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European heart journal. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 30.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, et al. Discrimination, racial bias, and telomere length in african-american men. American journal of preventive medicine. 2014;46:103–111. doi: 10.1016/j.amepre.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman DP, Wheaton AG, Anda RF, Croft JB, Edwards VJ, et al. Adverse childhood experiences and sleep disturbances in adults. Sleep medicine. 2011;12:773–779. doi: 10.1016/j.sleep.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Chasens ER, Twerski SR, Yang K, Umlauf MG. Sleepiness and health in midlife women: results of the National Sleep Foundation's 2007 Sleep in America poll. Behavioral sleep medicine. 2010;8:157–171. doi: 10.1080/15402002.2010.487462. [DOI] [PubMed] [Google Scholar]
- 35.Chee MW. Sleep, public health and wellness: the elephant in the room. Annals of the Academy of Medicine, Singapore. 2013;42:105–107. [PubMed] [Google Scholar]
- 36.Chervin RD, Dillon JE, Archbold KH, Ruzicka DL. Conduct problems and symptoms of sleep disorders in children. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:201–208. doi: 10.1097/00004583-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Committee on Assuring the Health of the Public in the 21st Century IoM. The Future of the Public’s Health in the 21st Century. Washington, DC: National Academy Press; 2002. [Google Scholar]
- 38.Coonrod DV, Bay RC, Balcazar H. Ethnicity, acculturation and obstetric outcomes. Different risk factor profiles in low- and high-acculturation Hispanics and in white non-Hispanics. The Journal of reproductive medicine. 2004;49:17–22. [PubMed] [Google Scholar]
- 39.Costa G. The 24-hour society between myth and reality. Journal of human ergology. 2001;30:15–20. [PubMed] [Google Scholar]
- 40.Danner F, Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2008;4:533–535. [PMC free article] [PubMed] [Google Scholar]
- 41.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA : the journal of the American Medical Association. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis AM, Vinci LM, Okwuosa TM, Chase AR, Huang ES. Cardiovascular health disparities: a systematic review of health care interventions. Medical care research and review : MCRR. 2007;64:29S–100S. doi: 10.1177/1077558707305416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deuster PA, Kim-Dorner SJ, Remaley AT, Poth M. Allostatic load and health status of African Americans and whites. American journal of health behavior. 2011;35:641–653. doi: 10.5993/ajhb.35.6.1. [DOI] [PubMed] [Google Scholar]
- 44.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 45.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 46.Ellingsen T, Bener A, Gehani AA. Study of shift work and risk of coronary events. The journal of the Royal Society for the Promotion of Health. 2007;127:265–267. doi: 10.1177/1466424007083702. [DOI] [PubMed] [Google Scholar]
- 47.Ertel KA, Berkman LF, Buxton OM. Socioeconomic status, occupational characteristics, and sleep duration in African/Caribbean immigrants and US White health care workers. Sleep. 2011;34:509–518. doi: 10.1093/sleep/34.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans GW. The environment of childhood poverty. The American psychologist. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 49.Evans GW, Marcynyszyn LA. Environmental justice, cumulative environmental risk, and health among low- and middle-income children in upstate New York. American journal of public health. 2004;94:1942–1944. doi: 10.2105/ajph.94.11.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fleck DE, Keck PE, Jr, Corey KB, Strakowski SM. Factors associated with medication adherence in African American and white patients with bipolar disorder. The Journal of clinical psychiatry. 2005;66:646–652. doi: 10.4088/jcp.v66n0517. [DOI] [PubMed] [Google Scholar]
- 51.Fox SRL. The Web at 25 in the U. S. The overall verdict: The internet has been a plus for society and an especially good thing for individual users. Pew Research Internet Project. 2014. [Google Scholar]
- 52.Gadegbeku CA, Lea JP, Jamerson KA. Update on disparities in the pathophysiology and management of hypertension: focus on African Americans. Med Clin North Am. 2005;89:921–933. doi: 10.1016/j.mcna.2005.05.003. 30. [DOI] [PubMed] [Google Scholar]
- 53.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. Journal of sleep research. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 54.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gellis LA, Lichstein KL, Scarinci IC, Durrence HH, Taylor DJ, et al. Socioeconomic status and insomnia. Journal of abnormal psychology. 2005;114:111–118. doi: 10.1037/0021-843X.114.1.111. [DOI] [PubMed] [Google Scholar]
- 56.Giordani B, Hodges EK, Guire KE, Ruzicka DL, Dillon JE, et al. Changes in neuropsychological and behavioral functioning in children with and without obstructive sleep apnea following Tonsillectomy. Journal of the International Neuropsychological Society : JINS. 2012;18:212–222. doi: 10.1017/S1355617711001743. [DOI] [PubMed] [Google Scholar]
- 57.Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117:417–424. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- 58.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 59.Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. The sleep and technology use of Americans: findings from the National Sleep Foundation's 2011 Sleep in America poll. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2013;9:1291–1299. doi: 10.5664/jcsm.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep medicine. 2014;15:42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep medicine reviews. 2007;11:341–360. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grandner MA, Gallagher RA, Gooneratne NS. The use of technology at night: impact on sleep and health. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2013;9:1301–1302. doi: 10.5664/jcsm.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grandner MA, Hale L, Jackson N, Patel NP, Gooneratne NS, Troxel WM. Perceived racial discrimination as an independent predictor of sleep disturbance and daytime fatigue. Behavioral sleep medicine. 2012;10:235–249. doi: 10.1080/15402002.2012.654548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grandner MA, Pack AI. Sleep disorders, public health, and public safety. JAMA : the journal of the American Medical Association. 2011;306:2616–2617. doi: 10.1001/jama.2011.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep medicine. 2010;11:470–478. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grunstein RR, Stenlof K, Hedner JA, Sjostrom L. Impact of self-reported sleep-breathing disturbances on psychosocial performance in the Swedish Obese Subjects (SOS) Study. Sleep. 1995;18:635–643. doi: 10.1093/sleep/18.8.635. [DOI] [PubMed] [Google Scholar]
- 67.Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 68.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hale L, Hale B. Treat the source not the symptoms: why thinking about sleep informs the social determinants of health. Health education research. 2010;25:395–400. doi: 10.1093/her/cyq027. [DOI] [PubMed] [Google Scholar]
- 70.Hale L, Hill TD, Burdette AM. Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Preventive medicine. 2010;51:275–278. doi: 10.1016/j.ypmed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 71.Hale L, Hill TD, Friedman E, Javier Nieto F, Galvao LW, et al. Perceived neighborhood quality, sleep quality, and health status: Evidence from the Survey of the Health of Wisconsin. Soc Sci Med. 2012 doi: 10.1016/j.socscimed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hale L, Hill TD, Friedman E, Nieto FJ, Galvao LW, et al. Perceived neighborhood quality, sleep quality, and health status: evidence from the Survey of the Health of Wisconsin. Soc Sci Med. 2013;79:16–22. doi: 10.1016/j.socscimed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hale L, Rivero-Fuentes E. Negative acculturation in sleep duration among Mexican immigrants and Mexican Americans. Journal of immigrant and minority health / Center for Minority Public Health. 2011;13:402–407. doi: 10.1007/s10903-009-9284-1. [DOI] [PubMed] [Google Scholar]
- 74.Haupt CM, Alte D, Dorr M, Robinson DM, Felix SB, et al. The relation of exposure to shift work with atherosclerosis and myocardial infarction in a general population. Atherosclerosis. 2008;201:205–211. doi: 10.1016/j.atherosclerosis.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 75.Heilemann MV, Choudhury SM, Kury FS, Lee KA. Factors associated with sleep disturbance in women of Mexican descent. Journal of advanced nursing. 2012;68:2256–2266. doi: 10.1111/j.1365-2648.2011.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hicken MT, Lee H, Ailshire J, Burgard SA, Williams DR. "Every shut eye, ain't sleep": The role of racism-related vigilance in racial/ethnic disparities in sleep difficulty. Race and social problems. 2013;5:100–112. doi: 10.1007/s12552-013-9095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill TD, Burdette AM, Hale L. Neighborhood disorder, sleep quality, and psychological distress: testing a model of structural amplification. Health & place. 2009;15:1006–1013. doi: 10.1016/j.healthplace.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Institute of Medicine. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington D: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 79.Jackson CL, Hu FB, Redline S, Williams DR, Mattei J, Kawachi I. Racial/ethnic disparities in short sleep duration by occupation: The contribution of immigrant status. Soc Sci Med. 2014;118:71–79. doi: 10.1016/j.socscimed.2014.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson CL, Redline S, Kawachi I, Williams MA, Hu FB. Racial Disparities in Short Sleep Duration by Occupation and Industry. American journal of epidemiology. 2013 doi: 10.1093/aje/kwt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jafari B, Mohsenin V. Polysomnography. Clinics in chest medicine. 2010;31:287–297. doi: 10.1016/j.ccm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 82.James SA. John Henryism and the health of African-Americans. Culture, medicine and psychiatry. 1994;18:163–182. doi: 10.1007/BF01379448. [DOI] [PubMed] [Google Scholar]
- 83.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep & breathing = Schlaf & Atmung. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 84.Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respiratory care. 2010;55:1155–1167. [PubMed] [Google Scholar]
- 85.Karasek RA, Theorell T. Healthy work: stress, productivity, and the reconstruction of working life. 1992. (1992). Basic books. [Google Scholar]
- 86.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occupational and environmental medicine. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karter AJ, Schillinger D, Adams AS, Moffet HH, Liu J, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE) Diabetes care. 2013;36:574–579. doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best practice & research. Clinical endocrinology & metabolism. 2010;24:731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;2:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 90.Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep medicine. 2010;11:652–658. doi: 10.1016/j.sleep.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 91.Krieger N, Waterman PD, Hartman C, Bates LM, Stoddard AM, et al. Social hazards on the job: workplace abuse, sexual harassment, and racial discrimination--a study of Black, Latino, and White low-income women and men workers in the United States. International journal of health services : planning, administration, evaluation. 2006;36:51–85. doi: 10.2190/3EMB-YKRH-EDJ2-0H19. [DOI] [PubMed] [Google Scholar]
- 92.Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. American journal of epidemiology. 2007;165:175–183. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 93.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. American journal of epidemiology. 2009;169:1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuhn P, Lozano F. The Expanding Workweek? Understanding Trends in Long Work Hours among U.S. Men, 1979–2006. Journal of Labor Economics. 2008;26:311–343. [Google Scholar]
- 95.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethnicity & disease. 2007;17:143–152. [PubMed] [Google Scholar]
- 96.Landrigan PJ, Rauh VA, Galvez MP. Environmental justice and the health of children. The Mount Sinai journal of medicine, New York. 2010;77:178–187. doi: 10.1002/msj.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lanfranchi P, Somers VK. Obstructive sleep apnea and vascular disease. Respiratory research. 2001;2:315–319. doi: 10.1186/rr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lara M, Gamboa C, Kahramanian MI, Morales LS, Bautista DE. Acculturation and Latino health in the United States: a review of the literature and its sociopolitical context. Annual review of public health. 2005;26:367–397. doi: 10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis TT, Troxel WM, Kravitz HM, Bromberger JT, Matthews KA, Hall MH. Chronic exposure to everyday discrimination and sleep in a multiethnic sample of middle-aged women. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2013;32:810–819. doi: 10.1037/a0029938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lian Y, Xiao J, Liu Y, Ning L, Guan S, et al. Associations between insomnia, sleep duration and poor work ability. Journal of psychosomatic research. 2014 doi: 10.1016/j.jpsychores.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 101.Lieu SJ, Curhan GC, Schernhammer ES, Forman JP. Rotating night shift work and disparate hypertension risk in African-Americans. Journal of hypertension. 2012;30:61–66. doi: 10.1097/HJH.0b013e32834e1ea3. [DOI] [PubMed] [Google Scholar]
- 102.Loredo JS, Soler X, Bardwell W, Ancoli-Israel S, Dimsdale JE, Palinkas LA. Sleep health in U.S. Hispanic population. Sleep. 2010;33:962–967. doi: 10.1093/sleep/33.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luckhaupt SE, Tak S, Calvert GM. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33:149–159. doi: 10.1093/sleep/33.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maia Q, Grandner MA, Findley J, Gurubhagavatula I. Short and long sleep duration and risk of drowsy driving and the role of subjective sleep insufficiency. Accident; analysis and prevention. 2013;59:618–622. doi: 10.1016/j.aap.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marco CA, Wolfson AR, Sparling M, Azuaje A. Family socioeconomic status and sleep patterns of young adolescents. Behavioral sleep medicine. 2011;10:70–80. doi: 10.1080/15402002.2012.636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marin JM, Agusti A, Villar I, Forner M, Nieto D, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA : the journal of the American Medical Association. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 108.Marshall JD, Brauer M, Frank LD. Healthy neighborhoods: walkability and air pollution. Environmental health perspectives. 2009;117:1752–1759. doi: 10.1289/ehp.0900595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annual review of psychology. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meetze K, Gillespie MB, Lee FS. Obstructive sleep apnea: a comparison of black and white subjects. The Laryngoscope. 2002;112:1271–1274. doi: 10.1097/00005537-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 111.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff (Millwood) 2007;26:38–48. doi: 10.1377/hlthaff.26.1.38. [DOI] [PubMed] [Google Scholar]
- 112.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 113.Moore PJ, Adler NE, Williams DR, Jackson JS. Socioeconomic status and health: the role of sleep. Psychosomatic medicine. 2002;64:337–344. doi: 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 114.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 115.Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, et al. Effect of shift work on body mass index and metabolic parameters. Scandinavian journal of work, environment & health. 2007;33:45–50. doi: 10.5271/sjweh.1063. [DOI] [PubMed] [Google Scholar]
- 116.Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Progress in brain research. 2012;199:337–358. doi: 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nanduri JPNR. Impact of Sleep and Sleep Disturbances on Obesity and Cancer. In: Redline SB, editor. Energy Balance and Cancer. New York: Springer; 2014. NA. [Google Scholar]
- 120.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. American journal of respiratory and critical care medicine. 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA : the journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep medicine reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 123.Olafiranye O, Jean-Louis G, Magai C, Zizi F, Brown CD, et al. Anxiety and cardiovascular symptoms: the modulating role of insomnia. Cardiology. 2010;115:114–119. doi: 10.1159/000258078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Partinen M. Epidemiology of sleep disorders. Handbook of clinical neurology. 2011;98:275–314. doi: 10.1016/B978-0-444-52006-7.00018-6. [DOI] [PubMed] [Google Scholar]
- 125.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pigeon WR, Heffner K, Duberstein P, Fiscella K, Moynihan J, Chapman BP. Elevated sleep disturbance among blacks in an urban family medicine practice. Journal of the American Board of Family Medicine : JABFM. 2011;24:161–168. doi: 10.3122/jabfm.2011.02.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pilcher JJ, Lambert BJ, Huffcutt AI. Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep. 2000;23:155–163. [PubMed] [Google Scholar]
- 128.Presser H. Race-ethnic and gender differences in nonstandard work shifts. Work and Occupations. 2003;30:412–439. [Google Scholar]
- 129.Program RA-BSM. https://sleep.med.harvard.edu/calendar/2153/ReCharge+America-+Because+Sleep+Matters.
- 130.Ralls FM, Grigg-Damberger M. Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Current opinion in pulmonary medicine. 2012;18:568–573. doi: 10.1097/MCP.0b013e328358be05. [DOI] [PubMed] [Google Scholar]
- 131.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. American journal of respiratory and critical care medicine. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Institute of Medicine Report. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. The National Academies Press; 2006. [PubMed] [Google Scholar]
- 133.Riedel BW, Durrence HH, Lichstein KL, Taylor DJ, Bush AJ. The relation between smoking and sleep: the influence of smoking level, health, and psychological variables. Behavioral sleep medicine. 2004;2:63–78. doi: 10.1207/s15402010bsm0201_6. [DOI] [PubMed] [Google Scholar]
- 134.Ruggiero JS, Redeker NS. Effects of Napping on Sleepiness and Sleep-Related Performance Deficits in Night-Shift Workers: A Systematic Review. Biological research for nursing. 2013 doi: 10.1177/1099800413476571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep medicine. 2011;12:209–214. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 136.Saliques S, Zeller M, Lorin J, Lorgis L, Teyssier JR, et al. Telomere length and cardiovascular disease. Archives of cardiovascular diseases. 2010;103:454–459. doi: 10.1016/j.acvd.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 137.Sarsour K, Van Brunt DL, Johnston JA, Foley KA, Morin CM, Walsh JK. Associations of nonrestorative sleep with insomnia, depression, and daytime function. Sleep medicine. 2010;11:965–972. doi: 10.1016/j.sleep.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 138.Sawyer AM, Canamucio A, Moriarty H, Weaver TE, Richards KC, Kuna ST. Do cognitive perceptions influence CPAP use? Patient education and counseling. 2011;85:85–91. doi: 10.1016/j.pec.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital and health statistics. Series 10, Data from the National Health Survey. 2012:1–207. [PubMed] [Google Scholar]
- 141.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, Liao D, Calhoun S, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep medicine. 2012;13:346–353. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Slopen N, Williams DR. Discrimination, other psychosocial stressors, and self-reported sleep duration and difficulties. Sleep. 2014;37:147–156. doi: 10.5665/sleep.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smedley BAS, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. 2003. [PubMed] [Google Scholar]
- 144.Soltani M, Haytabakhsh MR, Najman JM, Williams GM, O'Callaghan MJ, et al. Sleepless nights: the effect of socioeconomic status, physical activity, and lifestyle factors on sleep quality in a large cohort of Australian women. Archives of women's mental health. 2012;15:237–247. doi: 10.1007/s00737-012-0281-3. [DOI] [PubMed] [Google Scholar]
- 145.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. The Journal of clinical endocrinology and metabolism. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 146.Spiegelhalder K, Regen W, Feige B, Hirscher V, Unbehaun T, et al. Sleep-related arousal versus general cognitive arousal in primary insomnia. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2012;8:431–437. doi: 10.5664/jcsm.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spilsbury JC, Storfer-Isser A, Drotar D, Rosen CL, Kirchner HL, Redline S. Effects of the home environment on school-aged children's sleep. Sleep. 2005;28:1419–1427. doi: 10.1093/sleep/28.11.1419. [DOI] [PubMed] [Google Scholar]
- 148.Spilsbury JC, Storfer-Isser A, Kirchner HL, Nelson L, Rosen CL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. The Journal of pediatrics. 2006;149:342–347. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 149.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Annals of epidemiology. 2007;17:948–955. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Steffen PR, Bowden M. Sleep disturbance mediates the relationship between perceived racism and depressive symptoms. Ethnicity & disease. 2006;16:16–21. [PubMed] [Google Scholar]
- 151.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29:777–783. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 152.Swanson LM, Arnedt JT, Rosekind MR, Belenky G, Balkin TJ, Drake C. Sleep disorders and work performance: findings from the 2008 National Sleep Foundation Sleep in America poll. Journal of sleep research. 2011;20:487–494. doi: 10.1111/j.1365-2869.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 153.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 154.Thomas KS, Bardwell WA, Ancoli-Israel S, Dimsdale JE. The toll of ethnic discrimination on sleep architecture and fatigue. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2006;25:635–642. doi: 10.1037/0278-6133.25.5.635. [DOI] [PubMed] [Google Scholar]
- 155.Tomfohr L, Pung MA, Edwards KM, Dimsdale JE. Racial differences in sleep architecture: the role of ethnic discrimination. Biological psychology. 2012;89:34–38. doi: 10.1016/j.biopsycho.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tucker P, Smith L, Macdonald I, Folkard S. The impact of early and late shift changeovers on sleep, health, and well-being in 8- and 12-hour shift systems. Journal of occupational health psychology. 1998;3:265–275. doi: 10.1037//1076-8998.3.3.265. [DOI] [PubMed] [Google Scholar]
- 157.Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Hormone research. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- 158.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 159.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes care. 2009;32:1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vogtmann E, Levitan EB, Hale L, Shikany JM, Shah NA, et al. Association between sleep and breast cancer incidence among postmenopausal women in the women's health initiative. Sleep. 2013;36:1437–1444. doi: 10.5665/sleep.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Walia HK, Li H, Rueschman M, Bhatt DL, Patel SR, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10:835–843. doi: 10.5664/jcsm.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22(Suppl 2):S386–S393. [PubMed] [Google Scholar]
- 163.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. American journal of public health. 2008;98:1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Williams DR. Miles to go before we sleep: racial inequities in health. Journal of health and social behavior. 2012;53:279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff (Millwood) 2005;24:325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- 166.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. Journal of behavioral medicine. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Worsnop CJ, Naughton MT, Barter CE, Morgan TO, Anderson AI, Pierce RJ. The prevalence of obstructive sleep apnea in hypertensives. American journal of respiratory and critical care medicine. 1998;157:111–115. doi: 10.1164/ajrccm.157.1.9609063. [DOI] [PubMed] [Google Scholar]
- 168.Zanobetti A, Redline S, Schwartz J, Rosen D, Patel S, et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. American journal of respiratory and critical care medicine. 2010;182:819–825. doi: 10.1164/rccm.200912-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 170.Zimberg IZ, Damaso A, Del Re M, Carneiro AM, de Sa Souza H, et al. Short sleep duration and obesity: mechanisms and future perspectives. Cell biochemistry and function. 2012;30:524–529. doi: 10.1002/cbf.2832. [DOI] [PubMed] [Google Scholar]
- 171.Zizi F, Pandey A, Murrray-Bachmann R, Vincent M, McFarlane S, et al. Race/ethnicity, sleep duration, and diabetes mellitus: analysis of the National Health Interview Survey. The American journal of medicine. 2012;125:162–167. doi: 10.1016/j.amjmed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]